Mepolizumab (MPZ) is a therapeutic option for eosinophilic granulomatosis with polyangiitis (EGPA). However, the evidence on its efficacy, effectiveness, and safety is limited. Our results included 17 full-text articles evaluating the use of MPZ in patients diagnosed with EGPA, 47% (8/17) of the documents were case reports, 18% (3/17) retrospective cohort studies, 12% (2/17) clinical trials, and 6% corresponded to a prospective cohort (1/17), ambispective cohort (1/17), case series (1/17), and pilot study (1/17). The most frequently used dose of MPZ was 300 mg/month, described in 59% (10/17) of the included manuscripts. The decrease in the Birmingham Vasculitis Activity Score, reduction in the dose of corticosteroids, and modulation in the number of eosinophils in the blood were the variables most used to determine the clinical effect in patients. Finally, 10 clinical trial records were included describing the design, conduct, and administration of the ongoing studies. The use of MPZ in patients with EGPA shows a reduction in the Birmingham Vasculitis Activity Score, saving oral corticosteroids and modulating blood hypereosinophilia. The adverse events described were mild and moderate in all the included studies.
El mepolizumab (MPZ) es una opción terapéutica para la granulomatosis eosinofílica con poliangeítis (GEP). Sin embargo, la evidencia sobre su eficacia, efectividad y seguridad es limitada. Nuestros resultados incluyeron 17 artículos de texto completo que evalúan el uso de MPZ en pacientes con diagnóstico de GEP. El 47% (8/17) de los documentos eran reportes de caso, el 18% (3/17) estudios de cohorte retrospectiva, el 12% (2/17) ensayos clínicos y el 6% correspondió a cohorte prospectiva (1/17), cohorte ambispectiva (1/17), serie de casos (1/17) y estudio piloto (1/17). La dosis de MPZ utilizada con mayor frecuencia fueron 300 mg/mes, descrita en el 59% (10/17) de los manuscritos incluidos. La disminución en el puntaje del Birmingham Vasculitis Activity Score, la reducción de la dosis de corticoides y la modulación en el número de eosinófilos en sangre fueron las variables más utilizadas para determinar el efecto clínico en los pacientes. Finalmente se incluyeron 10 registros de ensayos clínicos que describen el diseño, la conducción y la administración de los estudios en curso. El uso del MPZ en pacientes con GEP muestra una reducción en el puntaje del Birmingham Vasculitis Activity Score, ahorro de corticoides orales y modulación de la hipereosinofilia en sangre. Los eventos adversos descritos fueron leves y moderados en la totalidad de los estudios incluidos.
Eosinophilic granulomatosis with polyangiitis (EGPA) is a small- to medium-sized vessel vasculitis associated with antineutrophil cytoplasmic antibodies. The disease has an estimated annual incidence of 0.5–4.2 cases per million people and a prevalence ranging from 2 to 38 cases per million people.1,2 It is a systemic condition with clinical manifestations typically occurring in three phases: the prodromal phase, characterized by asthma, allergic rhinitis, and sinusitis; a second phase mediated by eosinophils, marked by pulmonary infiltrates, cardiomyopathy, and gastrointestinal symptoms1,3; and a third phase, in which patients present with palpable purpura, glomerulonephritis, and mononeuritis multiplex. This third stage ultimately leads to chronic immune-mediated tissue damage and is responsible for a significant number of relapses in treated patients.3,4
The treatment of EGPA should be guided by the patient's risk of mortality and the potential for adverse effects. Initial therapy typically involves a combination of immunosuppressants and corticosteroids.4 However, the low incidence of the disease, combined with an incomplete understanding of its pathophysiology and the variability of its phenotypes, contributes to a high rate of therapeutic failure or refractoriness.2,4 Studies have explored new treatment regimens with more favorable clinical outcomes and reduced adverse effects compared to conventional therapies. These include treatment with humanized monoclonal antibodies such as omalizumab,5 rituximab,6 and mepolizumab (MPZ),7 among others.8
MPZ is a humanized monoclonal antibody that binds with high affinity to the α chain of interleukin-5 (IL-5), thereby preventing its interaction with the IL-5 alpha receptor (IL-5Rα) on the eosinophil membrane; this mechanism inhibits the activation of the signaling pathways involved in the pathogenesis of EGPA.9.10 IL-5 is a key therapeutic target in managing difficult-to-treat eosinophilia, as it plays an essential role in the maturation, recruitment, activation, proliferation, and survival of eosinophils.11 Bettiol et al.7 described the response to MPZ treatment between 3 and 24 months after initiation, noting a reduction in the Birmingham Vasculitis Activity Score (BVAS), a decrease in corticosteroid dosage, and modulation of eosinophil numbers. Due to its highly specific binding site and high affinity, MPZ offers an effective and safe pharmacological alternative for treating EGPA.7,12 Currently, limited evidence exists on the clinical outcomes of treating patients with this small- to medium-sized vessel vasculitis using humanized monoclonal antibodies, particularly MPZ.7–12 Therefore, this review aims to assess the efficacy, effectiveness, and safety of MPZ in patients diagnosed with EGPA.
MethodsThis systematic scoping review was conducted following the steps outlined by Arksey et al.13 and later modified by Levac et al.14: identifying the research question, searching for relevant documents, selecting the articles, extracting data, and reporting the results. The review aimed to answer the following question: How can the available medical evidence on the efficacy, effectiveness, and safety of using MPZ in patients with EGPA be described?
Eligibility criteriaClinical trials were included, as well as analytical and descriptive observational studies, that explored the use of MPZ in patients with EGPA, with no time limit, in either English or Spanish. Additionally, records of clinical trials that were in the recruitment or data analysis phases, or were close to publishing evidence, were included. We excluded manuscripts that presented theoretical data, such as narrative or systematic reviews, among others.
Search strategy and information sourcesThe search was conducted using the PubMed/Medline search engine and the Scopus database, employing Boolean operators and keywords appropriate to the data system. The search was last updated on October 1, 2023. The complete search strategy is detailed in Supplementary File 1. Records of studies from 17 databases within the World Health Organization (WHO) International Clinical Trials Registry Platform that assessed the use of MPZ in patients with EGPA were also included (Supplementary File 2).15
Study selection and data extractionThe Rayyan web application16 was used to select the abstracts of candidate publications independently. Two authors (JO, IP) then discussed and reached a consensus regarding the inclusion of documents. If there was any doubt about a manuscript's inclusion or exclusion, a third expert reviewer resolved the issue. Data were extracted into a table, with several meetings held to refine and modify the formats. Finally, the information was organized as shown below.
Summary and presentation of resultsThe results of this scoping review are presented according to the categories proposed by Grudniewicz et al.17: (i) a summary of the characteristics and distribution of the included documents, and (ii) a narrative synthesis of the findings. The extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting exploratory systematic reviews (PRISMA-ScR) was followed in the preparation of this manuscript (Supplementary File 3).18
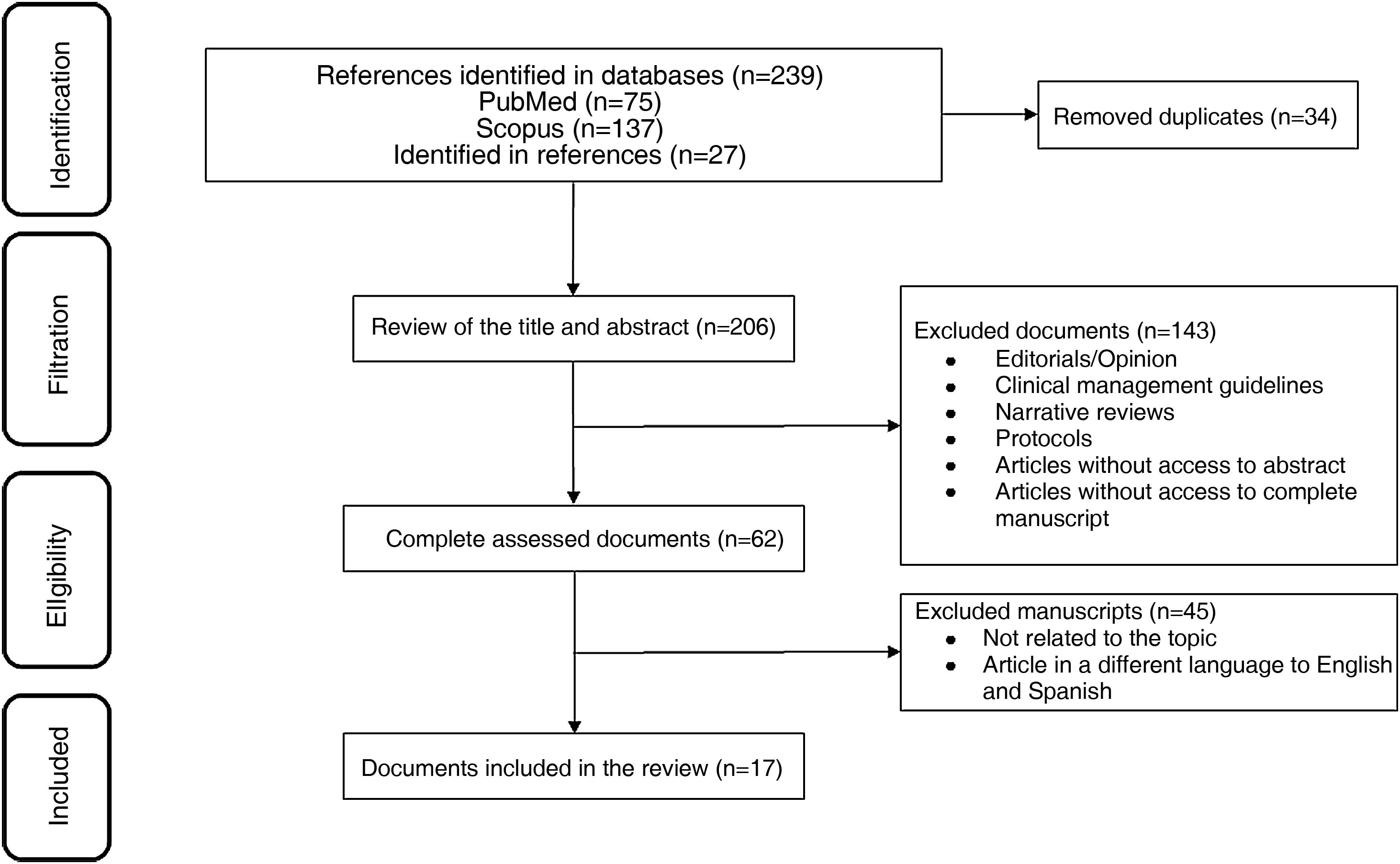
ResultsOut of 223 documents identified through the search strategies, 17 full-text articles evaluating the use of MPZ in patients diagnosed with EGPA were included (Fig. 1). Of these, 47% (8/17) were case reports, 18% (3/17) were retrospective cohort studies, 12% (2/17) were clinical trials, and 6% were a mix of prospective cohort (1/17), ambispective cohort (1/17), case series (1/17), and pilot study (1/17) (Table 1). The most frequently used dose of MPZ was 300 mg/month, reported in 59% (10/17) of the included articles. The most common variables used to assess clinical outcomes were the decrease in the BVAS score, reduction in corticosteroid dose, and changes in the number of eosinophils in the blood. Finally, 10 clinical trial records were included, detailing the design, conduct, and administration of ongoing studies (Table 2).
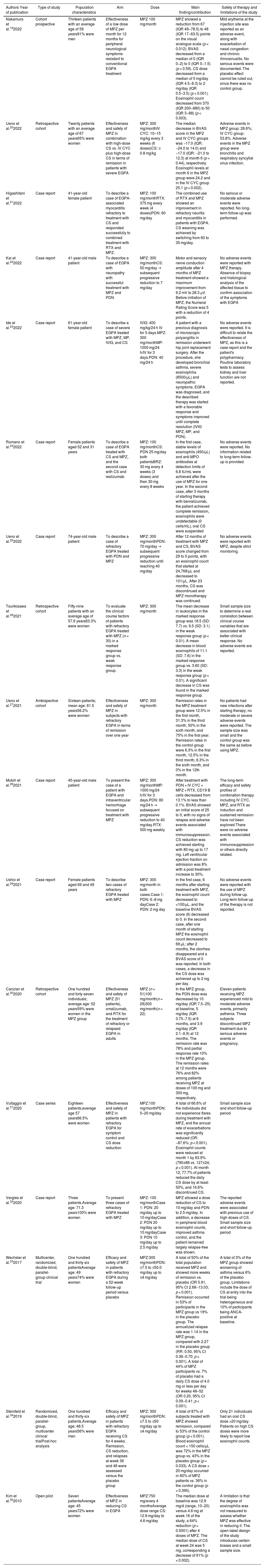
Characteristics of the included publications.
| Authors Year of publication | Type of study | Population characteristics | Aim | Dose | Main finding/contribution | Safety of therapy and limitations of the study |
|---|---|---|---|---|---|---|
| Nakamura et al.192022 | Cohort prospective | Thirteen patients with an average age of 59 years91% were men | Effectiveness of a low dose of MPZ per month for 12 months for peripheral neurological symptoms resistant to conventional EGPA treatment | MPZ 100 mg/month | MPZ showed a reduction from 67 (IQR 49−78.5) to 48 (IQR 17−63.5) points on the visual analogue scale (p < 0.012). BVAS decreased from a median of 0 (IQR 0−2) to 0 (IQR 0−1.5) (p < 0.59). CS dose decreased from a median of 5 mg/day (IQR 4.5−8.3) to 2 mg/day (IQR 0.5−3.5) (p < 0.001). Eosinophil count decreased from 370 (IQR 200−880) to 50 (IQR 3−88) (p < 0.003). | Mild erythema at the injection site was reported as an adverse event, along with exacerbation of nasal congestion and chronic rhinosinusitis. No serious events were documented. The placebo effect cannot be ruled out, since there was no control group. |
| Ueno et al.202022 | Retrospective cohort | Twenty patients with an average age of 67 years65% were women | Effectiveness and safety of MPZ in combination with high-dose CS vs. IV CYC plus high-dose CS in terms of remission in patients with severe EGPA | MPZ: 300 mg/monthIV CYC: 10–15 mg/kg every 3 weeks (6 doses)CS: ≥ 0.8 mg/kg | The median decrease in BVAS score in the MPZ and IV CYC groups was −17.0 (IQR: −24.0 to 14.0) and −17.0 (IQR: −21.0 to 12.3) at month 6 (p = 0.44), respectively. Eosinophil levels at month 6 in the MPZ group were 24.2 and in the IV CYC group 25.1 (p = 0.002). | Adverse events in MPZ group: 28.6%; IV CYC group: 53.8%. Adverse events in the MPZ group were bronchitis and respiratory syncytial virus infection. |
| Higashitani et al.212022 | Case report | 41-year-old female patient | To describe a case of EGPA-associated myocarditis refractory to treatment with CS and responded successfully to combined treatment with RTX and MPZ. | MPZ: 100 mg/monthRTX: 375 mg every week (4 doses)PDN: 60 mg/day | The combined use of RTX and MPZ showed an improvement in refractory neuritis and myocarditis in patients with EGPA. CS weaning was achieved by switching from 60 to 35 mg/day. | No serious or moderate adverse events were reported. No long-term follow-up was performed. |
| Kai et al.222022 | Case report | 41-year-old male patient | To describe a case of EGPA with neuropathy with successful treatment with MPZ and PDN | MPZ: 300 mg/monthCS: 50 mg/day → subsequent progressive reduction to 7 mg/day | Motor and sensory nerve conduction amplitude after 4 months of MPZ treatment showed a maximum improvement from 9.2 mV to 28.3 µV. Before initiation of MPZ, the Numeral Rating Score was 5 with a reduction of 4 points. | No adverse events were reported with MPZ therapy. Absence of biopsy and histological analysis of the affected tissue to confirm association of the symptoms with EGPA |
| Ide et al.232022 | Case report | 61-year-old female patient | To describe a case of severe EGPA treated with MPZ, MP, IVIG, and CS | IVIG: 400 mg/kg/24 h IV for 5 days.MPZ: 300 mg/monthMP: 1000 mg/24 h/IV for 3 days.PDN: 40 mg/24 h | A patient with a previous diagnosis of microscopic polyangiitis in remission underwent hip joint replacement surgery. After the procedure, she developed bronchial asthma, severe eosinophilia (8500/µL) and neuropathic symptoms. EGPA was diagnosed, and the described therapy was started with a favorable response and symptoms improved until complete resolution (IVIG MPZ, MP, and PDN). | No adverse events were reported. It is difficult to relate the effectiveness of MPZ, as this is a case report and the patient's polypharmacy. Routine laboratory tests to assess kidney and liver function are not reported. |
| Romano et al.242022 | Case report | Female patients aged 52 and 31 years | To describe a case of EGPA treated with CS and MPZ, and the second case with CS and reslizumab. | MPZ: 100 mg/monthCS: PDN 25 mg/day both patientsBRZ: 30 mg every 4 weeks (3 doses) and then 30 mg every 8 weeks | In the first case, stable levels of eosinophils (450/µL) and anti-MPO antibodies at detection limits of 6.8 IU/mL were achieved after the use of MPZ for one year. In the second case, after 3 months of starting therapy with benralizumab, the patient achieved complete remission, eosinophils were undetectable (0 cells/mL), oral CS were suspended | No adverse events were reported. No information related to long-term follow-up is provided. |
| Ueno et al.252022 | Case report | 74-year-old male patient | To describe a case of refractory EGPA treated with PDN and MPZ | MPZ: 300 mg/monthPDN: 70 mg/day → subsequent progressive reduction until reaching 40 mg/day | After 12 months of treatment with MPZ and CS, BVAS score changed from 29 to 0 points, with an eosinophil count that started at 24,768/μL and decreased to 101/μL. After 23 months, CS was discontinued and MPZ monotherapy was continued. | No adverse events were reported with MPZ, despite strict monitoring. |
| Tsurikisawa et al.262021 | Retrospective cohort | Fifty-nine patients with an average age of 57.6 years63.3% were women | To evaluate the clinical course factors of patients with refractory EGPA treated with MPZ (n = 30) in a marked response group vs. weak response group. | MPZ: 300 mg/month | The mean decrease in leukocytes in the marked response group was 18.5 (SD: 7.7) vs. 9.5 (SD: 3.1) in the weak response group (p < 0.01). A mean decrease in blood eosinophils of 11.1 (SD: 7.6) in the marked response group vs. 3.60 (SD: 3.3) in the weak response group (p < 0.01). A significant decrease in CS was found in the marked response group. | Small sample size to determine a real correlation between clinical course variables that are associated with better clinical response. No adverse events are reported. |
| Ueno et al.272021 | Ambispective cohort | Sixteen patients; mean age: 61.5 years56.2% were women | Effectiveness and safety of MPZ in subjects with refractory EGPA in terms of remission over one year | MPZ: 300 mg/month | Remission rates in the MPZ treatment group were 12.5% in the first month, 31.3% in the third month, 50% in the sixth month, and 75% in the first year. Remission rates in the control group were 6.3% in the first month, 12.5% in the third month, 6.3% in the sixth month, and 0% in the 12th month. | No patients had new infections after starting therapy; no moderate or severe adverse events were reported. The sample size was small and the control group was the same as before using MPZ. |
| Mutoh et al.282021 | Case report | 45-year-old male patient | To present the case of a patient with EGPA and intraventricular hemorrhage focused on treatment with MPZ | MPZ: 300 mg/monthMP: 1000 mg/24 h/IV for 3 days.PDN: 80 mg/24 h → subsequent progressive reduction to 40 mg/day.RTX: 500 mg weekly | After treatment with PDN + IV CYC + MPZ + RTX, CD19 B cells decreased from 13.1% to less than 0.1%. BVAS showed an initial score of 25 to 0, with no signs of relapse and adverse events associated with immunosuppression. CS reduction was achieved starting with 80 mg up to 17 mg. Left ventricular ejection fraction on admission was 8% with a post-treatment increase to 30%. | The long-term efficacy and safety profiles of combination therapy including IV CYC, MPZ, and RTX as induction and sustained remission have not been explored.There were no adverse events associated with immunosuppression or others directly related. |
| Ushio et al.292021 | Case report | Female patients aged 69 and 49 years | To describe two cases of refractory EGPA treated with MPZ | MPZ: 300 mg/month in both cases.Case 1: PDN: 6−8 mg dayCase 2: PDN: 2 mg day | In the first case, 6 months after starting treatment with MPZ, the eosinophil count decreased to <100/μL, and the baseline BVAS score (6) decreased to 0. In the second case, after one month of starting MPZ the eosinophil count decreased to 69 μL; after 2 months, the otorrhea disappeared and a BVAS score of 0 was reported. In both cases, a decrease in the CS dose was achieved up to 2 mg per day. | No adverse events were reported with the use of MPZ during follow-up. Long-term follow-up of the therapy is not reported. |
| Canzian et al.302020 | Retrospective cohort | One hundred and forty-seven individuals; average age: 52 years59% were women in the MPZ group | Effectiveness and safety of MPZ (51 patients), omalizumab, and RTX for the treatment of refractory or relapsed EGPA in adults | MPZ (n = 51)100 mg/month(n = 29)300 mg/month(n = 22) | In the MPZ group, the PDN dose was decreased by 10 mg/day (IQR 7.5−25) at baseline, 5 mg/day (IQR 3.75−7.5) at 6 months, and 3.9 mg/day (IQR 2.1−6.9) at 12 months. The remission rate was 78% and partial response rate 10% in the MPZ group. The remission rates at 12 months were 76% and 82% among patients receiving MPZ at doses of 100 mg and 300 mg, respectively. | Eleven patients receiving MPZ experienced mild to moderate adverse events, primarily asthenia. Three subjects discontinued MPZ treatment due to serious adverse events or pregnancy. |
| Vultaggio et al.312020 | Case series | Eighteen patients;average age 57 years66.5% were women | Effectiveness and safety of MPZ in patients with refractory EGPA for symptom control and CS dose reduction | MPZ:100 mg/monthPDN: 5–20 mg/day | A total of 66.6% of the individuals did not experience flares during treatment with MPZ, and the annual rate of exacerbations was significantly reduced (OR: −87.6%; p < 0.001). Eosinophil counts were reduced at month 1 by 83.9% (790±88 vs. 127±24; p < 0.001). At month 12, 77.7% of patients reduced the daily CS dose by at least 50%, and 16.6% discontinued CS. | Small sample size and short follow-up period |
| Vergles et al.322020 | Case report | Three patients.Average age: 71.3 years100% were women | To present three cases of refractory EGPA treated with MPZ | MPZ: 100 mg/monthCase 1: PDN: 20 mg/day up to 10 mg/dayCase 2: PDN 20 mg/day up to 10 mg/dayCase 3: PDN 10 mg/day up to 2.5 mg/day | MPZ allowed a dose reduction of CS to 10 mg/day and PDN to 2.5 mg/day. In addition, a decrease in peripheral blood eosinophil counts, improved asthma control, and the patient remained largely relapse-free was shown. | The reported adverse events were associated with previous use of high doses of CS. Small sample size and short follow-up period |
| Wechsler et al.332017 | Multicenter, randomized, double-blind, parallel-group clinical trial | One hundred and thirty-six patientsAverage age: 49 years74% were women | Efficacy and safety of MPZ in patients with refractory EGPA during a 52-week follow-up period versus placebo | MPZ:300 mg/monthPDN: ≥7.5 to ≤50.0 mg/day up to ≤4 mg/day | A total of 50% of the total population received MPZ and showed more weeks of remission vs. placebo (OR 5.91, 95% CI 2.68−13.03; p < 0.001). Remission occurred in 53% of participants in the MPZ group vs 19% in the placebo group. The annualized relapse rate was 1.14 in the MPZ group, compared with 2.27 in the placebo group (RR: 0.50, 95% CI 0.36−0.70; p < 0.001). A total of 44% of MPZ participants vs. 7% of placebo had a daily CS dose of 4.0 mg or less per day for weeks 48–52 (OR 0.20, 95% CI 0.09−0.41; p < 0.001). | A total of 3% of the MPZ group showed worsening of asthma versus 6% of the placebo group. Limitations include the dose of CS at entry into the trial being heterogeneous and 10% of participants being ANCA-positive at baseline. |
| Steinfeld et al.342019 | Randomized, double-blind, parallel-group, multicenter clinical trialPost-hoc analysis | One hundred and thirty-six patients,Average age: 48.5 years56% were men | Efficacy and safety of MPZ in patients with refractory EGPA receiving CS for 4 weeks. Remission, CS reduction, and relapses at week 36 and 48 were assessed versus the placebo group | MPZ: 300 mg/monthPDN: ≥7.5 to ≤50 mg/day up to ≤4 mg/day | A total of 87% of subjects treated with MPZ showed remission, compared to 53% of the control group (p < 0.001). Blood eosinophil count < 150 cells/μL was 72% in the MPZ group vs. 43% in the placebo group (p = 0.033). A CS dose > 20 mg/day occurred in 60% of MPZ patients vs. 36% in the control group (p = 0.395). | Only 21 individuals had an oral CS dose >20 mg/day. Patients on high CS doses were more likely to report low eosinophil counts. |
| Kim et al.352010 | Open pilot | Seven patientsAverage age: 45 years72% were women | Effectiveness of MPZ in reducing CS in EGPA | MPZ:750 mg/every 4 monthsAverage dose range CS: 12.9 mg/day to 4.6 mg/day | The median dose at baseline was 12.9 mg/d (range, 10−20) versus 4.6 mg at week 16 of the study, a 64% reduction (p < 0.0001) after 4 doses of MPZ. The median dose of CS at week 24 was 5 mg, corresponding a decrease of 61% (p < 0.002). | A limitation is that the degree of eosinophilia was not measured to assess whether MPZ was effective in reducing it. The open-label design of the study introduces certain biases and a small sample size. |
ANCA: Antineutrophil Cytoplasmic Antibodies; BRZ: benralizumab; BVAS: Birmingham Vasculitis Activity Score; IV CYC: Intravenous cyclophosphamide; CS: corticosteroids; SD: standard deviation; EGPA: Eosinophilic Granulomatous with Polyangiitis; CI: confidence interval; IVIG: Immunoglobulin; MPO: myeloperoxidase; IQR: interquartile range; IV: intravenous; MP: Methylprednisolone; MPZ: Mepolizumab; OR: odds ratio; PDN: Prednisolone; RR: Relative Risk; RTX: Rituximab; SC: Subcutaneous route.
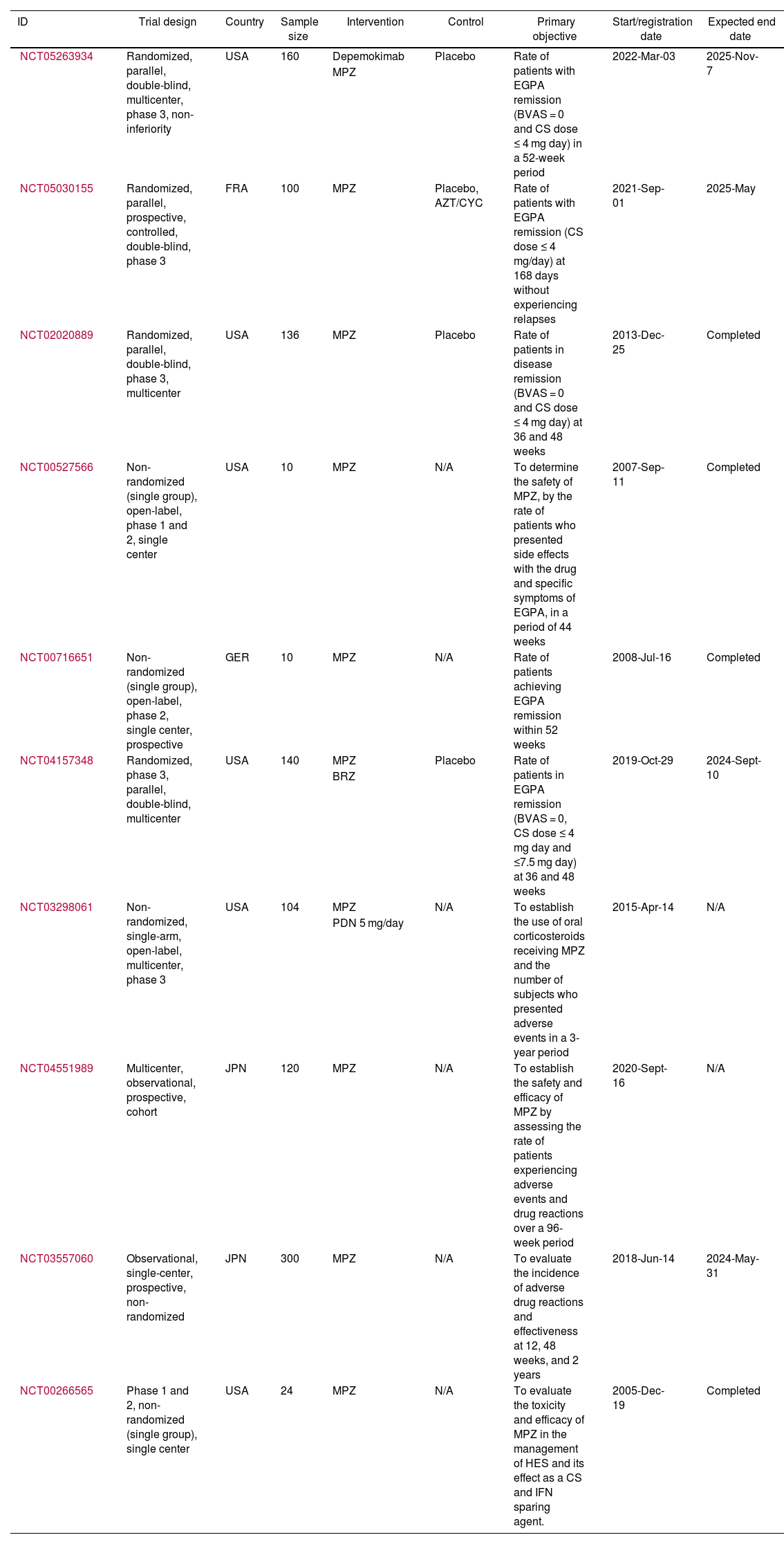
Features of the included clinical trial registries.
| ID | Trial design | Country | Sample size | Intervention | Control | Primary objective | Start/registration date | Expected end date |
|---|---|---|---|---|---|---|---|---|
| NCT05263934 | Randomized, parallel, double-blind, multicenter, phase 3, non-inferiority | USA | 160 | Depemokimab | Placebo | Rate of patients with EGPA remission (BVAS = 0 and CS dose ≤ 4 mg day) in a 52-week period | 2022-Mar-03 | 2025-Nov-7 |
| MPZ | ||||||||
| NCT05030155 | Randomized, parallel, prospective, controlled, double-blind, phase 3 | FRA | 100 | MPZ | Placebo, AZT/CYC | Rate of patients with EGPA remission (CS dose ≤ 4 mg/day) at 168 days without experiencing relapses | 2021-Sep-01 | 2025-May |
| NCT02020889 | Randomized, parallel, double-blind, phase 3, multicenter | USA | 136 | MPZ | Placebo | Rate of patients in disease remission (BVAS = 0 and CS dose ≤ 4 mg day) at 36 and 48 weeks | 2013-Dec-25 | Completed |
| NCT00527566 | Non-randomized (single group), open-label, phase 1 and 2, single center | USA | 10 | MPZ | N/A | To determine the safety of MPZ, by the rate of patients who presented side effects with the drug and specific symptoms of EGPA, in a period of 44 weeks | 2007-Sep-11 | Completed |
| NCT00716651 | Non-randomized (single group), open-label, phase 2, single center, prospective | GER | 10 | MPZ | N/A | Rate of patients achieving EGPA remission within 52 weeks | 2008-Jul-16 | Completed |
| NCT04157348 | Randomized, phase 3, parallel, double-blind, multicenter | USA | 140 | MPZ | Placebo | Rate of patients in EGPA remission (BVAS = 0, CS dose ≤ 4 mg day and ≤7.5 mg day) at 36 and 48 weeks | 2019-Oct-29 | 2024-Sept-10 |
| BRZ | ||||||||
| NCT03298061 | Non-randomized, single-arm, open-label, multicenter, phase 3 | USA | 104 | MPZ | N/A | To establish the use of oral corticosteroids receiving MPZ and the number of subjects who presented adverse events in a 3-year period | 2015-Apr-14 | N/A |
| PDN 5 mg/day | ||||||||
| NCT04551989 | Multicenter, observational, prospective, cohort | JPN | 120 | MPZ | N/A | To establish the safety and efficacy of MPZ by assessing the rate of patients experiencing adverse events and drug reactions over a 96-week period | 2020-Sept-16 | N/A |
| NCT03557060 | Observational, single-center, prospective, non-randomized | JPN | 300 | MPZ | N/A | To evaluate the incidence of adverse drug reactions and effectiveness at 12, 48 weeks, and 2 years | 2018-Jun-14 | 2024-May-31 |
| NCT00266565 | Phase 1 and 2, non-randomized (single group), single center | USA | 24 | MPZ | N/A | To evaluate the toxicity and efficacy of MPZ in the management of HES and its effect as a CS and IFN sparing agent. | 2005-Dec-19 | Completed |
GER: Germany; AZT: Azathioprine; BRZ: Benralizumab; BVAS: Birmingham Vasculitis Activity Score; CYC: Cyclophosphamide; CS: Corticosteroids; USA: United States; FRA: France; EGPA: Eosinophilic Granulomatous with Polyangiitis; IFN: Interferon; JPN: Japan; MPZ: Mepolizumab; PDN: Prednisolone; HES: Hypereosinophilic syndromes.
Wechsler et al.33 assessed the efficacy and safety of MPZ in 136 subjects, with 68 in the intervention and 68 in the placebo groups, respectively. The primary endpoint was achieving remission, defined by a BVAS score of 0 and a prednisolone or prednisone dose ≤ 4 mg for 52 weeks. In the MPZ group, cumulative remission was achieved by 28% of individuals compared to 3% in the placebo group. Among the 79 patients treated with MPZ and an absolute eosinophil count ≥ 150 cells/mm³, a higher remission rate was observed compared to the control group (33% vs. 0%). The intervention group also required a lower average dose of prednisolone or prednisone from weeks 48 to 52 compared to the placebo group (odds ratio [OR] 0.20; 95% confidence interval [CI] 0.09–0.41; p < 0.001).
Steinfeld et al.34 performed a post hoc analysis of a clinical trial in patients with EGPA treated with MPZ, who had not achieved remission as defined by the protocol established by Wechsler et al.33 This multicenter, double-blind, randomized clinical trial assessed MPZ in 136 subjects with EGPA, 68 of whom were assigned to the intervention group and the remaining 68 to the placebo group. Participants were required to have a diagnosis of refractory EGPA for at least six months and be receiving a stable oral dose of corticosteroids (prednisolone or prednisone: ≥7.5 to ≤50 mg/day), with or without immunosuppressive therapy for ≤4 weeks before study enrollment. Disease remission was defined as a BVAS score of 0 and an oral corticosteroid dose ≤ 4 mg/day. In the MPZ group, 59% (36/68) achieved disease remission, compared with 19% (13/68) in the placebo group (p < 0.001). Additionally, 57% (39/68) of those in the intervention group achieved a reduction in oral corticosteroid dose ≥ 50%, compared to 21% (14/68) in the control group (p < 0.001). The proportion of relapse-free patients was 26% higher in the MPZ group than in the placebo group (44% vs. 18%; p = 0.001).
Observational studiesCanzian et al.30 evaluated the effectiveness and safety of MPZ in 147 individuals diagnosed with EGPA, of whom 63 received rituximab, 51 MPZ, and 33 omalizumab. The study focused on reductions in BVAS score and oral corticosteroid dose. The MPZ group showed a median reduction in corticosteroid dose of 5 mg/day (interquartile range [IQR] 3.75–7.5) at six months and 3.9 mg/day (IQR 2.1–6.9) at 12 months. The initial BVAS score was 2 (IQR 1–5), with a median reduction of 0 points (IQR 0–0.5) at six months. In the MPZ group, remission rates were 76% with the 100-mg dose and 82% with the 300-mg dose at 12 months. Of the patients on MPZ, 6% discontinued due to adverse events or pregnancy. In comparison, patients treated with rituximab had an initial BVAS score of 8.5 (IQR: 5–13), which reduced to 1 point (IQR: 0–4.5) at six months and to 0 points (IQR: 0–2) at 12 months. The prednisone dose decreased from 20 mg/day (IQR: 15–37.5) to 7.5 mg/day (IQR: 5–10) at both six and 12 months of follow-up.
Tsurikisawa et al.26 analyzed the effectiveness of MPZ in 30 subjects in terms of BVAS score reduction. The study was divided into two groups: a "marked effect" group (n = 20), in which the daily corticosteroid dose was reduced or the interval between treatments with intravenous immunoglobulin was extended, and a "weak effect" group (n = 10), in which the dose of immunomodulators could not be reduced. In the marked effect group, the initial BVAS score was 33.5 (SD 8.3), with a reduction to 8.1 points (SD 4.2) at the last medical assessment after starting MPZ. In the weak effect group, the initial BVAS score was 32.2 (SD 6.6), with a final score of 14.0 (SD 4.9).
World Health Organization clinical trial registries evaluating mepolizumabTen clinical trials were found registered in the United States National Library of Medicine, including a total of 1104 patients (Table 2). The primary objective of these studies is to assess the efficacy and safety of MPZ, specifically focusing on the proportion of subjects achieving disease remission and the incidence of adverse reactions over a period defined by each study. In general, EGPA remission was defined as a BVAS score of 0 and oral corticosteroid doses ≤ 4 mg/day. However, in the Mandara trial (https://clinicaltrials.gov/study/NCT04157348), clinical remission was also accepted for patients with oral corticosteroid doses ≤ 7.5 mg/day. Currently, six studies are active, two of which (https://clinicaltrials.gov/study/NCT05263934 and https://clinicaltrials.gov/study/NCT05030155) are still in the patient recruitment phase.
Safety and limitations of included documentsAdverse events reported after MPZ administration included injection-site erythema, exacerbation of nasal congestion, asthenia, and respiratory tract infections such as respiratory syncytial virus infection (Table 1). No serious adverse events were documented in any of the included studies. Of the 17 included manuscripts, only two were clinical trials, while 88% (15/17) were observational studies, which have inherent limitations such as small sample sizes and short follow-up periods. No cost analyses of MPZ were found in the publications included in this review.
DiscussionThis manuscript presents an exploratory review of the clinical impact of mepolizumab (MPZ) in patients diagnosed with eosinophilic granulomatosis with polyangiitis (EGPA). The review highlights favorable outcomes in terms of modulating disease activity, along with a good safety profile. Studies19–35 consistently show a reduction in the BVAS score across most of the included studies, a decrease in the initial corticosteroid dose following the initiation of MPZ, and a significant role for blood eosinophil counts as a biological marker for assessing disease modulation.19–35 Adverse events were mild to moderate in severity in all studies. However, due to the observational design of most of the trials, there are limitations in interpreting and extrapolating the results.19–32,35
The clinical data derived from the included trials initially came from an assessment by Wechsler,33 followed by a post hoc analysis by Steinfeld two years later.34 The latter analysis helped complete the data on patients with recurrent or refractory EGPA who had not achieved remission. Both publications confirmed the efficacy and safety of MPZ, particularly in reducing corticosteroid doses, improving systemic involvement as assessed by BVAS scores, and preventing relapses.33,34 However, it should be noted that none of the clinical studies assessed the cost-effectiveness of MPZ compared to placebo or other available treatments. Therefore, further research is needed to compare these therapies in terms of health economics for EGPA.
Objective clinical assessment of EGPA activity remains challenging for rheumatologists. Severity scores or inflammatory markers are commonly used.36 The BVAS is a validated tool for assessing disease activity in individuals with various types of active vasculitis, objectively measuring involvement in nine organ systems to capture a broad spectrum of clinical manifestations.37,38 Diagnostic performance studies of the BVAS show an acceptable Receiver Operating Characteristic (ROC) curve analysis of 0.60 (95% CI 0.54–0.65), but the data are inconsistent for predicting one- (ROC 0.49) and five-year mortality (ROC 0.56).39 BVAS has been extensively evaluated in clinical trials and observational studies to estimate disease severity at diagnosis and during clinical follow-up, helping guide therapeutic approaches based on local and systemic manifestations.40 Furthermore, the relationship between initial EGPA activity and subsequent mortality risk may vary across studies, depending on the efficacy, effectiveness, and safety of the therapeutic strategies adopted.19–32,35
Refractory EGPA is defined as persistent or worsening disease activity despite at least four weeks of optimal treatment aimed at achieving remission.36,41 Before diagnosing EGPA as refractory, it is essential to confirm the primary diagnosis, exclude other organic diseases such as neoplasms or infectious processes, and assess patient adherence to therapy.36,41 Additionally, it must be determined whether the observed signs or symptoms are due to disease activation or irreversible chronic damage to organs affected by vasculitis or other coexisting conditions.36,41 Treatment for remission induction and maintenance in EGPA should be based on the severity of clinical manifestations and the prognosis, which can be assessed using the Five-Factor Score.41,42 Clinical practice guidelines recommend MPZ in combination with corticosteroids for non-severe refractory relapsed EGPA and as maintenance therapy for patients with predominantly respiratory symptoms and daily corticosteroid doses ≥ 7.5 mg to reduce the effective dose and minimize deleterious side effects.36,41
Relapse of EGPA is defined as the reappearance of signs and symptoms attributable to disease activity after a period of remission, accompanied by an increased need for corticosteroids or the initiation/increase in another immunosuppressant.36,41 It is important to distinguish systemic relapses from isolated exacerbations of asthma or rhinosinusitis, as these require different therapeutic approaches.36,41 The combined use of two monoclonal antibodies along with conventional therapy can reduce the need for high doses of corticosteroids, with rituximab and MPZ being the best-supported agents in the medical literature.21,43,44
EGPA presents with variable signs and symptoms, and treatment must be tailored to the individual’s specific presentation.44 The American College of Rheumatology recommends oral corticosteroids as the first-line therapy, with dose adjustments to achieve remission at optimal and safe levels for patients with EGPA.36,42,45 However, many patients fail to achieve remission and experience adverse events due to high corticosteroid doses, making the combination of monoclonal antibodies with stable corticosteroid doses a promising approach for managing difficult-to-control cases and reducing corticosteroid use without compromising clinical remission or increasing the risk of relapse.36,45
Biomarkers for differentiating EGPA activity from other systemic conditions are limited. Peripheral eosinophilia may reflect EGPA activity, particularly in cases of atopy with asthma.46,47 However, eosinophilic and vasculitic diseases can complicate the interpretation of systemic involvement and inflammatory status.46,47 It is crucial to rule out other conditions that can cause a hypereosinophilic state, such as drug reactions (DRESS), myeloid neoplasms, helminth infections (e.g., Strongyloides stercoralis), and others, to ensure proper use of blood eosinophil counts as biomarkers.3,36,46–48 While these counts are widely used to monitor treatment response with monoclonal antibodies in EGPA, their interpretation must account for other diseases and conditions that the rheumatologist should be aware of.
Other anti-IL-5 agents, such as benralizumab and reslizumab, exhibit pharmacokinetic and pharmacodynamic profiles similar to MPZ. Clinical trials suggest these agents are effective and safe for reducing oral corticosteroid use and disease activity.49,50 However, the available data on these alternatives is substantially limited compared to MPZ, and there is a lack of studies directly comparing the outcomes of different anti-IL-5 agents. The ongoing Mandara-NCT04157348 study, currently recruiting participants, aims to assess the efficacy and safety of benralizumab versus MPZ. Evidence from South American countries is still scarce, which limits the extrapolation and interpretation of results for this population, underscoring the need for additional clinical studies in this region.
Monoclonal antibodies are rapidly gaining acceptance due to their efficacy, effectiveness, and safety in treating diseases with significant immune-mediated components, including EGPA.51 The most reported adverse events are hypersensitivity reactions, malaise, headache, and dizziness, all of which are typically mild to moderate in severity.51,52 A review of the FDA database identified 104 reported cases of anaphylaxis associated with MPZ, with an odds ratio of 4.65 (95% CI 3.85–5.65) for anaphylaxis.53 Infections are estimated to occur in 4–7% of patients receiving MPZ, including those caused by the herpes zoster virus.52 The use of multiple medications, along with the study methodologies, limits the ability to directly link adverse events to MPZ in EGPA patients.
One of the main limitations of this review is the lack of an analysis of the quality of evidence from the included studies, as recommended by the PRISMA-ScR guidelines and Arksey et al.13,14,18 Search strategies were developed with the assistance of an expert librarian to enhance sensitivity, specificity, and reproducibility, and were employed to query search engines and databases containing extensive information on the topic. The restriction to English and Spanish publications represents another limitation in evidence collection.
Additionally, the high proportion of observational studies included in this review presents a major limitation due to small sample sizes and short clinical follow-up periods.19–32,35 Notably, the clinical trials included in this review compared MPZ with placebo, while most observational studies compared it only with standard therapy, which limits the extrapolation of the results and introduces bias when comparing MPZ to other biological agents.19–35 To better inform clinical practice for patients with EGPA, continued active recruitment for clinical registries, such as those described on the WHO platform,15 along with the design of new protocols and studies, is necessary. Expanding the sample size, considering a broader range of variables, and extending clinical follow-up would strengthen the evidence base.
ConclusionThe use of MPZ in patients with EGPA leads to a reduction in the BVAS score, a decrease in oral corticosteroid usage, and modulation of blood hypereosinophilia. The adverse events reported were mild to moderate across all the included studies. To obtain and extrapolate new clinical results, continued recruitment and randomization of patients with the disease treated with MPZ is necessary, along with an extended period of clinical follow-up. The development of new protocols and clinical trials that increase the sample size, consider a broader range of variables, and extend the duration of clinical follow-up is essential for the generalization and interpretation of results across different populations and geographic regions.
FundingThis work was funded by the Universidad de La Sabana.
We acknowledge the Universidad de La Sabana.