Rheumatoid arthritis (RA) is a high-cost disease, which allows patients to be classified into early or established phase approaches.
ObjectiveThe purpose of this work was to perform a cost-effectiveness analysis comparing both phases with patient data at a 6-month time horizon from a third-party payer perspective.
Materials and methodsThe population was delimited. The costs and effectiveness of each of the phases were estimated. A decision tree-type economic evaluation model was developed, and the Incremental Cost-Effectiveness Ratio (ICER) was calculated with the respective sensitivity analyses, both deterministic and probabilistic.
ResultsIn terms of costs, it was found that for effectiveness in goals, the cost was 85% higher in the established than in the early phase. Similarly, for non-target effectiveness, the cost was 77% higher in the established than in the early phase. On the other hand, the effectiveness results were better in the early phase compared to the established phase. Regarding the ICER, it was determined that the early phase approach saves $2,326,389 COPcte (colombian pesos current currency) per patient in goals at 6 months of treatment, compared to the established phase approach.
ConclusionThe clinical approach to early-stage rheumatoid arthritis is a less costly and more effective alternative vs. the established phase, as it generates savings for the third-party payer over a 6-month time horizon, from a third-party payer perspective.
La artritis reumatoide (AR) es una enfermedad de alto costo, que permite clasificar a los pacientes en fase temprana o establecida.
ObjetivoEl propósito de este trabajo fue hacer un análisis de costoefectividad, comparando ambas fases con datos de pacientes a un horizonte temporal de 6 meses, desde la perspectiva del tercer pagador.
Materiales y métodosSe delimitó la población. Se estimaron los costos y las efectividades de cada una de las fases. Se desarrolló un modelo de evaluación económica de tipo árbol de decisión y se calculó la razón incremental costoefectividad (RICE) con sus respectivos análisis de sensibilidad, tanto determinístico como probabilístico.
ResultadosEn términos de costos, se encontró que para una efectividad en metas el costo fue un 85% mayor en fase establecida que en fase temprana. Igualmente, para una efectividad en no metas, el costo fue un 77% mayor en fase establecida que en fase temprana. Por otra parte, los resultados de efectividad salieron a favor de la fase temprana en comparación con la fase establecida. Con respecto a la RICE, se determinó que el abordaje en fase temprana ahorra $2.326.389 COPcte (pesos colombianos moneda corriente) por paciente en metas a los 6 meses de tratamiento, en comparación con el abordaje en fase establecida.
ConclusiónEl abordaje clínico de la artritis reumatoide en fase temprana es una alternativa menos costosa y más efectiva vs. la fase establecida, ya que genera ahorros para el tercer pagador en un horizonte temporal de 6 meses, desde la perspectiva del tercer pagador.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that, untreated, leads to disability, pain, reduced quality of life, and premature mortality.1 According to the Colombian Fund for High-Cost Diseases (CAC by its acronym in Spanish), even though the global prevalence of RA is low (ranges from 0.2% to 5%), Colombia reported a prevalence of 0.23 cases per 100 inhabitants and an incidence of 13.78 per 100,000 inhabitants in 2018. The regions with the highest prevalence included Bogotá DC, Risaralda, Caldas, and Antioquia. The average age of reported patients was 57.8 years, with a male-to-female ratio of 1:5, and the mean age range at RA diagnosis was 36–64 years.2
Patients with RA can be classified into early or established phases.3–7 The early phase refers to ≤12 months from symptom onset to starting treatment with disease-modifying drugs (DMARDs), while, in the established phase, this time is longer than 12 months.5 These phases are mutually exclusive and not consecutive and occur only once in a patient's lifetime.3,5,7
However, in the early course of the disease, RA presents a phenotype with immunoregulatory alterations that can be temporarily or permanently blocked; thus, early treatment of RA is crucial due to its potential to prevent long-term joint damage and improve cumulative quality of life.8 Concerningly, the 2018 CAC report highlighted low compliance with opportunity indicators such as time of onset of symptoms to specialist care, diagnosis, and DMARD treatment initiation.2
Also, it is important to address RA activity levels, assessed by Disease Activity Score-28 (DAS28), categorizing subjects into remission (<2.4), low (2.4–3.6), moderate (3.6–5.5), and severe activity (≥5.5).8 Achieving remission or low activity is an international therapeutic target, including Colombia, to prevent complications, disability, and improve patient quality of life.9,10 Furthermore, this is a therapeutic goal that can be achieved in the short-term; therefore, after six months, the patient can be classified as target or non-target (moderate or severe activity).10 However, the 2018 CAC report indicated that only 44% of patients initially in high activity achieved remission, with 69% remaining in high activity, underscoring the need for improved therapeutic outcomes.2
RA is categorized as a high-cost disease due to its impact on productivity, medical expenses, out-of-pocket costs, medications, hospitalizations, surgeries, and interdisciplinary care due to the high consumption of resources.1,5,8 Thus, based on the 2018 CAC report, the estimated average six-month cost per patient in remission was $15,885,885 Colombian pesos in current currency (COPcte). Extrapolating this to the entire cohort evaluated in the report (79,199 patients) 2 that had reached remission would suggest management costs of approximately 1.2 billion COP. This prompts the question: how can pharmacoeconomics help Colombia ensure most patients achieve treatment goals at a lower cost?
Similarly, the question arose: is there a cost-effectiveness analysis that compares the clinical approach to RA in the early vs. established phases in Colombia? At the University Hospital of the Fundacion Santa Fe de Bogotá (HUFSFB), a quaternary health service institution in Colombia, many patients are referred by the Health Promotion Entity (EPS for its acronym in Spanish) after 12 months of symptoms, classifying them in the established phase. The lack of cost-effectiveness studies comparing early versus established phase approaches in Colombia in a real cohort of patients with the relevance of providing viability to developing health programs that promote the early diagnosis and treatment of RA prompted this research.
The purpose of this research was to conduct an economic evaluation answering how costly and effective is treating early-phase RA patients compared to those in the established phase. The study aimed to identify costs, compare effectiveness, and estimate the incremental cost-effectiveness ratio (ICER) of these approaches.
This research contributes new insights into RA management in Colombia, informing health policies by the entities administering benefit plans, according to the incremental cost per unit of effectiveness, based on real cohort data rather than simulations. It provides a foundational basis for further research into the national budgetary impacts of early versus established phase approaches, aiming to enhance RA management programs across the country.
Materials and methodsDelimitation of the population under study and research problemFollowing the PICOT question format—Patient, Intervention, Comparison, Outcome, and Time—this study focused on adult patients diagnosed with RA. The intervention of interest was the early-phase approach, defined as the initial clinical management upon RA diagnosis, compared to the established-phase approach. The primary outcome evaluated was disease level categorized as “target”,11 with a follow-up period of six months ± one month for both cost and effectiveness assessments. Regarding the time horizon, exactly six months was not chosen, because not all patients attended follow-up during their first six months of treatment.10–15
Information collectionClinical approach data for patients were extracted from medical records at HUFSFB and validated through meticulous data mining processes.5
The study ensured consistency in clinical approaches across early and established phases at HUFSFB, minimizing bias in approach variability, and making it possible to analyze whether there is a discrepancy in the costs and effectiveness due to the phase of disease. The institution's standardized care model adheres closely to national clinical guidelines, ensuring uniformity in patient management protocols, from referral criteria by the EPS, admission, physical examination, and tests; the latter are taken and analyzed in the same center.5 Moreover, internal monitoring of this model guarantees adherence by healthcare professionals to defined care protocols.5 The comprehensive nature of this model allows direct comparison between early and established phases, mitigating potential biases arising from different care pathways based on disease phase.5 Importantly, resource prescription at HUFSFB is unrestricted, ensuring patients receive necessary treatments irrespective of disease phase.5 Therefore, the HUFSFB comprehensive care model is essential, since it allows us to assess whether the cost differential is due to the phase in which the patient is located.5
Cohort description and sample designThe study utilized data from HUFSFB spanning May 2013 to December 2018, comprising 971 subjects (181 in the early phase, 790 in the established phase). A non-probabilistic inclusion method encompassed patients meeting specific criteria5:
Inclusion criteria- a)
Complete and consistent data with medical records
- b)
Confirmed RA diagnosis
- c)
At least one follow-up
- d)
Age over 18
- e)
Absence of other immunological disorders.
Exclusion criteria included duplicate follow-ups.
Subsequent univariate analysis and Propensity Score matching were employed,16 and subjects within the six months ± one month follow-up timeframe were selected.
CostsDirect medical costs over six-month periods were estimated based on PICOT parameters. Thus, given that the database did not contain the cost of the approach, identification, quantification, and assessment of cost-generating events were conducted under the assumption that prescribed medications were dispensed and consumed by patients, without applying a discount rate.16,17
About the directionality of costs, the compilation of the use of cost-generating events used a bottom-up approach, until the total cost of the intervention for patients in each phase was obtained according to its final effectiveness (target or non-target)18 detailing expenditures related to medications, clinical laboratories, hospitalizations, and consultations.17 Costs were measured in COPcte.
For medication costs, the Anatomical Therapeutic Chemical Classification Code (ATC) was used, while other cost-generating events employed the Mandatory Traffic Accident Insurance (SOAT) code.18 Data sources included the Drug Price Information System 2018,19 SOAT Tariff Manual,20 and National Commission for Prices of Drugs and Medical Devices Circular 04 of 2018.21 Unit values from 2018 were applied uniformly across the study period, regardless of the period they had their six ± one month follow-up.
Regarding adverse drug reactions,18 the number of patients on target in both phases was compared and p-values were calculated, yet no difference was found in the proportion of subjects who presented them. Thus, these probabilities were not included in the model. In the database delivered and authorized for this assessment, there was only data on the number of subjects with adverse drug events, and it was specified which medication it was associated with, but detailed information about the type of event was missing; however, the HUFSFB explained that, according to confidential medical records, minor events were managed by reducing the medication dose or withdrawing it.
For the costing that will be presented in the analysis, the average semiannual cost of a patient per cost-generating event was calculated among the subjects who used the event, as follows: the amount of the event was multiplied by its unit value and divided between the number of patients who used the event; these results were then totaled by corresponding activity (medications, clinical laboratories, hospital admissions, and consultations). The costs that entered the model were calculated with the following difference: the amount was multiplied by the unit value of each cost-generating event, totaled, and then divided by the total number of patients in the corresponding subgroup (understood as target early phase, non-target early phase, target established phase, and non-target established phase) so that the cost was jointly assumed among all patients in the subgroup. This was determined in such a way the effectiveness probabilities used in the model were unique per subgroup.
EffectivenessEffectiveness was assessed by the proportion of patients achieving treatment goals10 within the initial six months ± one month follow-up period.18 The minimum and maximum effectiveness were calculated by adjusting base case effectiveness data, subtracting and adding 10%, respectively.
ModelAn analytical decision tree model was developed in Excel® to assess costs and effectiveness between early-phase and established-phase RA approaches, reflecting the study's defined time horizon and outcomes. Based on the time horizon defined for the study and the type of outcome, this was considered the best model following good modeling practices.22–25
Incremental cost-effectiveness ratio (ICER)ICER calculations were performed in Excel®.26
Sensitivity analysisDeterministic and probabilistic sensitivity analyses in Excel® assessed the impact of variable uncertainties on ICER outcomes.27–29
Error and bias control strategiesAll patients were included regardless of initial activity level, considering potential changes by the study's time horizon. Activity level was factored into matching processes to balance target and non-target subjects at baseline.
ResultsDescriptive statistical analysisTo characterize the population (971 patients: 181 in the early phase and 790 in the established phase), the database was reviewed for sociodemographic and clinical variables. Sociodemographic variables included age, education, marital status, population group, and gender. Clinical variables included body mass index (BMI), smoking (SMK), rheumatoid factor (RF), cyclic citrullinated peptide (CCP) antibodies, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), DAS28 (to determine RA activity level), Health Assessment Questionnaire (HAQ), and active alcohol consumption.
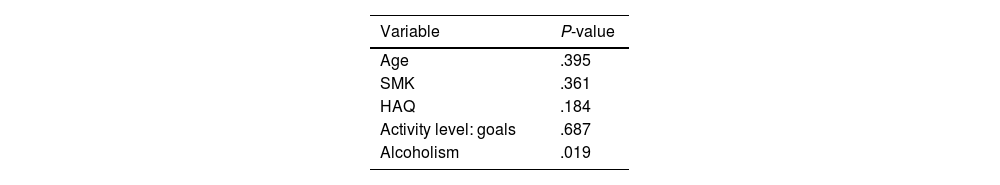
The inclusion and exclusion criteria described in the methodology were applied to the patient records, resulting in 128 patients in the early phase and 551 in the established phase. Univariate analysis revealed significant differences between the phases for age, SMK, HAQ, disease activity levels (in goals and non-goals), and active alcohol consumption, with P-values below .05 (see Table 1).
PairingGiven the observed differences in variables, the nearest neighbor Propensity Score method was used for matching to ensure homogeneity between the early and established phase patients.30 Variables showing statistical differences, as per literatura,31–37 were included in the matching process to correlate with the effectiveness outcome (disease activity).
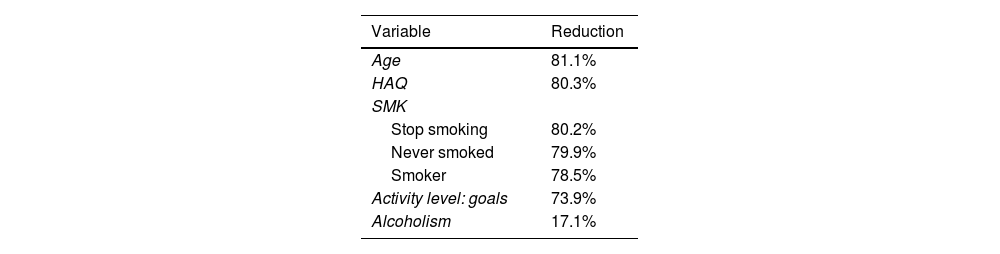
Propensity score matching methodCases consisted of patients from the early phase, while controls were from the established phase, since the first is the one of interest and with the smallest number of people, with two controls per case for increased robustness.38 This resulted in 128 trios: 128 subjects in the early phase (cases) and 256 in the established phase (controls). Matching reduced mean differences between early and established phase groups by 81% to 17% compared to unmatching (see Table 2).
Post-matching univariate analysisNo significant differences were found between early and established phases for age, SMK, HAQ, and disease activity levels in goals and non-goals. The p-value for active alcoholism was not corrected due to low numbers (nine individuals in the early phase and five in the established phase), which may not influence study outcomes significantly (Table 3).
Application of time horizonApplying the time horizon to matched patients with a follow-up of six months (±1 month), there were 103 trios, resulting in 103 patients in the early phase and 206 in the established phase, given the 2:1 ratio.
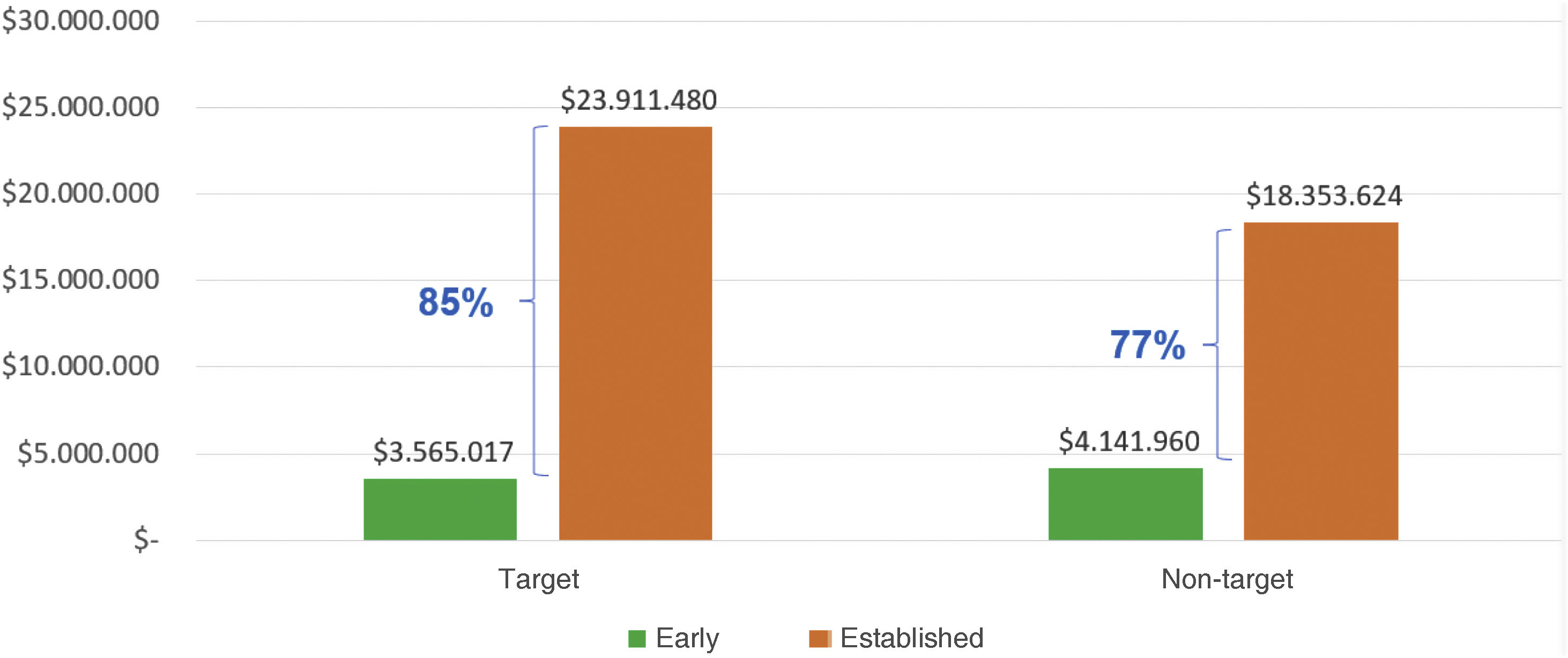
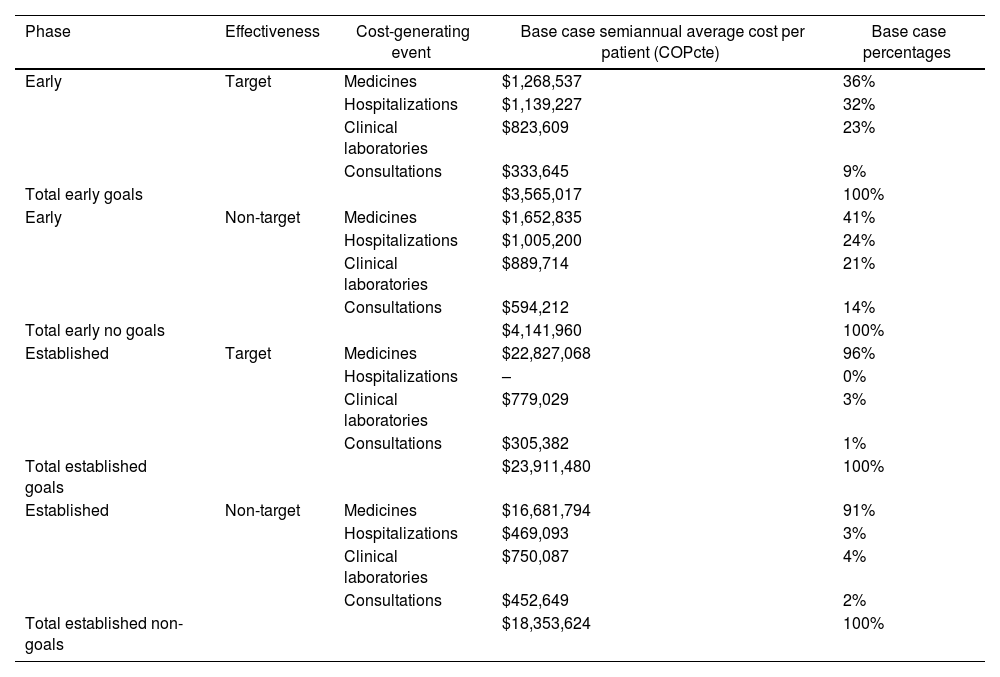
CostsAs shown in Table 4, costs in the early phase for targeted goals were higher in medications by 36%, followed by hospitalizations (32%), clinical laboratories (23%), and consultations (9%). Average six-month costs per patient in targeted goals were $3,565,017 COP cte. Conversely, in the early non-target phase, costs were highest in medications (41%), followed by hospitalizations (24%), clinical laboratories (21%), and consultations (14%), with an average six-month cost per patient of $4,141,960 COPcte.
Cost Summary.
| Phase | Effectiveness | Cost-generating event | Base case semiannual average cost per patient (COPcte) | Base case percentages |
|---|---|---|---|---|
| Early | Target | Medicines | $1,268,537 | 36% |
| Hospitalizations | $1,139,227 | 32% | ||
| Clinical laboratories | $823,609 | 23% | ||
| Consultations | $333,645 | 9% | ||
| Total early goals | $3,565,017 | 100% | ||
| Early | Non-target | Medicines | $1,652,835 | 41% |
| Hospitalizations | $1,005,200 | 24% | ||
| Clinical laboratories | $889,714 | 21% | ||
| Consultations | $594,212 | 14% | ||
| Total early no goals | $4,141,960 | 100% | ||
| Established | Target | Medicines | $22,827,068 | 96% |
| Hospitalizations | – | 0% | ||
| Clinical laboratories | $779,029 | 3% | ||
| Consultations | $305,382 | 1% | ||
| Total established goals | $23,911,480 | 100% | ||
| Established | Non-target | Medicines | $16,681,794 | 91% |
| Hospitalizations | $469,093 | 3% | ||
| Clinical laboratories | $750,087 | 4% | ||
| Consultations | $452,649 | 2% | ||
| Total established non-goals | $18,353,624 | 100% |
COPcte: Colombian pesos current currency.
Therefore, the difference in average semiannual cost per person when comparing the early phase between goals and non-goals is 14 percentage points less in goals, equivalent to $576,943 COPcte. This indicates that costs were higher for non-goals, reflecting a higher percentage of medication use during the early phase without goals. Notably, neither targets nor non-targets received biological DMARDs during this period. Non-biological medications used included prednisolone, folic acid, methotrexate, chloroquine, sulfasalazine, leflunomide, azathioprine, and cyclosporine.
In the established goals phase, medication costs accounted for 96%, followed by clinical laboratories (3%), consultations (1%), and hospitalizations (0%), with an average six-monthly patient cost of $23,911,480 COPcte. Conversely, in the established non-goal phase, medication costs were 91%, clinical laboratories 4%, hospitalizations 3%, and consultations 2%, with an average cost of $18,353,624 COPcte per patient. This resulted in a 23.2 percentage points higher average semiannual cost per person in goals compared to non-goals, totaling $5,557,856 COPcte, highlighting higher expenses in the goals group due to increased biological DMARDs use (etanercept 2.29% and adalimumab 0.76%).
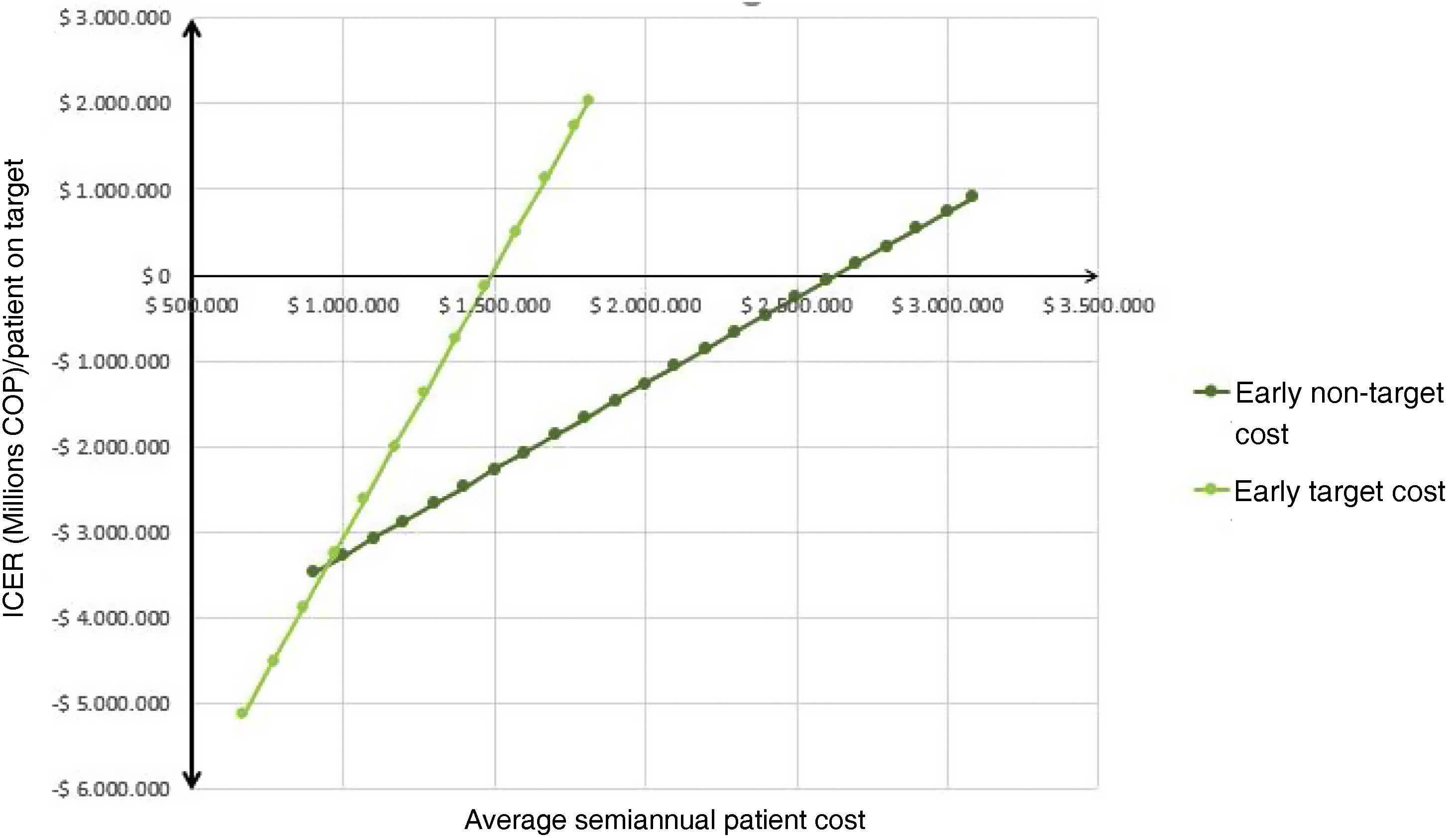
Considering the above analysis, the difference in average semiannual cost per patient between the early and established phases was significant: the cost was 85% higher in the established phase for goals compared to early phase goals, indicating higher expenses per patient in the established phase for goals. Similarly, for non-goals, the average cost increased by 77% in the established phase compared to the early phase, showing increased expenses in the established phase. Thus, subjects in the early phase were generally less expensive compared to those in the established phase, regardless of goal achievement (Fig. 1).
EffectivenessAs shown in Table 5, at the six-month follow-up, 75.7% of patients in the early phase achieved goals compared to 63.6% in the established phase, with a statistically significant difference (P = .043) at a significance level of 0.05, indicating effectiveness variations.11
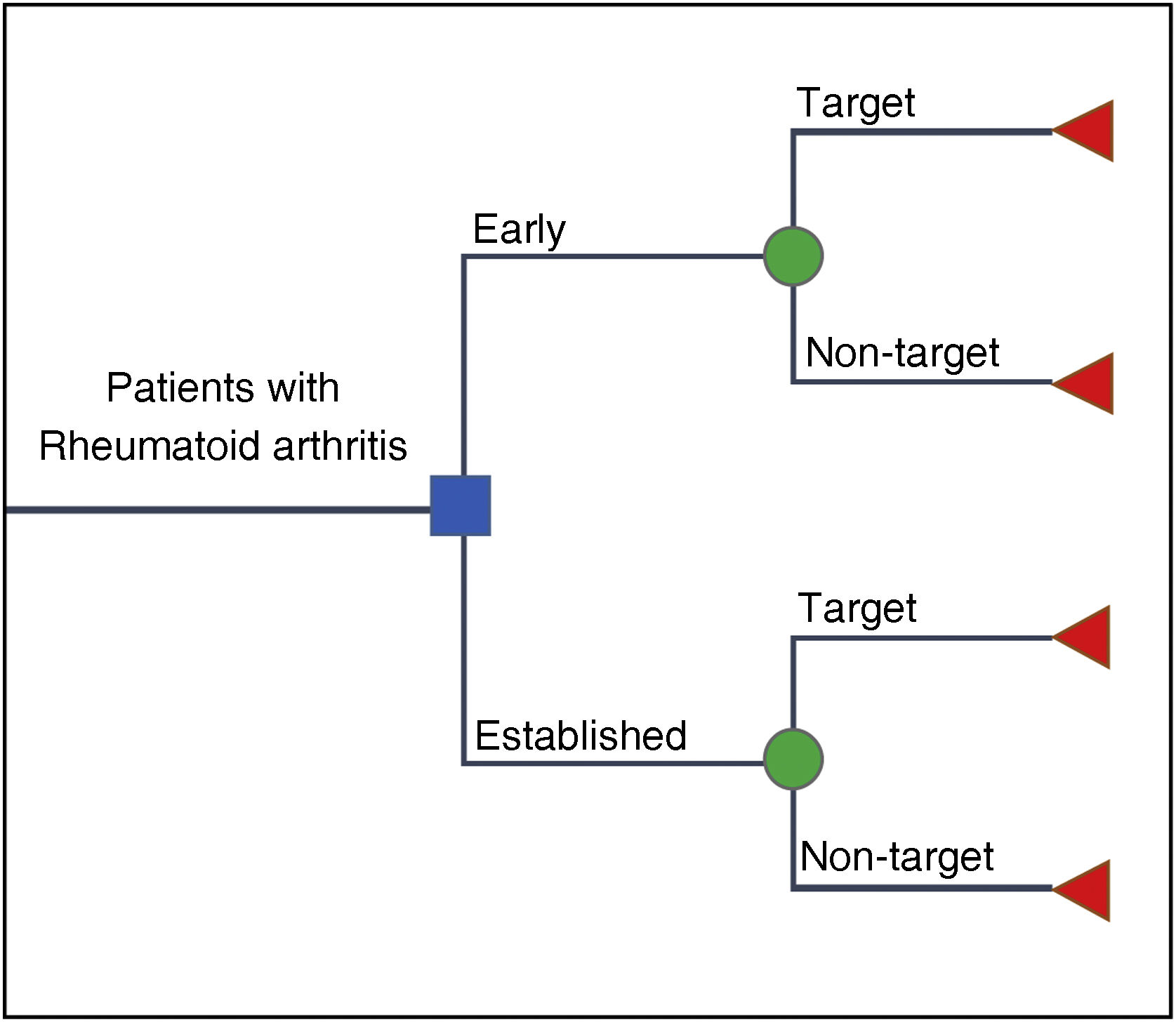
ModelA decision tree24,25 (Fig. 2) was designed for RA, assuming no interaction between individuals, mutually exclusive phases, and it is not a contagious disease. Likewise, for this case, it was required to compare two cohorts and it was assumed that there is no resource restriction for patient care. The model incorporated data on goal effectiveness probabilities and associated costs to derive ICER results.
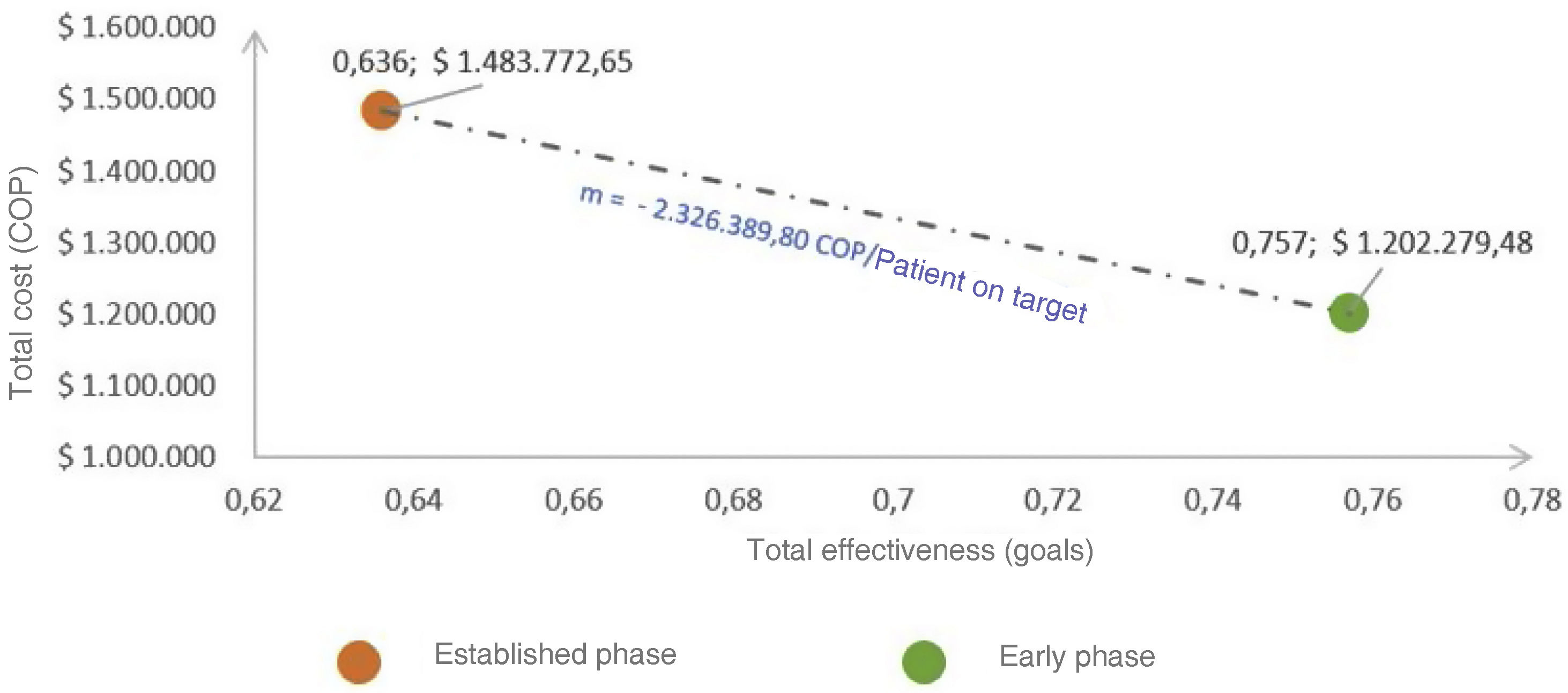
Incremental cost-effectiveness ratio (ICER)The cost-effectiveness plane (Fig. 3) revealed a dominant ICER of −$2,326,389 COPcte (savings) per patient in goals addressed in the early versus the established phase in the base case scenario.
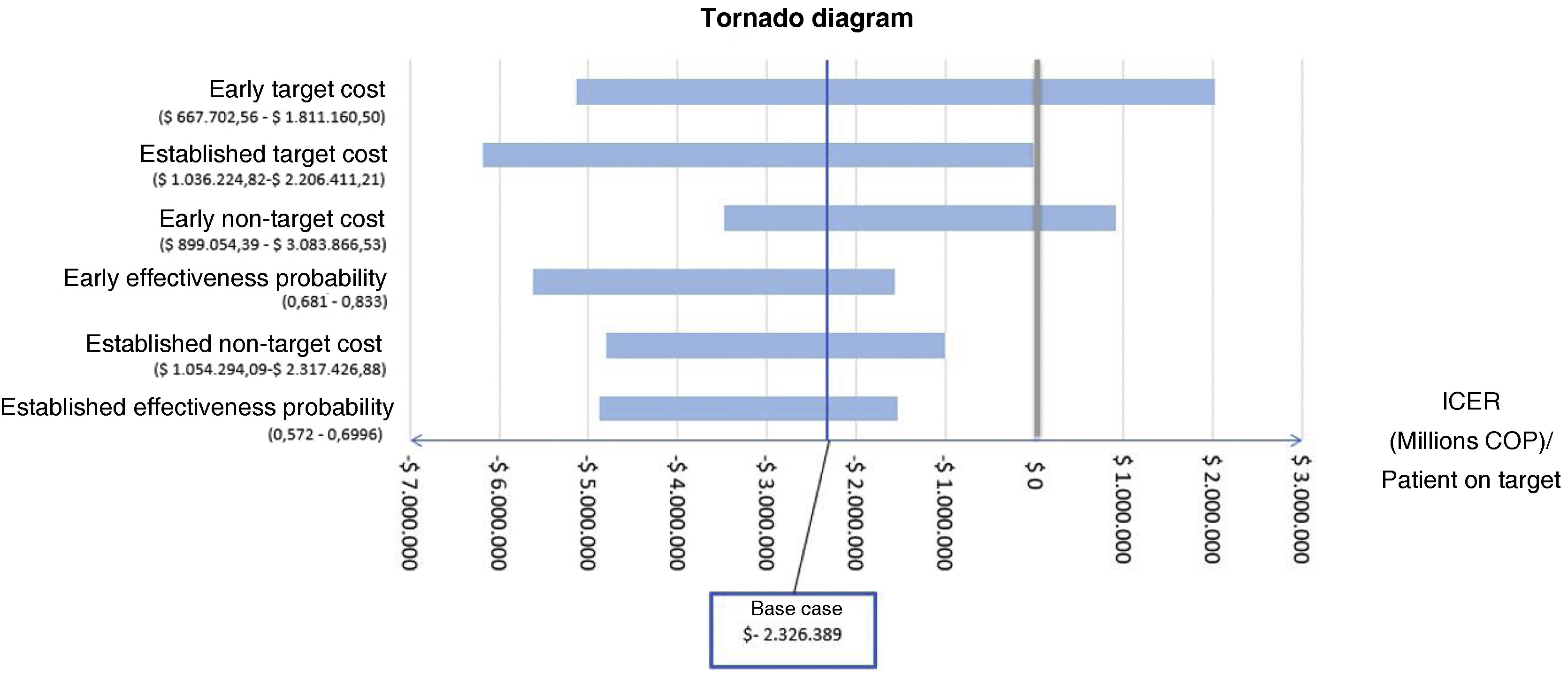
Deterministic sensitivity analysisThe tornado diagram (Fig. 4) identified “cost of early goals,” “cost of established goals,” and “cost of early non-goals” as the top contributors to model uncertainty. The only variables affecting the ICER conclusion were “cost of early goals” and “cost of non-early goals” (Fig. 5); when the cost exceeded approximately $1,500,000 COPcte for early goals or $2,650,000 COPcte for non-early goals, the ICER turned positive.27
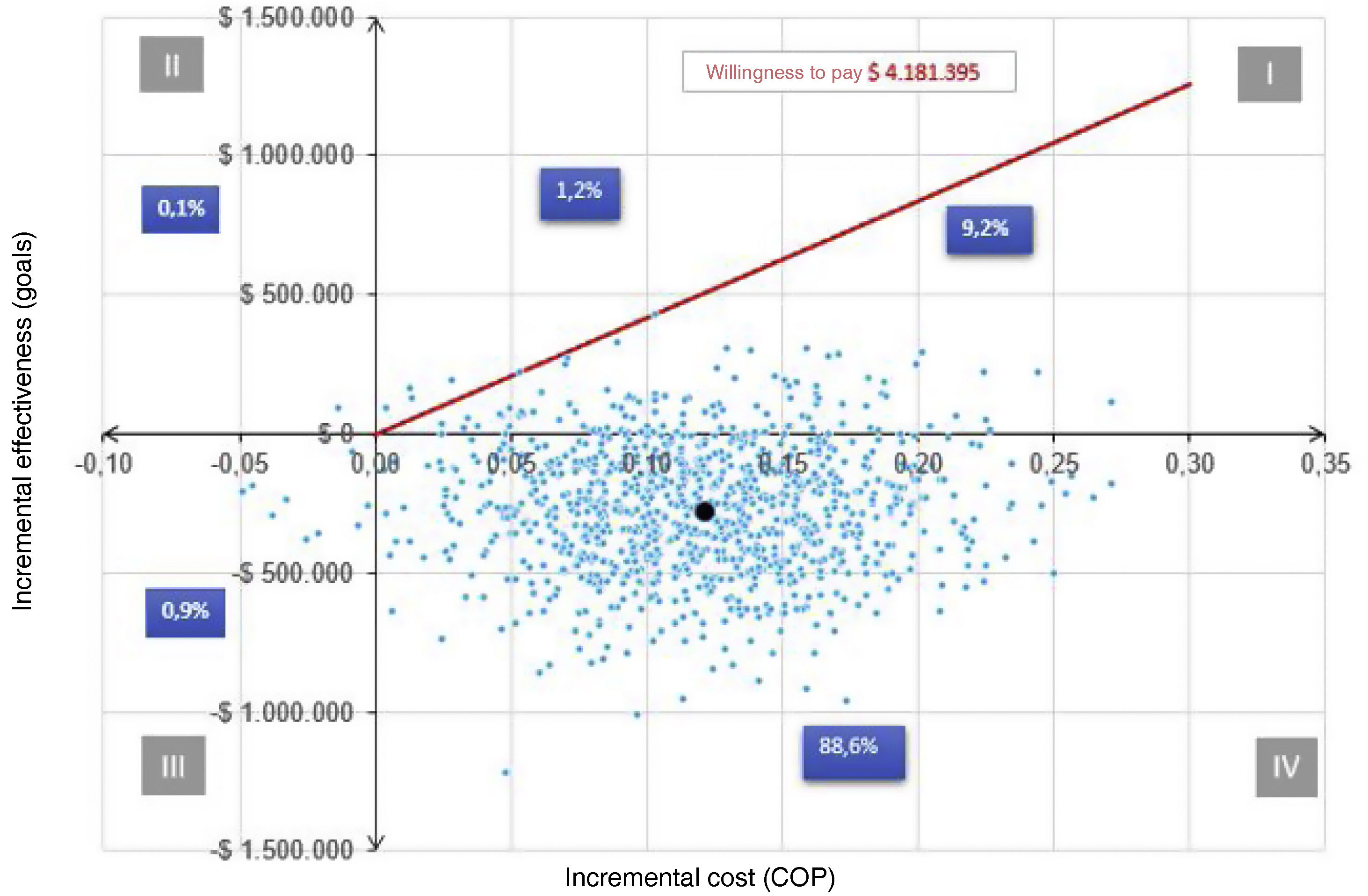
Probabilistic sensitivity analysisTo analyze the behavior of the ICER around a willingness to pay, the CAC reported that the annual cost of DMARDs not in the Basic Health Plan (PBS by its acronym in Spanish) was $5,783,292 COPcte, and the annual cost of care was $2,585. 497 COPcte. Considering a willingness to pay would be the semiannual cost of $4,184,395 COPcte (threshold). Thus, according to Fig. 6, for the first quadrant, 1.2% of the ICER were above the threshold (non-cost-effective) and 9.2% were below (cost-effective). Secondly, 88.6% are in the fourth quadrant; hence, addressing a patient in the early phase is dominant in 88.6% (less expensive and more effective) over the established phase. Additionally, 0.1% of the calculated ICERs are in the second quadrant. In that sense, in 0.1% of the results, the early phase is dominated by the established phase. Consequently, 97.8% of the RICE is below the chosen willingness to pay, being cost-effective in favor of the early phase.1
Acceptability curveAccording to Fig. 7, at zero willingness to pay, there was an 88.7% probability that the early phase approach was cost-effective, compared to 11.3% for the established phase. Likewise, the acceptability curve allows us to infer that, with a willingness to pay of $4,184,395 COPcte, (threshold - red dot), the probability of cost-effectiveness for the early phase approach rose to 98.7%.
DiscussionIn this research, a comprehensive cost-effectiveness analysis was conducted, revealing that the early phase approach is both less costly and more effective compared to the established phase. However, due to the absence of prior cost-effectiveness studies directly comparing these phases, the results cannot be directly contrasted with similar investigations. Nevertheless, our chosen economic assessment method was ideal, since enabled us to evaluate differential costs and effectiveness, calculating the incremental cost-effectiveness ratio and aligning it with willingness-to-pay thresholds.39
Moreover, we assert the relevance of this research to national health policies, underscoring the importance of implementing strategies that ensure timely access to specialized services for managing RA during its early stages. Furthermore, it holds significant implications for clinical practice, as early intervention improves patient prognosis. Clinical experience at HUFSFB suggests that some patients can achieve sustained remission without requiring biological DMARDs, a benefit less observed in the established phase.5,40 The standardized comprehensive care model at HUFSFB5 likely contributes to these positive outcomes and should be promoted alongside protocols prioritizing timely care.
Additionally, the potential long-term return on investment in early intervention may pique the interest of decision-makers, given the savings to third-party payers.8,41,42 While the findings are specific to the context of the third payer, the cohort, and the care model at HUFSFB, it is important to think that, if the average semiannual cost per patient in remission goes from $15,885,885 COPcte2 to $3,565,017 (calculated in this research), and all the prevalence in 2018 would have reached goals, the total cost could be reduced from $1.2 trillion to $282,345 million COPcte, broadly speaking. All of this would indicate that prioritizing care in the early phase could make a difference in cost issues for the treatment of RA in Colombia.
ConclusionThe average semiannual costs per patient achieving goals were as follows: $3,565,017 COPcte for target early phase, $4,141,960 COPcte for non-target early phase, $23,911,480 COPcte for target established phase, and $18,353,624 COPcte for non-target established phase. Effectiveness, measured by the proportion of patients achieving goals at six months, was 75.7% in the early phase and 63.6% in the established phase.
The clinical approach to RA in the early phase proves to be less expensive and more effective compared to the established phase. In the base case, the research indicates that the ICER resulted in cost savings of −$2,326,389 COPcte per patient achieving goals in the early phase compared to the established phase costs in 2018, over a six-month ± one-month follow-up in the cohorts. It is important to note that the model does not include probabilities of adverse events due to their similarity between cohorts, and the small sample size did not allow correction of the p-value for active alcoholism.
Previous cost-effectiveness studiesA literature review was conducted across several databases (LILACS, SCIELO, Redalyc, Web of Science, Google Scholar, PubMed, and SCOPUS), in which the search terms: “cost-effectiveness”, “early arthritis”, “established arthritis” were introduced, and the Boolean term “AND” focusing on studies comparing early and established phases of arthritis in terms of cost-effectiveness. The search encompassed articles and clinical cohort studies published in English and Spanish up until 2018, reflecting the data analyzed for this study. Additionally, recent studies from 2022 to 2023 were considered for comparative purposes in this publication.
Ethical considerationsThe study obtained informed consent from patients at HUFSFB and adhered to bioethical research regulations, receiving approval from both the HUFSFB and Universidad Nacional de Colombia ethics committees.
FinancingThe study was conducted with data collection supported by HUFSFB. The study design, analysis, interpretation, article writing, and the decision to submit the article for publication were performed by the authors independently under a master's thesis in pharmacology at the Universidad Nacional de Colombia.27 While the research did not receive direct funding during its execution, it was recognized with the 2020 National Rheumatology Award, securing second place and an associated financial award from the Colombian Association of Rheumatology Congress.
Conflict of interestsThe authors declare no conflicts of interest.
We acknowledge Jorge Augusto Díaz Rojas, Jairo Alexander Moreno Calderón, José Ricardo Urrego Novoa, Douglas Jair Castiblanco Ángel, Julián Mauricio Cruz, Jorge Bruce Flórez, Jhon Jairo Arévalo Vargas, and the institutions National University of Colombia, Santa Fe de Bogotá Foundation, Pharmacoeconomics Seminar National University of Colombia – Health Technology Assessment Group (GETS), Seminar - Reumavance Research Group, and Paul Méndez for their contributions and support.