Salivary gland biopsy is the gold standard for the diagnosis of Sjogren’s syndrome. There are several approaches for the realization of the biopsy, being generally used an approach to the mucosa with the use of various instruments and incisions that vary in length, passing through trucut biopsy to scraping with needle.
Materials and methodsWe conducted a descriptive study that included adult patients undergoing salivary gland biopsy between 2017 and 2022. Medical records and salivary gland biopsy reports were used to collect information and registered in the Magpi platform. Sociodemographic, clinical, and histopathological variables were recorded. The qualitative variables were expressed in absolute and relative frequencies; the quantitative ones were by median and interquartile range. Seventy-two biopsies were included.
ResultsRegarding demographic characteristics, 87,5% were women with a median age of 52 years. The biopsy characteristics showed that 70 (97,2%) corresponded to a representative sample. Twenty-seven (38,5%) biopsies showed lymphocytic infiltration and reported a classification system, of which the most used one was the Chisholm Mason used in 18 (66,6%) of such biopsies. Complications were present in four (5,7%) patients.
ConclusionsDespite the recommendation of using Focus Score for classification criteria, the Chisholm Mason system is still the most widely used. The present technique is a safe and effective for the realization of minor salivary gland biopsy.
La biopsia de glándulas salivales es el estándar por excelencia para el diagnóstico del síndrome de Sjögren. Existen varias aproximaciones para su realización, siendo de forma general utilizado un abordaje por la mucosa con uso de diversos instrumentos e incisiones que varían en su longitud, pasando por biopsia por trucut hasta raspado con aguja.
Materiales y métodosSe llevó a cabo un estudio descriptivo que incluyó pacientes adultos sometidos a biopsia de glándulas salivales entre el 2017 y el 2022. Se utilizaron historias clínicas e informes de biopsia de glándula salival para recopilar información y se registraron en la plataforma Magpi. Se registraron variables sociodemográficas, clínicas e histopatológicas. Las variables cualitativas se expresaron en frecuencias absolutas y relativas; las cuantitativas por mediana y rango intercuartílico. Se incluyeron 72 biopsias.
ResultadosEn cuanto a las características demográficas, el 87,5% de los pacientes eran mujeres, con una edad media de 52 años. Las características de las biopsias mostraron que 70 (97,2%) de estas correspondían a una muestra representativa. Veintisiete (38,5%) biopsias mostraron infiltración linfocítica y reportaron un sistema de clasificación, de los cuales el más utilizado fue el Chisholm Mason, utilizado en 18 (66,6%) de dichas biopsias. Hubo complicaciones en cuatro (5,7%) pacientes.
ConclusionesA pesar de la recomendación de utilizar el Focus Score como criterio de clasificación, el sistema Chisholm Mason sigue siendo el más utilizado. La presente técnica es segura y eficaz para la realización de biopsias de glándulas salivales menores.
Minor salivary gland biopsy (MSGB) is one of the fundamental pillars for the diagnosis of Sjögren's syndrome, as it provides histological evidence of the autoimmune process. For this reason, it is one of the main tests that, together with the evidence of anti-Ro antibodies, belong to the classification criteria of the American College of Rheumatology and the European League Against Rheumatism (ACR/EULAR).1
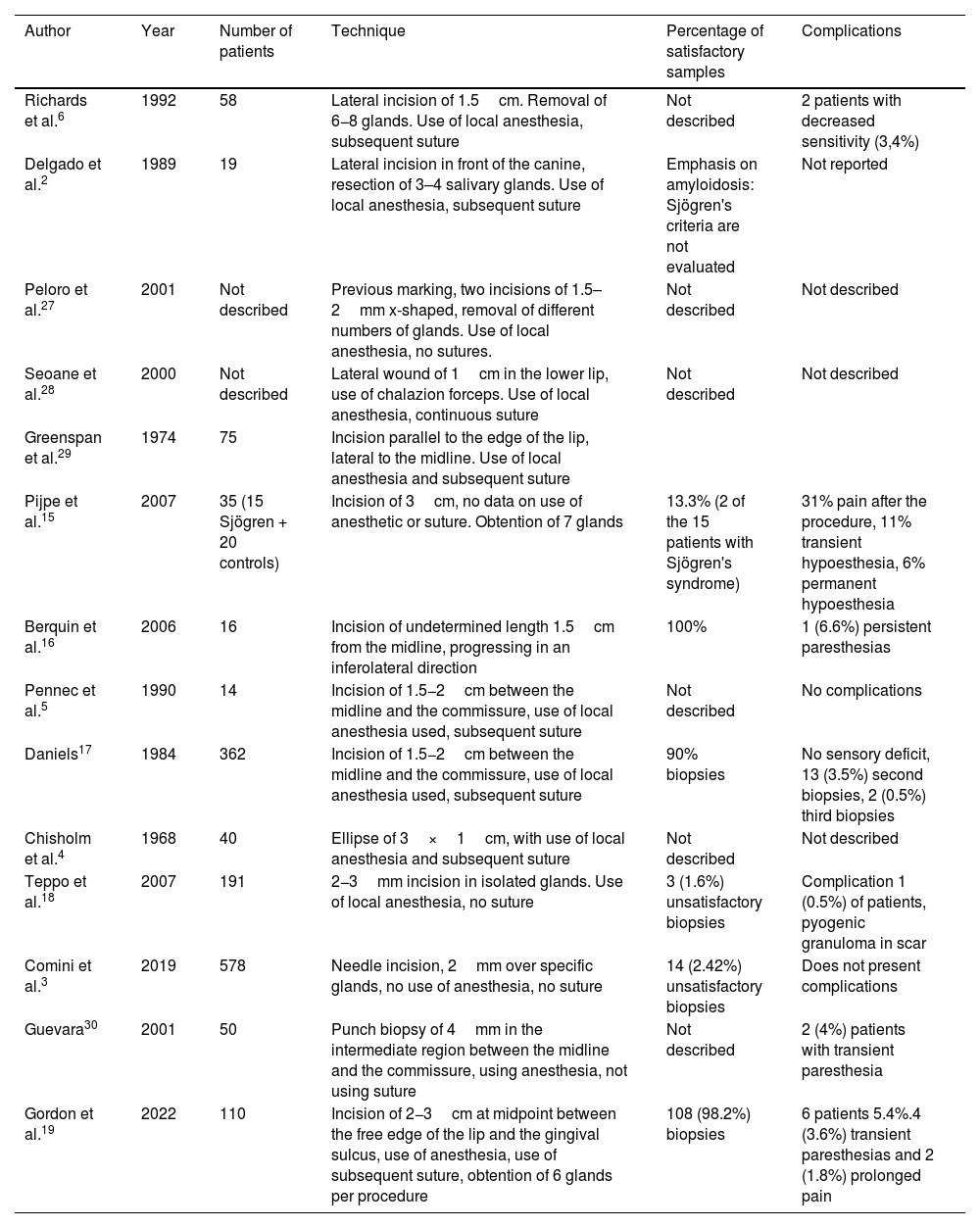
From the technical point of view, there are several approaches for the realization of the biopsy. Normally, a mucosal approach is used with the use of various instruments and incisions that vary in length, ranging from trucut biopsy to needle scraping (Table 1Table 1).2–5 In general terms, the reports of the techniques present a sensitivity and specificity higher than 80%, although they usually lack external validation studies. From the point of view of complications, these vary according to the type of technique, but can be summarized as infection, healing disorders and alteration of sensitivity, both temporary and permanent.2,6
Summary of the main studies on salivary gland biopsy technique.
| Author | Year | Number of patients | Technique | Percentage of satisfactory samples | Complications |
|---|---|---|---|---|---|
| Richards et al.6 | 1992 | 58 | Lateral incision of 1.5cm. Removal of 6−8 glands. Use of local anesthesia, subsequent suture | Not described | 2 patients with decreased sensitivity (3,4%) |
| Delgado et al.2 | 1989 | 19 | Lateral incision in front of the canine, resection of 3–4 salivary glands. Use of local anesthesia, subsequent suture | Emphasis on amyloidosis: Sjögren's criteria are not evaluated | Not reported |
| Peloro et al.27 | 2001 | Not described | Previous marking, two incisions of 1.5–2mm x-shaped, removal of different numbers of glands. Use of local anesthesia, no sutures. | Not described | Not described |
| Seoane et al.28 | 2000 | Not described | Lateral wound of 1cm in the lower lip, use of chalazion forceps. Use of local anesthesia, continuous suture | Not described | Not described |
| Greenspan et al.29 | 1974 | 75 | Incision parallel to the edge of the lip, lateral to the midline. Use of local anesthesia and subsequent suture | ||
| Pijpe et al.15 | 2007 | 35 (15 Sjögren + 20 controls) | Incision of 3cm, no data on use of anesthetic or suture. Obtention of 7 glands | 13.3% (2 of the 15 patients with Sjögren's syndrome) | 31% pain after the procedure, 11% transient hypoesthesia, 6% permanent hypoesthesia |
| Berquin et al.16 | 2006 | 16 | Incision of undetermined length 1.5cm from the midline, progressing in an inferolateral direction | 100% | 1 (6.6%) persistent paresthesias |
| Pennec et al.5 | 1990 | 14 | Incision of 1.5−2cm between the midline and the commissure, use of local anesthesia used, subsequent suture | Not described | No complications |
| Daniels17 | 1984 | 362 | Incision of 1.5−2cm between the midline and the commissure, use of local anesthesia used, subsequent suture | 90% biopsies | No sensory deficit, 13 (3.5%) second biopsies, 2 (0.5%) third biopsies |
| Chisholm et al.4 | 1968 | 40 | Ellipse of 3×1cm, with use of local anesthesia and subsequent suture | Not described | Not described |
| Teppo et al.18 | 2007 | 191 | 2−3mm incision in isolated glands. Use of local anesthesia, no suture | 3 (1.6%) unsatisfactory biopsies | Complication 1 (0.5%) of patients, pyogenic granuloma in scar |
| Comini et al.3 | 2019 | 578 | Needle incision, 2mm over specific glands, no use of anesthesia, no suture | 14 (2.42%) unsatisfactory biopsies | Does not present complications |
| Guevara30 | 2001 | 50 | Punch biopsy of 4mm in the intermediate region between the midline and the commissure, using anesthesia, not using suture | Not described | 2 (4%) patients with transient paresthesia |
| Gordon et al.19 | 2022 | 110 | Incision of 2−3cm at midpoint between the free edge of the lip and the gingival sulcus, use of anesthesia, use of subsequent suture, obtention of 6 glands per procedure | 108 (98.2%) biopsies | 6 patients 5.4%.4 (3.6%) transient paresthesias and 2 (1.8%) prolonged pain |
The interpretation of the biopsy was described for the first time by Chisholm et al.,4 but over time other systems such as the Focus Score (FS) and the modified Chisholm-Mason were implemented, leading to a lack of uniformity in the pathology reports and diversity in the classifications used.7 Given this situation, societies such as EULAR have issued guidelines in which they recommend FS as the reference methodology when evaluating the salivary gland to determine the presence of lymphocytic sialadenitis.8 Briefly, the histological report can be classified into normal histology, non-specific chronic sialadenitis, focal lymphocytic sialadenitis (FLS), chronic sclerosing sialadenitis and granulomatous inflammation; FLS constitutes the characteristic finding of Sjögren's syndrome.7
Salivary gland biopsy, in addition to being a diagnostic element in Sjögren's syndrome, has also been associated with some manifestations of the disease, such as interstitial lung disease, hematological involvement and hypergammaglobulinemia.9 The presence of FS≥1 has been useful to predict adverse outcomes, such as extraglandular manifestations and the development of lymphoma. Risselada et al. observed that a FS≥3 has a predictive value for the development of non-Hodgkin's lymphomas.10,11
In the present study, the members of the rheumatology service perform the salivary gland biopsy procedure with a minimally invasive process modified from the technique described by Delgado et al.12 For this reason, we wanted to answer three questions: How effective is this technique in obtaining satisfactory material for analysis? How frequent are its complications? And what are the main histological reporting systems used in the media?
MethodologyIt was conducted a descriptive cross-sectional study which included patients over 18 years of age, who consulted between the years 2017 and 2020 and underwent a MSGB in the rheumatology service of two institutions in Medellín, Antioquia. Those in whom the salivary gland biopsy report was not available were excluded. For pragmatic reasons, the sample size was not estimated, but rather all those who met the study criteria during the established period were included.
The information, which was obtained from the review of clinical records and histopathological results, was recorded in a format designed for this purpose in the Magpi tool version 6.2.6. The variables collected were sociodemographic (age, sex and place of residence), clinical (patient complications: bleeding, second incision, local pain, syncope, infection at the wound site, edema, granulomas or hematomas), quality of the sample (whether the pathologist defined it as satisfactory or if it was representative) and the histological findings of the biopsy.
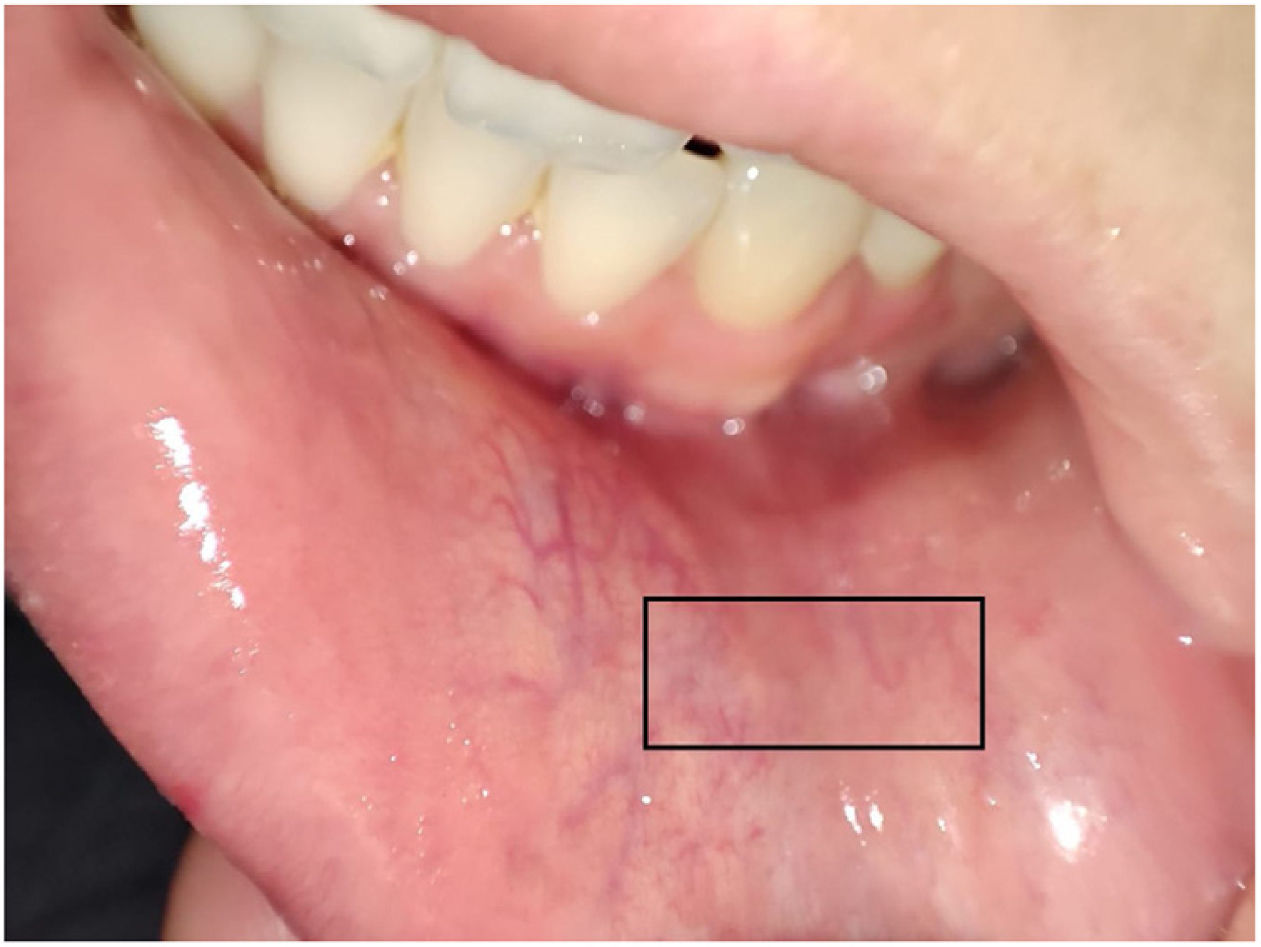
The MSGBs were performed by Miguel Antonio Mesa and Marcela Posada and the technique used in both institutions was the following (Fig. 1): In the jugal mucosa adjacent to the anterior canine, in an intermediate region between the midline and the edge of the mouth and between the base of the mouth and the vermilion edge, the salivary glands were located by palpation. Once located, they proceeded to infiltrate with a 30-gauge needle (insulin needle) using 1 cc of 2% lidocaine without epinephrine. The infiltration started at an angle of 30° and the needle was relocated at more acute angles until the infiltration was completed, in order to separate the mucosal layer from the submucosa to minimize bleeding. Subsequently, using a number 11 scalpel blade, an incision was made from medial to lateral with little pressure, trying to incise only the mucosal layer, with a length of 1cm, and with the help of a gauze, pressure was applied to the opposite side of the incision. The glands were denuded using a number 30 needle and, once located, were sectioned at their base with the aid of a scalpel and placed in a jar with formaldehyde prepared in advance for this purpose. The anatomical area selected for sample collection was chosen due to the abundance of salivary glands and the relative safety regarding the variations of the branches of the mentonian nerve, which minimizes the risk of paresthesia after the procedure.13 At the end, pressure was applied to the wound area for 5min or until bleeding was controlled and the wound was approximated without using sutures. In case that a cut did not obtain enough glands or it was considered technically unfeasible to continue the exploration, a second incision was made on the contralateral side following the same method. The minimum number of glands required to consider the process as satisfactory was four.
From the histopathological point of view, the sample was considered representative when it met one of the three conditions due to the difficulty of obtaining a uniform histological report:
- 1
Presence of at least four salivary glands.8
- 2
Description of area of analysis of 8mm.2,8
- 3
Description by the pathologist of a satisfactory specimen.
To follow-up complications, the patients were observed for a period of 30min after the biopsy and, at the time of discharge, they received contact information and were instructed and encouraged to report any inconvenience.
Statistical analysisQualitative variables were expressed in absolute and relative frequencies, while quantitative variables were described using mean and standard deviation (SD), due to compliance with the assumption of normality evaluated with the Kolgomorov–Smirnov test. The analyses were carried out in the IBM SPSS 25.0 statistical package.
ResultsDuring the study period, 72 patients who had a biopsy indication were attended, most of them from a more complex institution and 10 in an outpatient management institution.
Regarding the characteristics of the studied population, 63 patients (87.5%) were female and the average age was 52 years (SD: 13.1). With respect to the characteristics of the biopsies, 70 (97.2%) were considered representative; in two cases only sebaceous tissue was obtained. From this point on, the results are reported based on these 70, from which a significant sample was obtained (n=70).
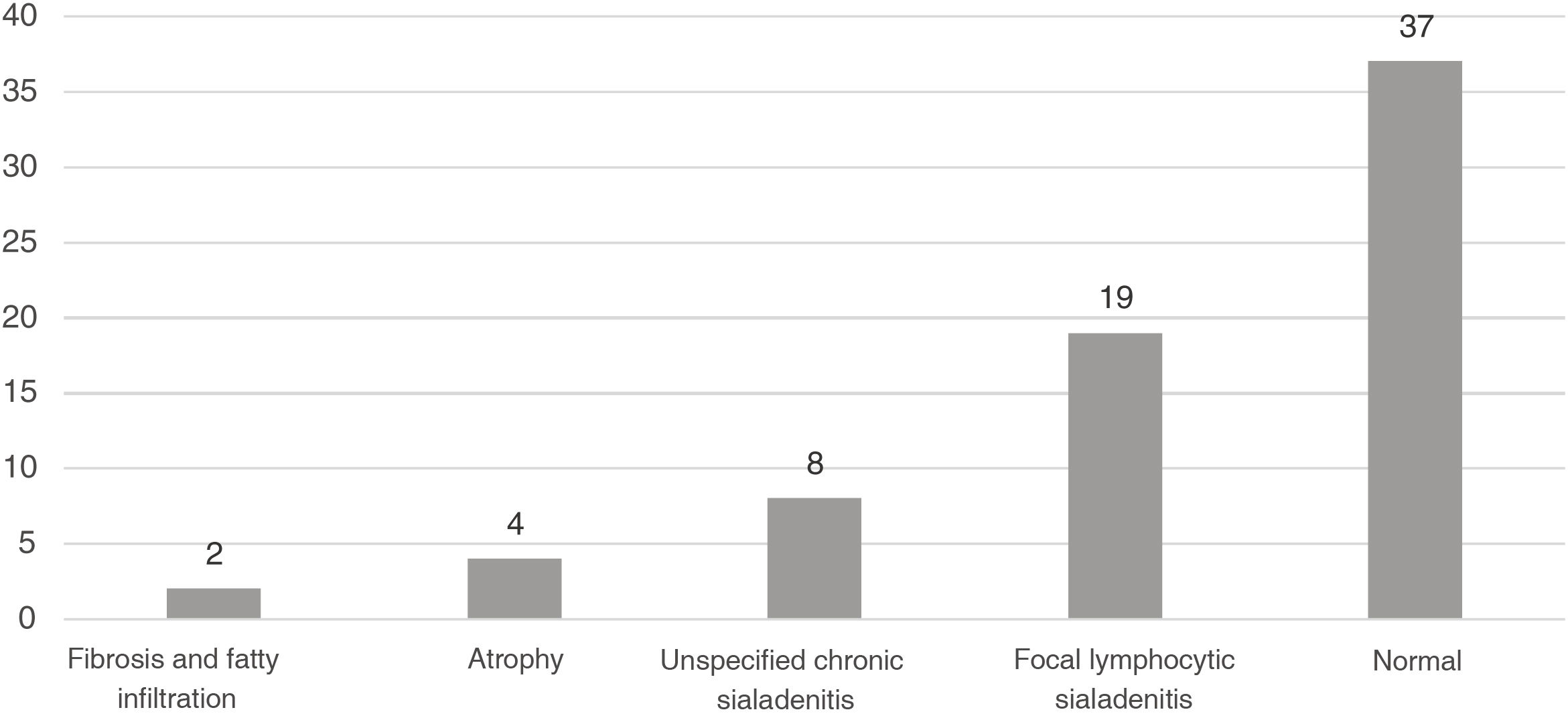
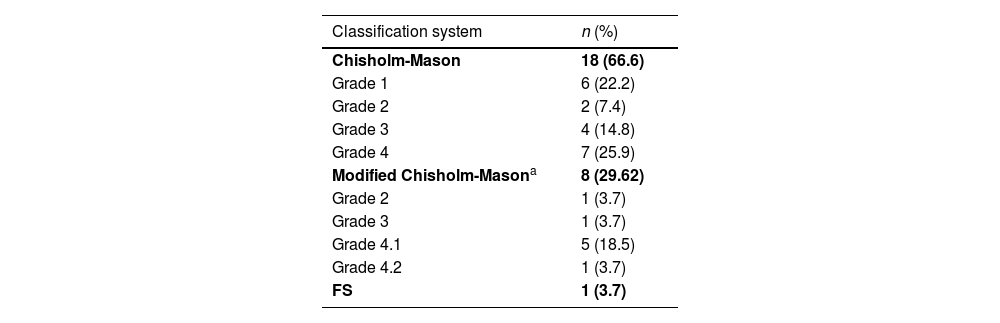
Sialadenitis was documented in 27 biopsies (38.5%) (Fig. 2), with a report of the classification system used, of which the most common was the Chisholm-Mason system in 18 of these (66.6%); the other data are reported in Table 2.
Classification system for biopsies with sialadenitis n=27.
| Classification system | n (%) |
|---|---|
| Chisholm-Mason | 18 (66.6) |
| Grade 1 | 6 (22.2) |
| Grade 2 | 2 (7.4) |
| Grade 3 | 4 (14.8) |
| Grade 4 | 7 (25.9) |
| Modified Chisholm-Masona | 8 (29.62) |
| Grade 2 | 1 (3.7) |
| Grade 3 | 1 (3.7) |
| Grade 4.1 | 5 (18.5) |
| Grade 4.2 | 1 (3.7) |
| FS | 1 (3.7) |
FS: Focus Score.
The only patient who used FS had a score of 1 therein.
4.1=2–3 foci.
4.2=4–6 foci.
4.3=7–12 foci.
Score for the foci, according to Daniels and Whitcher.31 Use this scheme if the size of the glandular lobules cannot be measured.
The most frequent finding was the normal salivary gland, which corresponded to 37 cases (52.8%), followed by focal sialadenitis, on 19 occasions (27.1%), and nonspecific sialadenitis, of which eight were found (11.4%); all findings are described in Fig. 1.
Complications occurred in four of the cases (5.7%), these being: an episode close to syncope, on one occasion that occurred in the immediate recovery period when the patient moved from the prone position to the sitting position. This episode improved when the patient returned to the prone position for a period of 5min. Two cases required a second incision because insufficient material was obtained on the first attempt. Finally, there was one case of intense pain in the postoperative period, which improved with the administration of acetaminophen 1,000mg and the use of ice for 20min in the incision region; At subsequent follow-up, the patient did not have a recurrence of the pain. It is important to emphasize that no difficulties in healing, requirement for the use of suture, persistent bleeding or transient or permanent paresthesia were reported.
DiscussionThe present article shows the experience with a MSGB technique and the type of histological report used. A predominance in the female sex was found, a finding expected due to the higher prevalence of Sjögren's syndrome in this group, and an age expected for a population with suspected Sjögren's syndrome.14
The first finding to mention is the effectiveness of the procedure to obtain satisfactory material for histological analysis; this is similar to the series described by other authors with different techniques, where values above 90% are reached.3,15–19 It is important to keep in mind that EULAR recommends to obtain four glands and six if they are small and that this standard should be used to achieve the highest percentage possible of representative biopsies.8 One of the striking findings of our study was to find sebaceous glands in the biopsies on two occasions. These glands in the oral cavity have historically been described as Fordyce spots and are typically located at the edge of the vermilion, which could generate doubts regarding the correct location of the biopsy area; however, in all cases the authors strictly followed the protocol for selecting the incision area to minimize the risk of paresthesia. In addition, pathology studies in the oral cavity have described the sporadic presence of sebaceous glands as a normal finding in healthy individuals, present even in up to 80% of cases.20
The reporting system in patients in whom lymphocytic infiltration was found was the Chisholm-Mason system, contrary to what was indicated by the international recommendations of using the FS.1,8 Despite the changes over time in the classification systems, the Chisholm–Mason system, which has lower performance, especially when classifying confluent foci of inflammation and suffering a ceiling effect in cases of increased inflammation, is still widely used.8,19,21 In a study conducted.at the San José Hospital in Bogotá, it was found that there was little agreement between the two and that Chisholm Manson's system overestimates the foci count, since all of these are taken into account regardless of the state of the glandular parenchyma, which determines a probable systematic bias.19
These findings cannot be generalized, since when evaluating only biopsies from two centers, this may not represent the entire local reality; however, it does generate a call for attention to evaluate and update this system, in addition to being the main finding of our study and the one that can most immediately impact rheumatological practice, through communication between rheumatologists and pathology services.
Regarding the complications found, these were shown in a low proportion, being minor and without the presence of paresthesias, which are one of the most feared due to the deterioration of the quality of life that they produce. This study demonstrates that the present technique is safe and, as it does not require suture, it avoids a second time for its removal.
Among the strengths of this research, the sample size can be highlighted, which is in the middle range of the different similar studies (Table 2). In addition, the surgical technique was unified, which allows us to assert that there was homogeneity in the procedure.
When interpreting our study, it is important to clarify that its intention is not to make a comparison with other techniques, but rather to describe the local experience and, therefore, it cannot be concluded in relation to other techniques described in the literature.
Additionally, it is a retrospective work, which limits the information available, such as, for example, the number of glands obtained to define whether the international guidelines for biopsy were met. Another weakness is the difficulty in monitoring mediate complications, since patients did not have a follow-up visit. Despite this, everyone was instructed about possible complications and received a contact telephone number to report the presence of any inconvenience.
Another complication, more typical of the literature than of the study, is the heterogeneity at the time of reporting the technique, the representativeness of the sample obtained and the associated problems, which is described more broadly in Table 1 and demonstrates the difficulty in making comparisons with other articles.
Finally, an aspect that is worth addressing is whether currently, with the appearance of new technologies such as salivary gland ultrasound, it is still relevant to perform histological studies, even more so when there is a concordance of more than 78% between ultrasound and the presence of an inflammatory focus.22 In our opinion, histological study remains necessary for the following reasons:
- 1
The ultrasound criteria for Sjögren's syndrome have already been homogenized through the Omeract guidelines. It is necessary that all the personnel that perform this procedure adopt these definitions in order for it to be truly effective.23
- 2
Seronegative Sjögren's syndrome, at least for anti-Ro antibodies, corresponds to about 30% of cases. They have a behavior and natural histories different from those of their positive counterpart. Given the wide variety of clinical pictures that simulate dry symptoms, the biopsy becomes necessary to confirm the diagnosis.24
- 3
The salivary gland biopsy provides information on the course of the disease, the risk of progression to lymphoma and extraglandular manifestations.24,25
- 4
Replacing the biopsy with ultrasound of the major salivary gland generates a slight decrease in sensitivity (85 vs. 85.6%) and specificity (79.8 vs. 82.2%) which, although by itself does not imply a significant change, is important if we consider the loss of the information described in numeral three.26
This article presents the experience of a technique of salivary gland biopsy with a 10mm incision without subsequent suture, a procedure that provides more than 90% representative samples with a low rate of complications. At the local level, the preferred histological reporting system is the Chisholm-Mason system, contrary to international recommendations, and reflects the need to update these reports.
FundingThis study was not funded.
Thanks to Dr. Rubén Mantilla, who instructed Miguel Antonio Mesa Navas, one of the authors of this article, in the procedure for performing the biopsy.