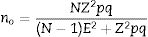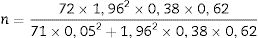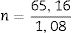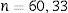Psoriatic arthritis is a chronic inflammatory arthritis associated with psoriasis. Its clinical presentation is diverse and heterogeneous with a spectrum of axial, peripheral and extra-articular involvement (dactylitis, enthesitis, skin psoriasis, nail disease). Clinimetric tools including simple and composite domains have been designed and used in clinical trials and clinical practice. In recent years, there has been much progress in the development of assessments tools, these provide an objective measure of disease activity and treatment response involving clear benefits if applied in routine clinical practice. This paper presents a thorough and up-to-date review of clinimetric tools for psoriatic arthritis reported in the literature.
La artritis psoriásica es una artritis inflamatoria crónica asociada con la psoriasis. Su presentación clínica es diversa y heterogénea, con un espectro de afectación axial, periférica y extraarticular (dactilitis, entesitis, psoriasis cutánea, enfermedad ungueal). Se han diseñado y utilizado herramientas clinimétricas que incluyen dominios simples y compuestos en ensayos clínicos y en la práctica clínica. En los últimos años se ha avanzado mucho en el desarrollo de herramientas de evaluación, las cuales proporcionan una medida objetiva de la actividad de la enfermedad y la respuesta al tratamiento que implican claros beneficios si se aplican en la práctica clínica habitual. Este artículo presenta una revisión exhaustiva y actualizada de las herramientas clinimétricas para la artritis psoriásica reportadas en la literatura.
Psoriatic arthritis was acknowledged as a disease in 1964. Moll and Wright defined it as an inflammatory arthritis plus the presence of psoriasis, in the absence of a rheumatoid factor.1 Domains of psoriatic arthritis include axial involvement, peripheral arthritis, enthesitis, dactylitis, as well as skin, nail, and scalp features. In 70% of cases, psoriatic arthritis involves skin first and joints after.2 Prevalence is highest in patients aged 30–60, with an equal proportion of males and females.3
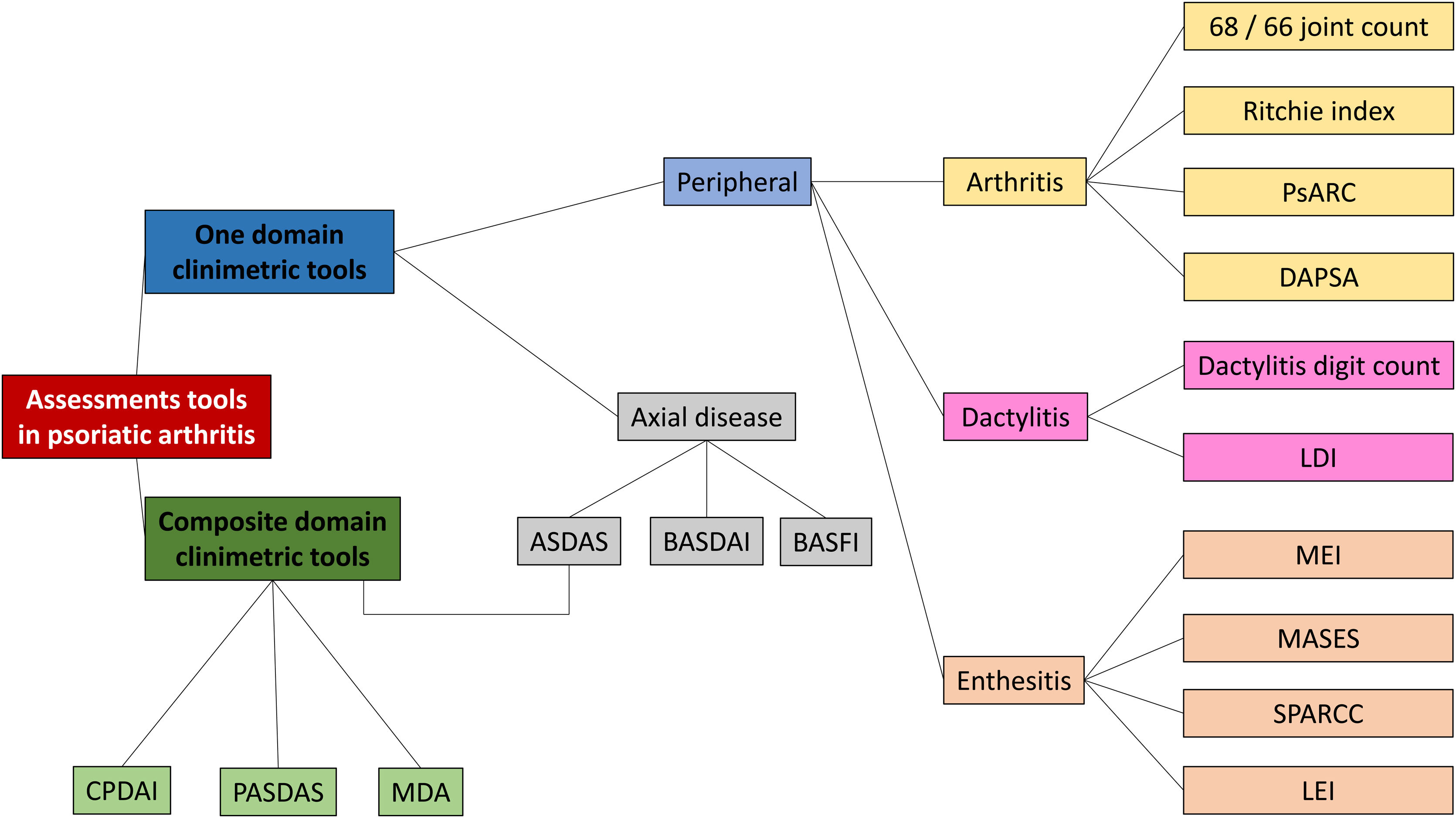
Psoriatic arthritis exhibits a destructive and progressive course associated with increased risk of cardiovascular comorbidities, functional limitation, and mortality.4 Even so, timely diagnosis remains a challenge due to lack of biomarkers and to the heterogeneous course of the disease, which may present with axial, peripheral (arthritis, enthesitis, or dactylitis), or extraarticular involvement. Several clinimetric scores have been designed for clinical follow-up and to assess response to treatment in research and clinical practice.5 This paper presents a thorough and up-to-date review of clinimetric tools reported in the literature, along with a description of the components and use of such tools (Fig. 1).
Clinimetric tools in psoriatic arthritis. PsARC: Psoriatic Arthritis Response Criteria. DAPSA: Disease Activity in Psoriatic Arthritis. LDI: Leeds Dactylitis Index. MEI: Mander/Newcasttle Enthesitis Index. MASES: Maastricht Ankylosing Spondylitis Enthesitis Index. SPARCC: Spondyloarthritis Research Consortium of Canada. LEI: Leeds Enthesitis Index. ASDAS: Ankylosing Spondylitis Disease Activity Score. BASDAI: Bath Ankylosing Spondylitis Disease Activity Index. BASFI: Bath Ankylosing Spondylitis Functional Index. CPDAI: Composite Psoriatic Arthritis. PASDAS: Psoriatic Arthritis Disease Activity Score. MDA: Minimal Disease Activity.
The authors searched for references in PubMed, Embase, and SciELO using the terms (Clinimetry OR “clinimetric scale*” OR Instrument* OR scale*) AND ((“Psoriasis Arthritis*” OR “Arthritic Psoriasis”) AND (Arthritis OR Spondylitis* OR Enthesitis OR Dactylitis)).
The authors considered relevant articles in English and Spanish providing information on clinimetric tools used for detection of joint or axial involvement in psoriatic arthritis.
Clinimetrics for psoriatic arthritis domainsClinimetrics for peripheral involvementSingle-domain clinimetric toolsThese tools assess only one domain of psoriatic arthritis (arthritis, enthesitis, dactylitis, or axial involvement), whether or not they include acute-phase reactants.
Clinimetrics for arthritisAssessment of articular involvement uses a count of swollen and tender joints. Psoriatic arthritis, unlike rheumatoid arthritis, usually presents with oligoarticular, asymmetric involvement. Psoriatic arthritis, however, may also cause polyarticular involvement that includes the distal interphalangeal joints.6
Swollen joint countPeripheral joint involvement differs in psoriatic arthritis and rheumatoid arthritis. A study by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) showed that reduced joint counts do not properly assess active psoriatic arthritis.7 Reduced counts of 28 or 44 swollen joints are not suitable for this condition.
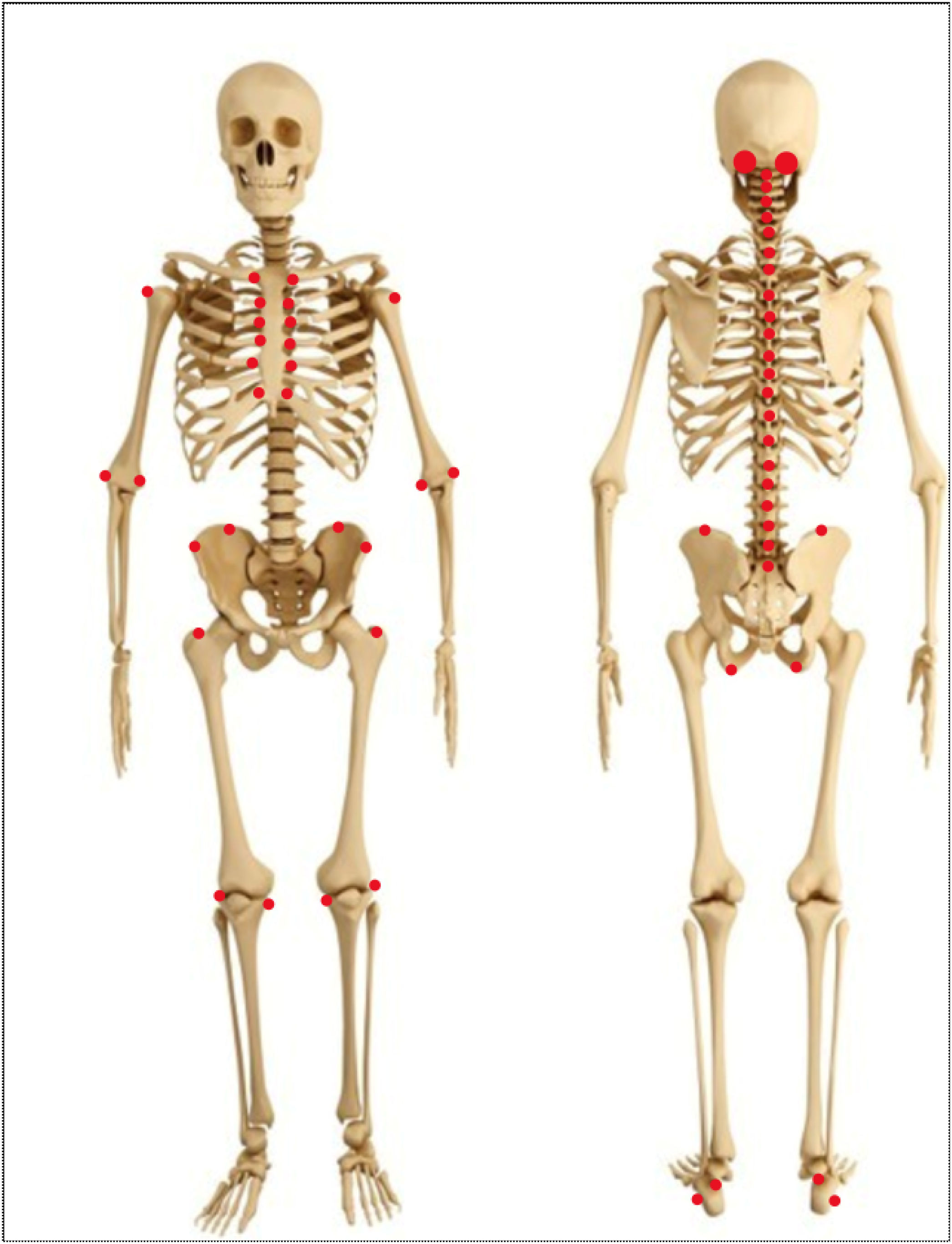
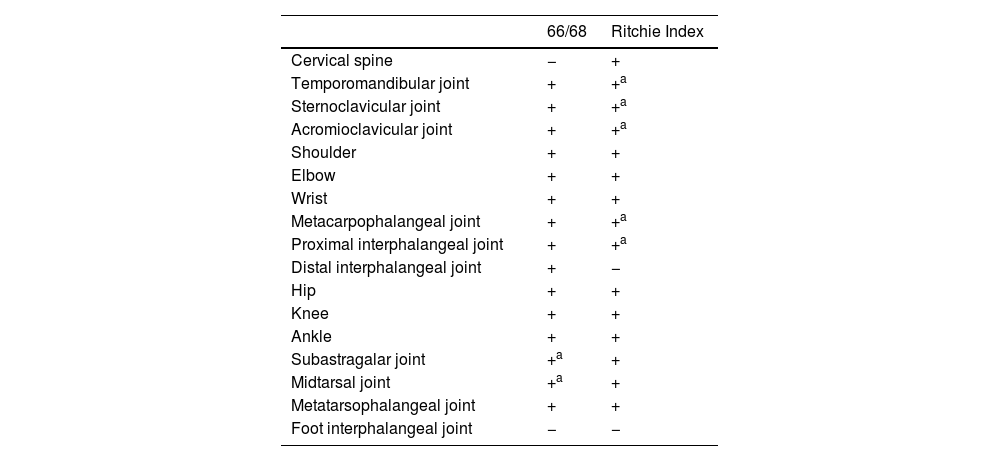
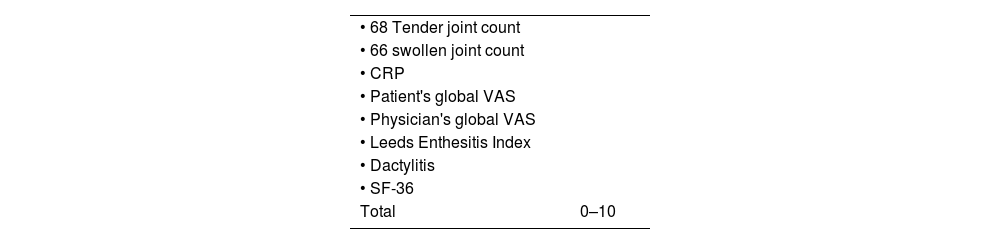
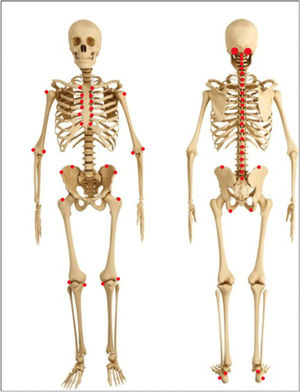
Other joint countsThe 68/66 Joint CountThe 68/66 Joint Count is the most thorough clinimetric. It assesses 68 joints for tenderness and 66 joints for swelling. Evaluation of swelling excludes hips and considers subastragalar and midtarsal joints as one.8Table 1 describes the joint groups in the 68/66 Joint Count.
Joint count clinimetric tools.
| 66/68 | Ritchie Index | |
|---|---|---|
| Cervical spine | − | + |
| Temporomandibular joint | + | +a |
| Sternoclavicular joint | + | +a |
| Acromioclavicular joint | + | +a |
| Shoulder | + | + |
| Elbow | + | + |
| Wrist | + | + |
| Metacarpophalangeal joint | + | +a |
| Proximal interphalangeal joint | + | +a |
| Distal interphalangeal joint | + | − |
| Hip | + | + |
| Knee | + | + |
| Ankle | + | + |
| Subastragalar joint | +a | + |
| Midtarsal joint | +a | + |
| Metatarsophalangeal joint | + | + |
| Foot interphalangeal joint | − | − |
The Ritchie Index scores from 0 to 3 the presence of pain elicited by pressure on the joint or by joint movement (7): 0=absence of pain. 1=pain. 2=pain and wince. 3=pain, wince, and withdrawal. The Ritchie is the equivalent to the 44 Joints assessment of pain elicited by pressure or motion. Since the Ritchie Index counts some articular groups (temporomandibular, sternoclavicular, acromioclavicular, metacarpophalangeal, and proximal interphalangeal joints) as one joint, it includes 26 evaluations comprising a maximal score of 78. Table 1 describes the evaluated articular groups of the Ritchie Index. This clinimetric assigns a group the highest score of a joint in the group.7 This score measures pain caused by movement in cervical spine, hip, subastragalar, and midtarsal joints.
Disease Activity Score (DAS)The DAS was developed in Europe through a prospective study on rheumatoid arthritis patients. That study used statistical methods with discriminant factors and logistic regression analyses.9 The DAS was designed to assess the activity status of rheumatoid arthritis and to define treatment adjustments, though its use has been extrapolated for psoriatic arthritis in daily clinical practice and in clinical trials.9 The DAS combines in one tool the assessment of four variables: swollen joint count (SJC); RAI (which includes tender joint count); erythrocyte sedimentation rate (ESR); and the patient's general health (GH)10 The formula to calculate DAS is:
SJC: (Swollen joint count). ln: natural logarithmDisease Activity Score 28 (DAS 28)The DAS 28 assesses four components: swollen joint count; tender joint count; an acute-phase reactant (CRP or ESR); and the patient's general health.10 Unlike DAS, it counts 28 joints, including shoulders, elbows, wrists, metacarpophalangeal, and proximal interphalangeal joints. Proposed cutoff points for DAS 28 are: 2.6, indicating remission; 3.2 for low disease activity; and 5.1 for high disease activity (10*).
The formulas to calculate DAS 28 are:
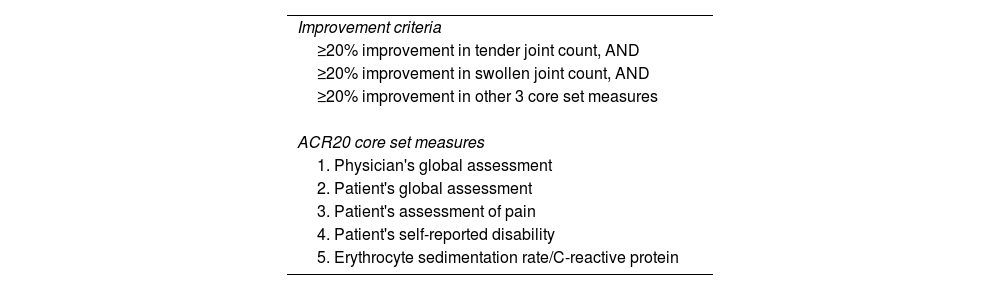
ln: natural logarithm.American College of Rheumatology Response (ACR Response)In the 1990s, the American College of Rheumatology (ACR)3 selected ‘ACR Response’ as a core set of measures to assess the response of rheumatoid arthritis to treatments in clinical trials.11 The ACR20 Response requires a 20% reduction in the disease manifestations listed in Table 2. The ACR50 and ACR70 responses require a 50% and 70% improvement, respectively. The United States Food and Drug Administration has approved ACR Response to evaluate treatments for rheumatoid arthritis and other conditions, such as psoriatic arthritis.12
American College of Rheumatology definition of improvement (ACR20).
| Improvement criteria |
| ≥20% improvement in tender joint count, AND |
| ≥20% improvement in swollen joint count, AND |
| ≥20% improvement in other 3 core set measures |
| ACR20 core set measures |
| 1. Physician's global assessment |
| 2. Patient's global assessment |
| 3. Patient's assessment of pain |
| 4. Patient's self-reported disability |
| 5. Erythrocyte sedimentation rate/C-reactive protein |
The PsARC is a composite tool designed to assess response to treatment of psoriatic arthritis. It was developed in a study conducted in 1996 on sulfasalazine treatment for psoriatic arthritis.13
The PsARC considers the following parameters6:
- •
Swollen joint count, assessed in 66 joints (Table 1)
- •
Tender joint count, assessed in 68 joints (Table 1)
- •
Patient global assessment, scored 0–5 by answering the question: “Considering all the ways arthritis affects you, how are you feeling today?”
- ∘
0: Feeling well, with no symptoms or limitations on daily activity.
- ∘
5: Feeling bad, with severe, intolerable symptoms that generate incapacity to perform normal activities.
- ∘
- •
Physician global assessment, scored from 0 to 5
The PsARC considers a response to treatment has occurred with at least 2 of the following events6:
- •
30% reduction in the swollen joint count
- •
30% reduction in the tender joint count
- •
Improvement of patient global assessment for at least one point
- •
Improvement of physician global assessment for at least one point
This tool does not include laboratory parameters. The European Medicines Agency recommends use of PsARC in day-to-day clinical practice. The PsARC, however, is considered less efficient than the ACR Response.6
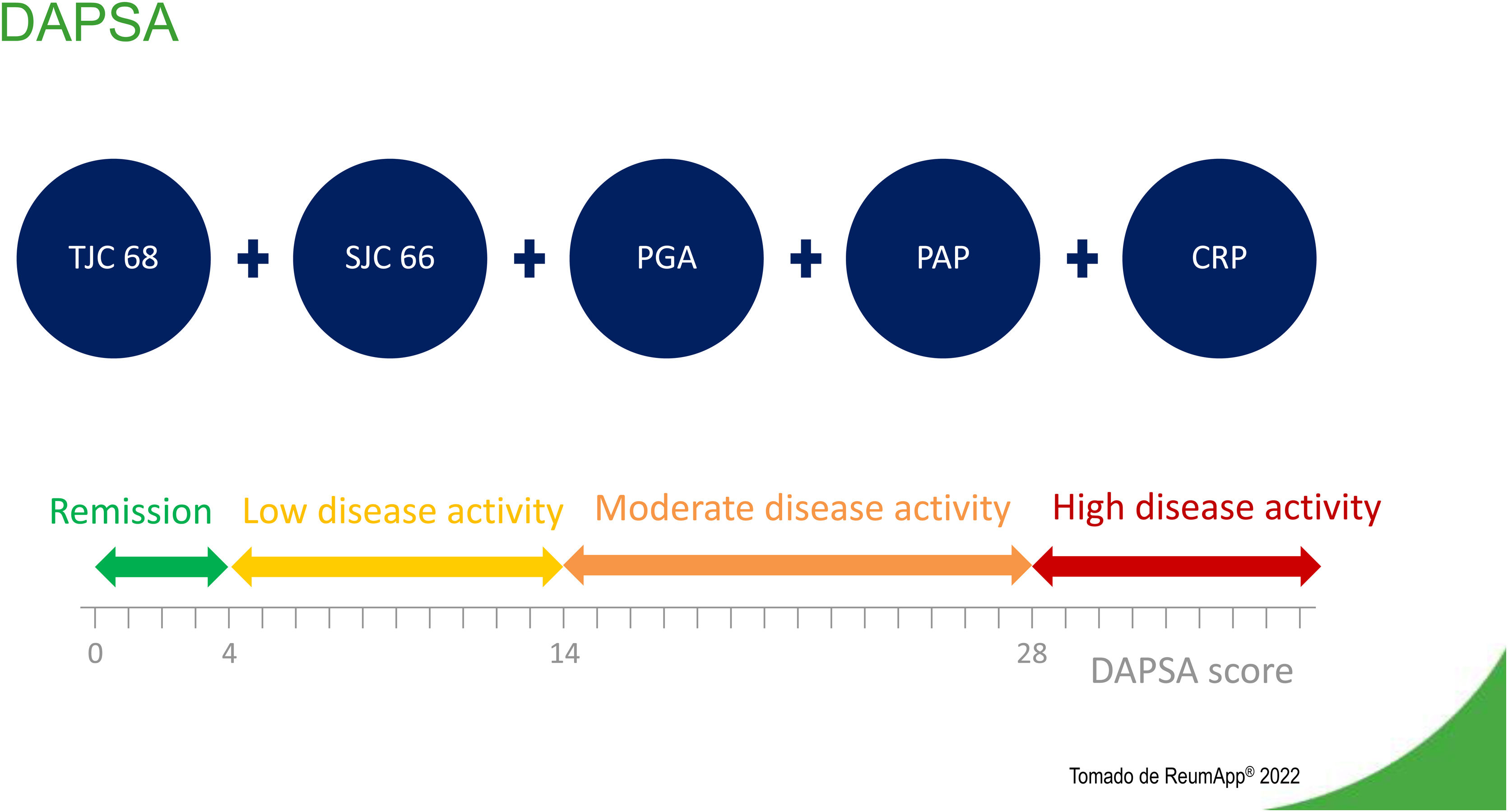
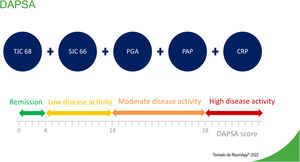
Disease Activity Index for Psoriatic Arthritis (DAPSA)The DAPSA is an adaptation for psoriatic arthritis of the Disease Activity Index for Reactive Arthritis. Validation of this adaptation used data from a clinical trial of infliximab conducted in Vienna in 2005.14 The study analyzed clinical and laboratory data of 105 patients with psoriatic arthritis and established four main components.15Fig. 2 describes components and levels of disease activity, according to DAPSA.
- •
Joint Count
- ∘
Swollen Joint Count (SJC): 0–66
- ∘
Tender Joint Count (TJC): 0–68
- ∘
- •
Patient Assessment of Pain (PAP) assessed with a visual analog scale (VAS): 0–10
- •
Patient Global Assessment (PGA) assessed with a visual analog scale (VAS): 0–10
- •
C-Reactive Protein (CRP): in mg/dl or mg/L
Formula for DAPSA is:
The sum of variables determines whether a patient is in remission or the degree of a patient's disease activity (Fig. 2). The DAPSA focuses on peripheral arthritis, but it does not include other manifestations of psoriatic arthritis, such as enthesitis, dactylitis, or axial involvement.
Clinimetrics for dactylitisDactylitis is a uniform soft-tissue inflammation of the entire digit, from the metacarpophalangeal to the distal interphalangeal joint or from the metatarsophalangeal to the interphalangeal joint. Dactylitis involves feet more often than hands, and it may affect several digits simultaneously, with the second and fifth toes most frequently involved.16 Since relapsing dactylitis of one digit alone may be the first manifestation of psoriatic arthritis, a thorough physical examination is very important. Though dactylitis presents in 14–49% of patients with psoriatic arthritis,16 it is not specific for this condition. Dactylitis may present in other diseases, such as syphilis, tuberculosis, flexor sheath infections, sickle cell anemia, and sarcoidosis.16
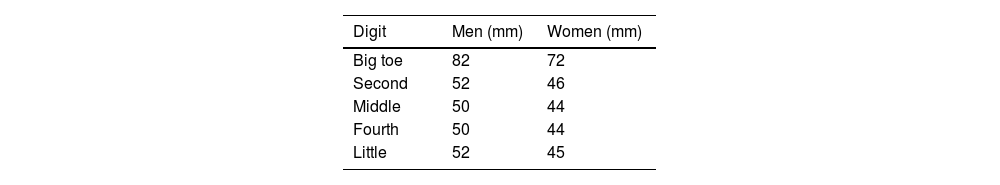
Objective assessment of swelling uses a dactylometer to measure the digit circumference at its base. Dactylitis is considered when the circumference at the base is more than 10% larger than that of the contralateral digit.17 Clinical trials use a count of fingers with dactylitis, and also the Leeds Dactylitis Index.
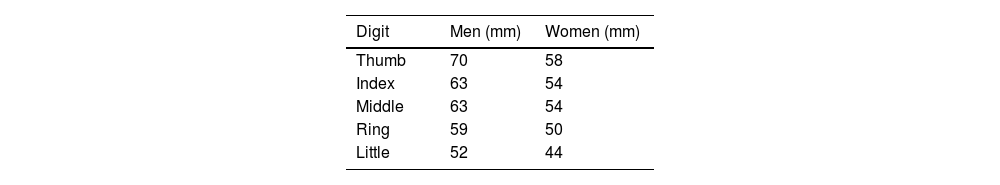
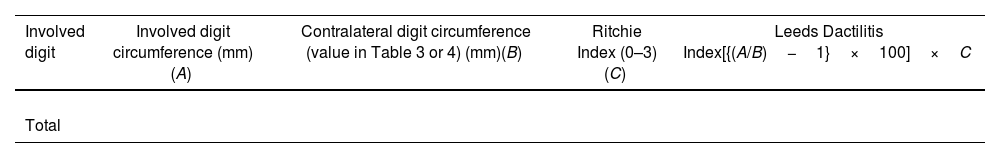
Leeds Dactylitis IndexThe Leeds Index aims for quantitative assessment of dactylitis. It uses a dactylometer to identify involved digits. The Leeds Dactylitis Index compares the circumferences of the affected digit with that of the digits of the contralateral hand or foot, using a table of normal reference values. Tables 3 and 4 describe values for fingers and toes. Since dactilometry may elicit pain with the compression of digits, calculation of the Leeds Index multiplies the quotient of digit circumferences by the Ritchie Index17 (Table 5). A basic Leeds Index that uses the Simplified Ritchie Index, a modification with binary scoring (0 for no pain/1 for pain).17 Interobserver reliability increases with a modified Leeds Dactylitis Index, since it simplifies the assessment of tenderness.
Leeds Dactylitis Index calculation.
| Involved digit | Involved digit circumference (mm)(A) | Contralateral digit circumference (value in Table 3 or 4) (mm)(B) | Ritchie Index (0–3)(C) | Leeds Dactilitis Index[{(A/B)−1}×100]×C |
|---|---|---|---|---|
| Total |
*Basic LDI uses the simplified Ritchie Index 0–1 scoring.
Enthesis is the site where tendon, ligament, or articular capsule insert into bone to ease joint movement. The enthesis may be fibrous or fibrocartilaginous, according to the type of tissue at the insertion site.18 The entheses are usually located out of the joints, in a periarticular area, or distant from any synovial joint. Enthesitis, inflammation of the enthesis, is acknowledged as a key feature of psoriatic arthritis.18 It is important to differentiate enthesitis from synovitis, an intraarticular inflammation of the synovial membrane. At the University of Toronto, a study of a prospective cohort of patients with psoriatic arthritis showed that enthesitis is a common condition, occurring in 35% of cases.19
Clinical evaluation of enthesitis may pose a challenge for clinicians. There are multiple clinical indexes, but those can be unspecific when other conditions, such as fibromyalgia, mechanical injuries, or tendinitis, overlap.20
Mander/Newcastle Enthesitis Index (MEI)The MEI was originally developed to assess all the entheses potentially involved in ankylosing spondylitis. The MEI scores examination of 66 entheses based on the patient response to firm palpation, using a 0–3 scale: 0: no pain. 1: mild pain. 2: moderate pain. 3: pain leading to muscle contraction or withdrawal. The MEI includes these entheses (Fig. 3):
- •
Nuchal crests
- •
Manubriosternal joints
- •
Costochondral joints
- •
Greater tuberosity and humerus medial and lateral epicondyles
- •
Iliac crests and anterior superior iliac spines
- •
Greater trochanter of the femur
- •
Tibial tuberosities
- •
Adductor tubercles
- •
Medial and lateral condyles of the femur and tibia
- •
Head of the fibula
- •
Calcaneal insertions of the plantar fascia and the Achilles tendons
- •
Sacroiliac joints
- •
Cervical, thoracic, and lumbar spinous processes
- •
Ischial tuberosities
- •
Anterior posterior iliac spines
Some entheseal sites get an individual score; others are grouped. A group of entheseal sites receives the highest score found in any entheses in the group. Sites considered as a group are: nuchal crests, costochondral joints, sacroiliac joints, and cervical, thoracic and lumbar spinous processes. The remaining entheseals sites receive an individual score for the right and left sides of the body. Calculation of the MEI score is done by adding up all the assessed entheses. The result ranges from 0 to 90.20 Limitations of MEI include its time-consuming examination, so randomized clinical trials have not used this index.
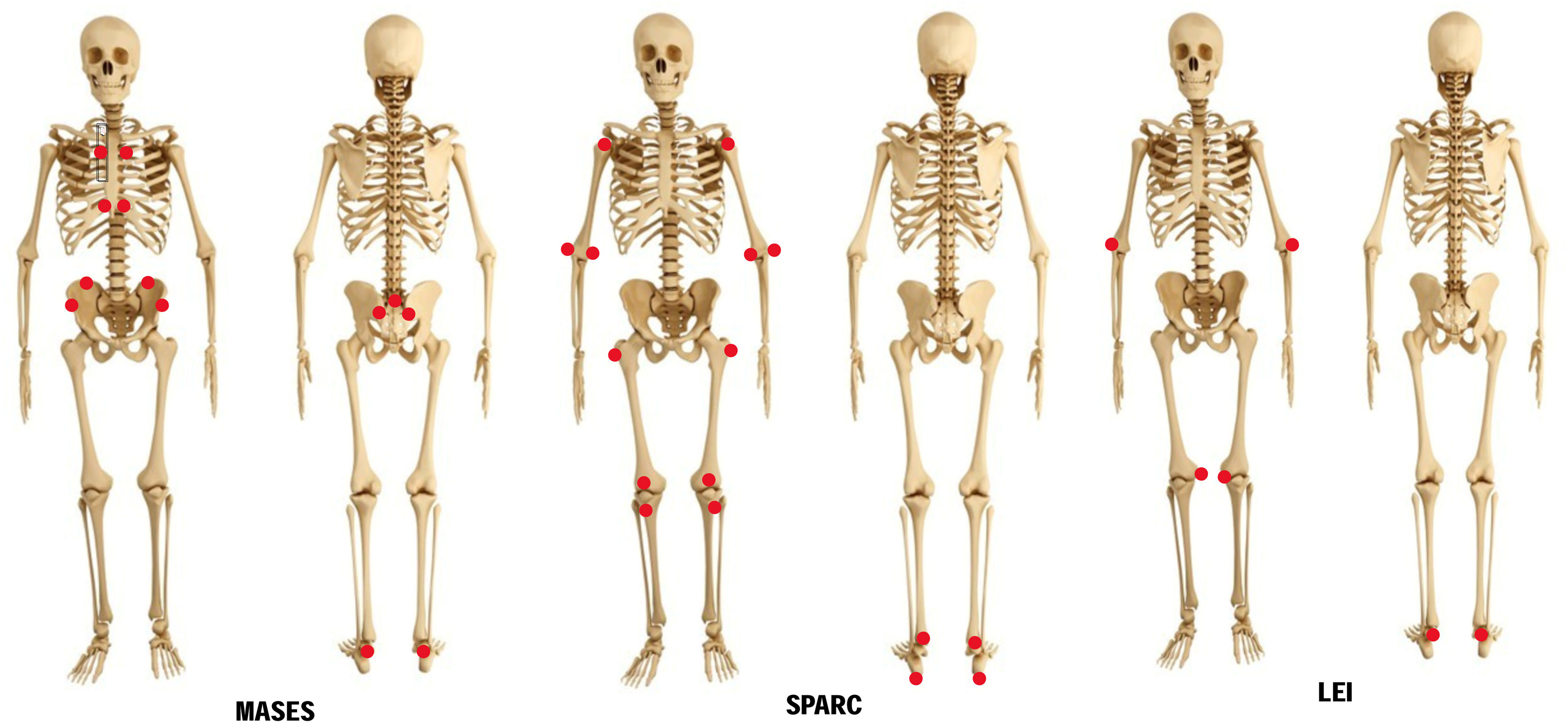
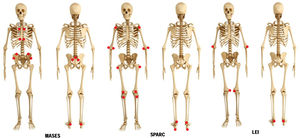
Maastricht Ahnkylosing Spondylitis Enthesitis Index (MASES)The MASES clinimetric was originally developed to assess enthesitis in ankylosing spondylitis, and it is currently used for psoriatic arthritis and spondyloarthritis, in general. It consists in application of pressure on 13 entheseal sites, grading the response with 0=no pain or 1=presence of pain,20 and adding up the points. Score ranges from 0 to 13. Since MASES examines less points than other indexes, it is more frequently used in day-to-day clinical practice. Also, the Assessment in Ankylosing Spondylitis (ASAS) working group recommends its use for randomized clinical trials on ankylosing spondylitis and psoriatic arthritis. The modified MASES adds two entheseal sites, right and left insertions of plantar fascia, for a maximum score of 15.21 The MASES examines these entheseal sites (Fig. 4):
- •
1st costochondral joint left/right
- •
7th costochondral joint left/right
- •
Posterior superior iliac spine left/right
- •
Anterior superior iliac spine left/right
- •
Iliac crest left/right
- •
Proximal insertion of Achilles tendon left/right
- •
5th lumbar spinous process
The SPARCC was developed to score enthesitis in general. It has been favorably compared to other instruments for ankylosing spondylitis and psoriatic arthritis assessment.6 The SPARCC scores pain at 16 entheseal sites as present (0 point) or absent (1 point), on the left and right sides of the body (Fig. 4):
- •
Medial epicondyle left/right
- •
Lateral epicondyle left/right
- •
Supraspinatus insertion in the greater tuberosity of the humerus
- •
Greater trochanter left/right
- •
Quadriceps tendon enthesis on the superior pole of the patella left/right
- •
Patellar ligament enthesis on the inferior pole of the patella or the tibial tuberosity, left/right
- •
Plantar fascia enthesis on the calcaneus left/right
To select of those entheseal sites, physicians used ultrasound examination of healthy individuals and patients with psoriatic arthritis.6 Total SPARCC score ranges from 0 to 16.
Leeds Enthesitis Index (LEI)The LEI is a tool specifically designed for assessment of enthesitis in patients with psoriatic arthritis. It was developed by the Bradford National Health Service Trust (West Yorkshire, United Kingdom) in a study that included 28 patients with psoriatic arthritis and enthesitis who did not receive or did not respond to modifying therapy.22 Clinical parameters of disease activity consistently correlated with LEI.23,24 The LEI assesses 6 easy-access entheseal sites (Fig. 4):
- •
Achilles tendon, left and right
- •
Humerus distal lateral, left and right
- •
Femur distal medial, left and right
The exam of each of the 6 sites registers tenderness as present (1) or absent (0), for a general score ranging from 0 to 6.
Clinimetrics for axial involvementIn 1973, Moll and Wright described the presence of spinal disease as one of the patterns of clinical involvement in psoriatic arthritis.25 Axial involvement presents in approximately 40% percent of patients with psoriatic arthritis.26 The majority of patients with axial psoriatic arthritis will also have peripheral joint disease,27 and only 2–5% of patients have only axial involvement. It is important to consider that some patients with axial psoriatic arthritis are asymptomatic but have radiologic characteristics. In these cases, the diagnosis is made by documentation of another involved domain. Clinical trials on psoriatic arthritis assess three key components: disease activity, functionality, and structural damage.
There are not specific measurements for axial psoriatic arthritis. Use of tools such as Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Score (ASDAS), and Bath Ankylosing Spondylitis Functional Index (BASFI) in psoriatic arthritis are an extrapolation from axial spondyloarthritis.
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)The BASDAI is a composite scoring system validated by the Assessment of Spondyloarthritis International Society (ASAS) to measure disease activity.27,28 It was developed in Bath, England, by a multidisciplinary team of rheumatologists, physical therapists, and researchers.29
The BASDAI assesses 4 aspects of the disease based on patient perception of pain in axial joints, pain in peripheral joints, tenderness to palpation of soft tissue, and severity/duration of morning stiffness.30 The BASDAI questionnaire asks patients 6 questions about subjective symptoms during the preceding week (Table 6). Questions 1–5 are scored from 0 (none) to 10 (very severe).16 The last question, regarding duration of morning stiffness, considers a time scale (0–2h or more).31
The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).
| In the past week… | Score 0–10 |
|---|---|
| 1. How would you describe the overall level of fatigue/tiredness you have experienced? | |
| 2. How would you describe the overall level of neck, back, or hip pain you have had? | |
| 3. How would you describe the overall level of pain/swelling in joints other than neck, back, hips you have had? | |
| 4. How would you describe the level of discomfort you have had from an area that is tender to touch or pressure? | |
| 5. How would you describe the level of morning stiffness you have had from the time you wake up? | |
| 6. How long does your morning stiffness last from the time you wake up? | |
| TOTALBASDAI=((Q1+Q2+Q3+Q4)+((Q5+Q6)/2))/5 |
Each question is scored on a scale of 0–10. Aside from the last question, 0 indicates none and 10 indicate very severe. For the last question, 0 is 0hours, 5 is one hour, and 10 is two or more hours. To calculate the BASDAI score, the formula is: BASDAI=((Q1+Q2+Q3+Q4)+((Q5+Q6)/2))/5.
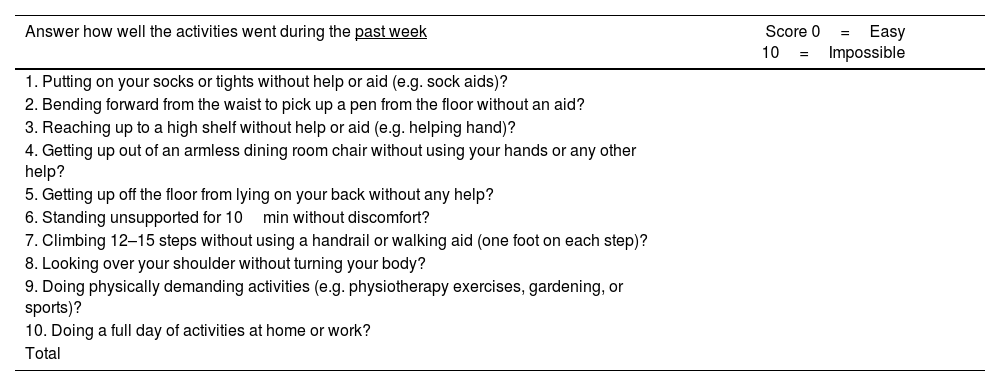
The BASFI is a self-assessment instrument that measures functionality of patients with ankylosing spondylitis. Like BASDAI, BASFI has been used to evaluate patients with psoriatic arthritis and axial involvement. Also, ASAS validated BASFI.32 The BASFI has 10 questions: 8 are specific on functionality and 2 reflect the patient's ability to perform activities of daily living (Table 7).33
The Bath Ankylosing Spondylitis Functional Index (BASFI).
| Answer how well the activities went during the past week | Score 0=Easy 10=Impossible |
|---|---|
| 1. Putting on your socks or tights without help or aid (e.g. sock aids)? | |
| 2. Bending forward from the waist to pick up a pen from the floor without an aid? | |
| 3. Reaching up to a high shelf without help or aid (e.g. helping hand)? | |
| 4. Getting up out of an armless dining room chair without using your hands or any other help? | |
| 5. Getting up off the floor from lying on your back without any help? | |
| 6. Standing unsupported for 10min without discomfort? | |
| 7. Climbing 12–15 steps without using a handrail or walking aid (one foot on each step)? | |
| 8. Looking over your shoulder without turning your body? | |
| 9. Doing physically demanding activities (e.g. physiotherapy exercises, gardening, or sports)? | |
| 10. Doing a full day of activities at home or work? | |
| Total |
Experts of the ASAS group developed this score and published it in 2008.34 The design used data of patients in the ISSAS (International Study On Staring Tumor Necrosis Factor Blocking Agent in Ankylosing Spondylitis) cohort, and a cross validation with the OASIS (Outcome in Ankylosing Spondylitis International Study) cohort.34 The ASDAS includes evaluation of back pain referred by the patient (question 2 of BASDAI), global patient assessment, peripheral joint pain and/or inflammation (question 3 of BASDAI) assessment, length of morning stiffness (question 6 of BASDAI), and a serological marker of inflammation (preferably PCR, and alternatively ESR.33 The score in ASDAS establishes four levels of disease activity34:
- •
Lower than 1.3: Inactive disease
- •
1.3–2: Moderate disease activity
- •
2.1–3.5: High disease activity
- •
Higher than 3.5: Very high disease activity
Table 8 presents the formula to calculate ASDAS score with PCR or ESR.
ASDAS formulas.
| ASDAS-CRP | 0.12×Back Pain+0.06×Duration of Morning Stiffness+0.11×Patient Global+0.07×Peripheral Pain/Swelling+0.58×Ln (CRP+1) |
| ASDAS-ESR | 0.08×Back Pain+0.07×Duration of Morning Stiffness+0.11×Patient Global+0.09×Peripheral Pain/Swelling+0.29×√(ESR) |
| • ASDAS, Ankylosing Spondylitis Disease Activity Score• √(ESR), square root of the erythrocyte sedimentation rate (mm/h)• ln(CRP+1), natural logarithm of the C-reactive protein (mg/L)+1• Back pain, patient global, duration of morning stiffness, and peripheral pain/swelling assessments use a visual analog scale (from 0 to 10) or a numerical rating scale (from 0 to 10).• Back pain, BASDAI question 2: “How would you describe the overall level of AS neck, back, or hip pain you have had?”• Duration of morning stiffness, BASDAI question 6: “How long does your morning stiffness last from the time you wake up?”• Patient global: “How active was your spondylitis on average during the last week?” |
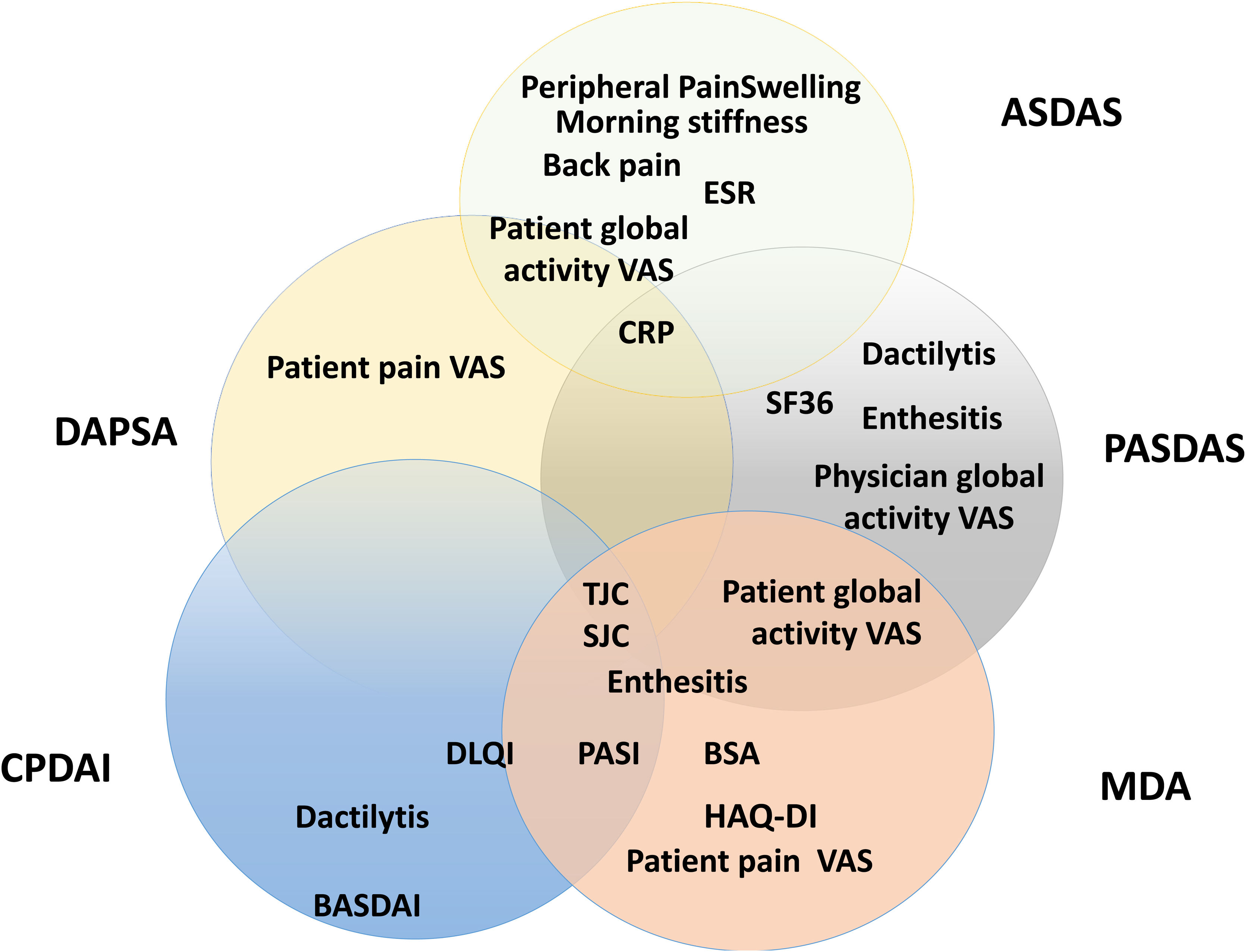
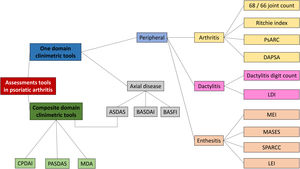
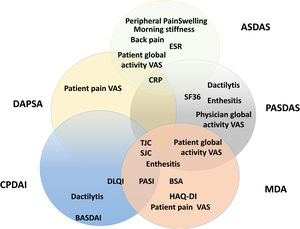
Composite measurements assess relevant clinical results with only one instrument that incorporates different domains of the disease in one scoring system. Fig. 5 presents a scheme of the composite clinimetric tools (CPDAI, PASDAS and MDA), ASDAS and DAPSA.
Items in the composite clinimetric tools (CPDAI, PASDAS and MDA), ASDAS and DAPSA. ASDAS: Ankylosing Spondylitis Disease Activity Score. DAPSA: Disease Activity in Psoriatic Arthritis. PASDAS: Psoriatic Arthritis Disease Activity Score. CPDAI: Composite Psoriatic Arthritis. MDA: Minimal Disease Activity. ESR: Erythrocyte Sedimentation Rate. CRP: C-Reactive Protein. VAS: Visual Activity Scale. SF 36: Short Form 36 Health Survey Questionnaire. TJC: Tender Joint Count. SJC: Swollen Joint Count. PGA: Patient Global Assessment. DLQI: Dermatology Life Quality Index. PASI: Psoriasis Area and Severity Index. BSA: Body Surface Area. HAQ-DI: Health Assessment Questionnaire Disability Index. BASDAI: Bath Ankylosing Spondylitis Disease Activity Index.
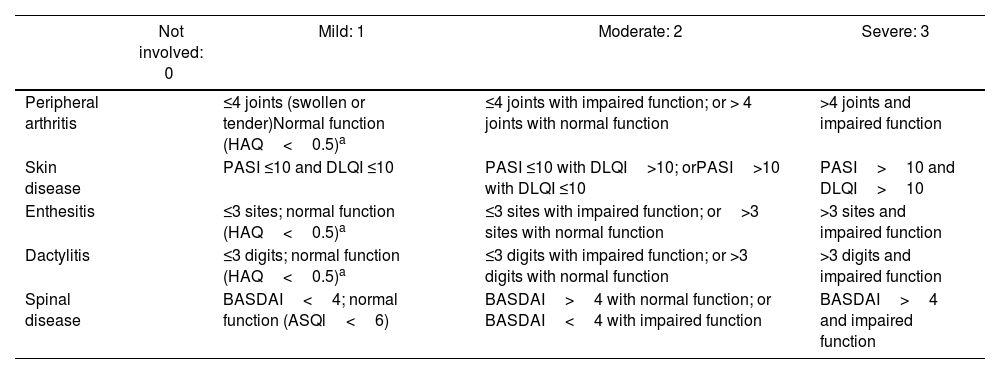
The GRAPPA has proposed CPDAI, a composite index of disease activity that uses several of the previously described instruments.35 The CPDAI assesses five psoriatic arthritis domains: (1) Peripheral arthritis with joint count (66 joints for swelling and 68 joints for tenderness) and functional disability with the health assessment questionnaire (HAQ). (2) Skin disease with Psoriasis Area Severity Index (PASI) and Dermatology Life Quality Index (DLQI). (3) Dactylitis, with simple count of involved digits. (4) Enthesitis, with LEI. (5) Spine manifestations with BASDAI and Ankylosing Spondylitis QoL Scale (ASQoL). Score of each domain ranges from 0–3, according to the disease activity and impact.36 Validation studies found that median CPDAI scoring was 9 points for patients needing treatment change, while the scoring was 3 points for patients not needing treatment change. Table 9 itemizes each domain assessed in CPDAI.
Composite Psoriatic Disease Activity Index (CPDAI).
| Not involved: 0 | Mild: 1 | Moderate: 2 | Severe: 3 | |
|---|---|---|---|---|
| Peripheral arthritis | ≤4 joints (swollen or tender)Normal function (HAQ<0.5)a | ≤4 joints with impaired function; or > 4 joints with normal function | >4 joints and impaired function | |
| Skin disease | PASI ≤10 and DLQI ≤10 | PASI ≤10 with DLQI>10; orPASI>10 with DLQI ≤10 | PASI>10 and DLQI>10 | |
| Enthesitis | ≤3 sites; normal function (HAQ<0.5)a | ≤3 sites with impaired function; or>3 sites with normal function | >3 sites and impaired function | |
| Dactylitis | ≤3 digits; normal function (HAQ<0.5)a | ≤3 digits with impaired function; or >3 digits with normal function | >3 digits and impaired function | |
| Spinal disease | BASDAI<4; normal function (ASQl<6) | BASDAI>4 with normal function; or BASDAI<4 with impaired function | BASDAI>4 and impaired function |
Health assessment questionnaire (HAQ) only counted if clinical involvement of domain (joint/enthesis/dactylitis) presented. ASQoL, ankylosing spondylitis quality of life. BASDAI, Bath Ankylosing Spondylitis Disease Activity Index. CPDAI, Composite Psoriatic Disease Activity Index. DLQI, Dermatology Life Quality Index. PASI, psoriasis area severity index.
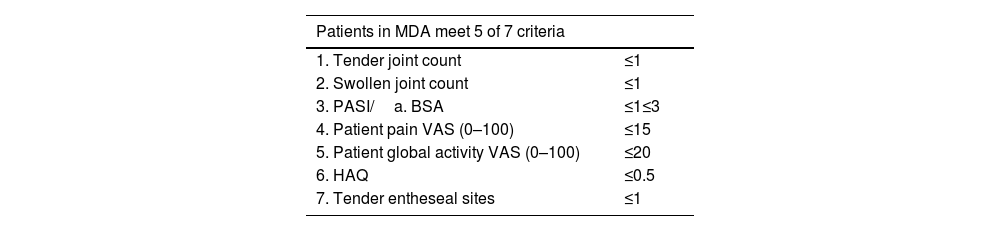
The MDA composite clinimetric tool was created to identify low or Minimal Disease Activity. It assesses symptoms and types of involvement by psoriatic arthritis and psoriasis. Dr. Laura Coates and GRAPPA led MDA design with the participation of other specialists in rheumatology and dermatology.
The MDA assesses 6 domains of psoriatic arthritis, using 7 measurements (Table 10)37:
- 1)
Tender joint count
- 2)
Swollen joint count
- 3)
Enthesitis count
- 4)
Skin (PASI or body surface area)
- 5)
Patient perception of pain (visual analog scale)
- 6)
Patient assessment of disease activity (visual analog scale)
- 7)
Functional capacity (HAQ-DI)
Five measurements belong in the ACR response criteria, and the remaining two assess cutaneous involvement (PASI≤1 or BSA≤3). Randomized clinical trials have used MDA to assess efficacy of treatments for psoriatic arthritis.38 After publication of MDA, its authors proposed a state of higher control called Very Low Disease Activity (VLDA). The patient in VLDA fulfills all 7 criteria presented in Table 10.
Psoriatic Arthritis Disease Activity Score (PASDAS)The PASDAS is a composite psoriatic arthritis activity measurement developed by GRAPPA.39 It includes a count of tender and swollen joints, Leeds enthesitis Index, dactylitis count, CRP, the SF-36 health questionnaire, and global assessment of the disease by patient and physician. A modified version of PASDAS extracts only 12 questions of the SF-36 health questionnaire to reduce the time required for filling out PASDAS. Established cut-off points are: <3.2, indicating low disease activity, and ≥5.4, indicating high activity disease.39Table 11 describes PASDAS variables.
Swollen joint count is 66 joints, and tender joint count 68. Score in PASDAS ranges from 0 to 10, with higher scores reflecting worse disease activity.
The formula for PASDAS calculation is:
ln=natural logarithm. PCS=physical component summary scale of SF36. CRP=C-reactive protein in mg/L. SF36=Medical Outcomes Study Short Form-36. All VAS scores are 0–100mm.Ethical aspectsThis review has no ethical considerations, no patient interventions were performed, informed consent is not required.
ConclusionPsoriatic arthritis is a heterogeneous disease with a wide spectrum of clinical manifestations. Clinical practice and clinical trials use clinimetric scales to determine the degree of disease activity and define changes in pharmaceutical therapy. This paper summarizes the features of each scale. The authors aim to promote the use of clinimetric scales in rheumatology for clinical practice and research.
Conflict of interestsThe authors declare they have no conflict of interest.