To identify the association between vitamin D (VD) concentrations and the activity of systemic lupus erythematosus (SLE) and determine a supplementation dose that allows patients to maintain adequate levels of VD.
MethodsLongitudinal, observational study. Serum levels of 25-hydroxy-VD were measured in 100 Paraguayan SLE patients from the Hospital de Clínicas between 2016 and 2018. To analyze the response to different doses of VD supplementation, 50 patients received 1000IU/day and the other 50 patients received 2000IU. SLE disease activity measured by SELENA-SLEDAIwas scored before and after supplementation.
ResultsThe mean age was 27.5±9.8 years, 88.9% of patients presented mild disease activity and 11.1% presented moderate to severe activity. The mean VD concentration was 30.8±11.8ng/mL. A total of 34% of patients presented VD insufficiency and 13% VD deficiency. There was an inverse relationship between VD concentrations and SLE disease activity (p=0.03). Increasing levels of serum VD are associated with supplementation of 2000IU/day (p=0.0224).
ConclusionSLE activity was associated with low levels of VD. In our cohort, SLE patients required a supplementation dose equal to or greater than 2000IU/day to increase their serum VD.
Identificar la asociación entre las concentraciones de vitamina D (VD) y la actividad del lupus eritematoso sistémico (LES), además de encontrar una dosis de suplementación que les permita a los pacientes mantener niveles adecuados de VD.
MétodosEstudio observacional longitudinal. Se midieron los niveles séricos de 25-hidroxi-VD en 100 pacientes paraguayos con LES, del Hospital de Clínicas, entre los años 2016 y 2018. Para analizar la respuesta a diferentes dosis de suplementación con VD, 50 pacientes recibieron 1.000 UI/día y los otros 50 pacientes recibieron 2.000 UI/día. La actividad de la enfermedad del LES medida por SELENA-SLEDAI se puntuó antes y después de la suplementación.
ResultadosLa media de edad fue de 27,5±9,8 años, el 88,9% de los pacientes presentó actividad leve de la enfermedad y el 11,1% presentó actividad moderada a severa. La concentración media de VD fue de 30,8±11,8 ng/ml. El 34% de los pacientes presentó insuficiencia de VD y el 13%, deficiencia de VD. Hubo una relación inversa entre las concentraciones de VD y la actividad de la enfermedad del LES (p=0,03). Los niveles crecientes de VD en suero se asocian con una suplementación de 2.000 UI/día (p=0,0224).
ConclusiónLa actividad del LES se asoció con niveles bajos de VD. En nuestra cohorte, los pacientes con LES requirieron una dosis de suplementación igual o superior a 2.000 UI/día para aumentar su VD sérica.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease associated with genetic, environmental, hormonal and immunological factors. Among environmental and hormonal factors, lack of vitamin D (VD) has been linked with increased SLE disease activity.1,2
Association with disease activity is based on the fact that several immune cells express VD receptors (VDR) on their surfaces,3 and many of them synthesize 1α-hydroxylase, responsible for the synthesis of the active form of VD in lymphatic tissues, with their respective relationship with immune modulation,4–6 affecting polarization of T cells and inhibiting secretion of antibodies by B lymphocytes.7–9
It has been postulated that both environmental and genetic reasons may be related to VD deficiency in patients with SLE. Previous environmental studies have postulated that sun avoidance, cumulative use of corticosteroids and creatinine levels could be related to low VD levels.10–12 On the other hand, Want et al., reported that genetic variation also contributes to VD insufficiency.13
Currently, there are controversial results on the role of VD concentration and disease activity in patients with SLE. While most have found an inverse relationship between VD concentration and disease activity, others have found no effect on it.
In this study, we analyzed the association between SLE activity and VD status in a cohort of South American lupus patients and the dose of supplementation with which patients can maintain adequate levels of VD.
Patients and methodsWe conducted a longitudinal observational study of 100 SLE patients, regularly seen at the outpatient rheumatology clinic of Hospital de Clínicas (a tertiary hospital of Asunción, Paraguay) during the November 2016 to March 2018 period. The present study was approved by the local ethics committee.
All patients fulfilled the Systemic Lupus International Collaborating Clinics (SLICC) criteria for SLE14 and gave written informed consent.
Exclusion criteria were: pregnancy and lactation period, diagnosis of malignant disease or suspected malignancy, diabetes, hepatitis B and C and HIV syndrome.
The following variables were recorded: age, sex, disease duration, sunscreen use, dietary intake habits. Use of glucocorticoids (GC), antimalarials, immunosuppressive agent, vitamin D and calcium supplements were also recorded. Serum concentration of 25-hydroxy-vitamin D, calcium, parathyroid hormone (PTH), C3, C4 and anti-DNA and disease activity were evaluated at the beginning of the study and 24 weeks after. The plasma levels of 25-OH-VD were quantified by chemiluminescent microparticle immunoassay (CMIA).15 VD deficiency was defined as plasma levels of 25-OH-VD<20ng/mL and insufficiency as 20–30ng/mL.16,17
The SELENA-SLEDAI score was used to evaluate disease activity. Patients were categorized into two groups for analysis: remission or low disease activity, defined as a SLEDAI score of ≤4 points or moderate to high disease activity, defined as a SLEDAI score >4 points.18
All patients received VD supplementation for 24 weeks. Patients with VD deficiency or insufficiency were supplemented with a loading dose every day for 4 weeks before receiving a maintenance dose for the remaining 20 weeks. Patients with BMI lower that 40 received a loading dose of 4000IU/day and patients with BMIs equal to or higher than 40 received 6000IU/day.19 Patients with adequate levels of VD at week 0 did not receive a loading dose and started VD supplementation at maintenance doses since week 0. Maintenance doses of 1000IU/day, 2000IU/day or 4000IU/day were administered to all patients (without regards to previous loadings doses). Participants were allocated to different treatment arms using a non-random method: The first half received 1000IU/day and the second half received 2000IU/day, with the exception of patients with BMIs equal to or higher than 40, who received 4000IU/day.19
Statistical analysisAll statistical tests were performed using the R statistical software version 3.4.2 (www.R-project.org). For the descriptive analysis, the mean and standard deviation in numerical data and the absolute and relative frequencies in categorical data were used. For the association study between vitamin D and the disease activity variables, univariate linear regression analyses using VD concentration as independent variable were performed. In addition, regression analyses were also performed using the VD insufficiency/deficiency phenotype to check that significant associations remained significant when using the discretized phenotype.
For the analysis of evolution between weeks 0 and 24 of the numerical variables studied, we used a linear regression with the values of week 24 as the dependent variable and those of week 0 as an independent variable. Finally, the relation between variation of VD concentrations between weeks 0 and 24 and VD supplementation doses at week 0 was studied using both VD concentration variation and categorized VD variation (−1: decrease; 0: equal; 1: increase) as dependent variables in a regression analysis.
ResultsDemographic and other baseline variablesA total of 100 patients were included in the study. There were 89 women, patient average age was 29.8±9.8 years and median disease duration was 31 (IQR, 18–60) months. 62.8% used sunscreen, 26.4% wore hats and 51.2% wore long sleeves. 94% consumed dairy products regularly. 55% of the patients had corticosteroids, with a mean dose of 17.05mg/day (±14). 89% were taking hydroxychloroquine, 44% patients were using immunosuppressive therapy at week 0: 22.6% of patients were on azathioprine, 15% on mycophenolate, 3.2% on methotrexate and 3.2% on cyclophosphamide. 89% consumed VD and calcium supplements.
In week 0, 11.1% had decreased serum calcium and 22% had elevated PTH values. Parathyroid hormone levels above 35.6pg/mL were considered elevated. 88.9% of patients had mild activity and 11.1% had moderate to severe activity. 22 patients presented positivity for anti-DNA antibodies, 32 and 64 patients presented decreased values of C3 and C4 respectively.
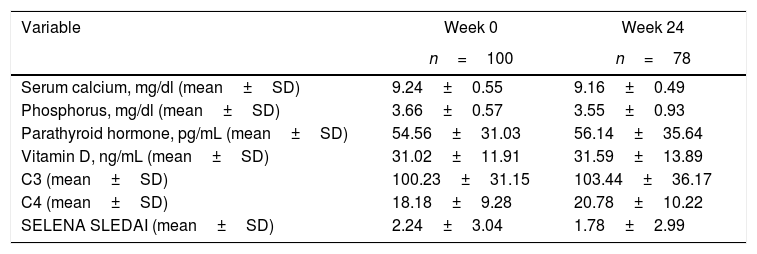
At the initial visit, 47% had reduced serum VD concentrations. 34 patients presented with VD insufficiency and 13 with deficiency. Table 1 shows the main analytical values of week 0 and week 24 of the studied cohort. Twenty-two patients were excluded from analysis at week 24: 10 due to inadequate VD supplementation and 12 to insufficient data.
Characteristics of SLE patients at week 0 and week 24.
| Variable | Week 0 | Week 24 |
|---|---|---|
| n=100 | n=78 | |
| Serum calcium, mg/dl (mean±SD) | 9.24±0.55 | 9.16±0.49 |
| Phosphorus, mg/dl (mean±SD) | 3.66±0.57 | 3.55±0.93 |
| Parathyroid hormone, pg/mL (mean±SD) | 54.56±31.03 | 56.14±35.64 |
| Vitamin D, ng/mL (mean±SD) | 31.02±11.91 | 31.59±13.89 |
| C3 (mean±SD) | 100.23±31.15 | 103.44±36.17 |
| C4 (mean±SD) | 18.18±9.28 | 20.78±10.22 |
| SELENA SLEDAI (mean±SD) | 2.24±3.04 | 1.78±2.99 |
SELENA SLEDAI: Systemic Lupus Erythematosus Disease Activity Index.
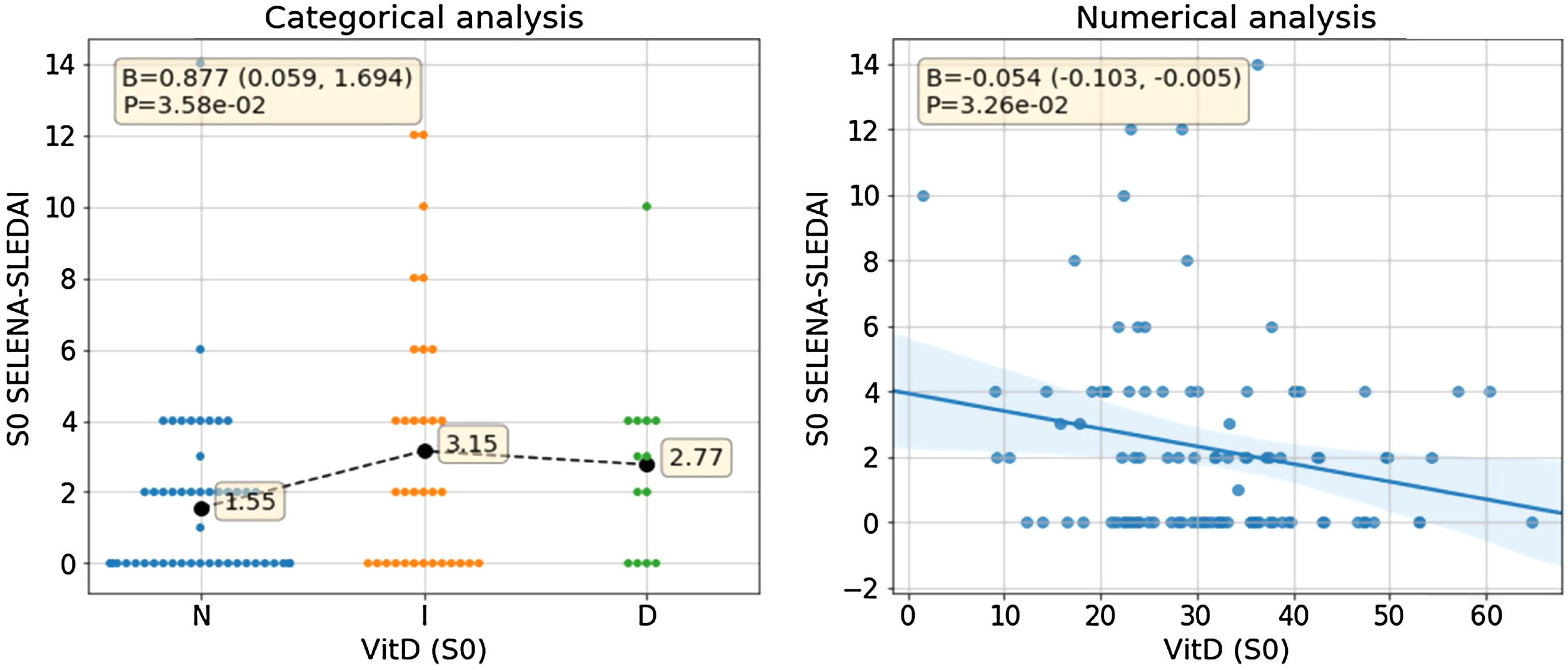
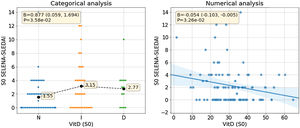
When analyzing the association between VD concentration and activity parameters, a statistically significant association with disease activity is observed. Among the patients with moderate-severe disease activity (SLEDAI score >4 points), we found 19 cases with low vitamin D values and 9 patients with normal values (Fig. 1). The association is statistically significant when taking into account both the VD concentration and the categorized VD phenotype. In the first case, a decrease of 0.54 SLEDAI activity points is observed for each increase of 10ng/mL in VD concentration (β=−0.054, CI 95% [−0.103, −0.005] SLEDAI points/VD [ng/mL], p=0.0326). In the second case, a statistically significant association is also observed between increased activity and the categorized VD phenotype (B=0.877 CI 95% [0.059, −1.694], p=0.0358). Note that normal, insufficiency and deficiency phenotypes were coded as 0, 1, and 2 for this analysis meaning that SLEDAI activity in patients with VD insufficiency is 0.877 points higher than in patients with normal phenotype. Same conclusion applies when comparing patients with VD deficiency and VD insufficiency. Fig. 1 shows the result of this analysis.
Association between serum VD levels and disease activity in SLE patients. The figure at left side shows the association of SELENA-SLEDAI activity with the categorized VD phenotype where a significant difference can be observed both statistically and visually between the normal phenotype and the insufficiency/deficiency phenotypes. The figure at right side shows de association of SELENA-SLEDAI activity with VD concentration. The negative slope of the regression line shows the inverse association between VD concentration and SELENA-SLEDAI activity. N: normal, I: insufficient, D: deficient, VitD: vitamin D, S0: W0 (week 0).
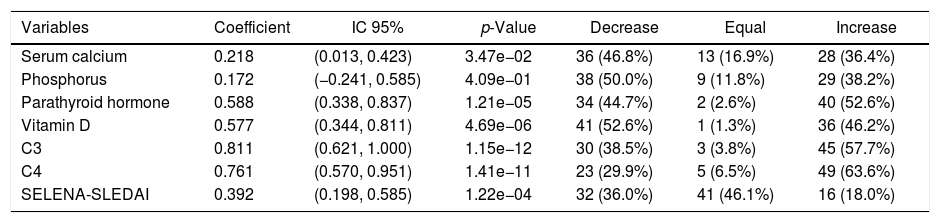
Significant changes were observed when analyzing the evolution of the metabolic and activity variables from week 0 to 24, considering that VD supplementation for maintenance was in the range from 1000 to 2000IU/day (Table 2).
Evolution of laboratory and activity variables at week 24.
| Variables | Coefficient | IC 95% | p-Value | Decrease | Equal | Increase |
|---|---|---|---|---|---|---|
| Serum calcium | 0.218 | (0.013, 0.423) | 3.47e−02 | 36 (46.8%) | 13 (16.9%) | 28 (36.4%) |
| Phosphorus | 0.172 | (−0.241, 0.585) | 4.09e−01 | 38 (50.0%) | 9 (11.8%) | 29 (38.2%) |
| Parathyroid hormone | 0.588 | (0.338, 0.837) | 1.21e−05 | 34 (44.7%) | 2 (2.6%) | 40 (52.6%) |
| Vitamin D | 0.577 | (0.344, 0.811) | 4.69e−06 | 41 (52.6%) | 1 (1.3%) | 36 (46.2%) |
| C3 | 0.811 | (0.621, 1.000) | 1.15e−12 | 30 (38.5%) | 3 (3.8%) | 45 (57.7%) |
| C4 | 0.761 | (0.570, 0.951) | 1.41e−11 | 23 (29.9%) | 5 (6.5%) | 49 (63.6%) |
| SELENA-SLEDAI | 0.392 | (0.198, 0.585) | 1.22e−04 | 32 (36.0%) | 41 (46.1%) | 16 (18.0%) |
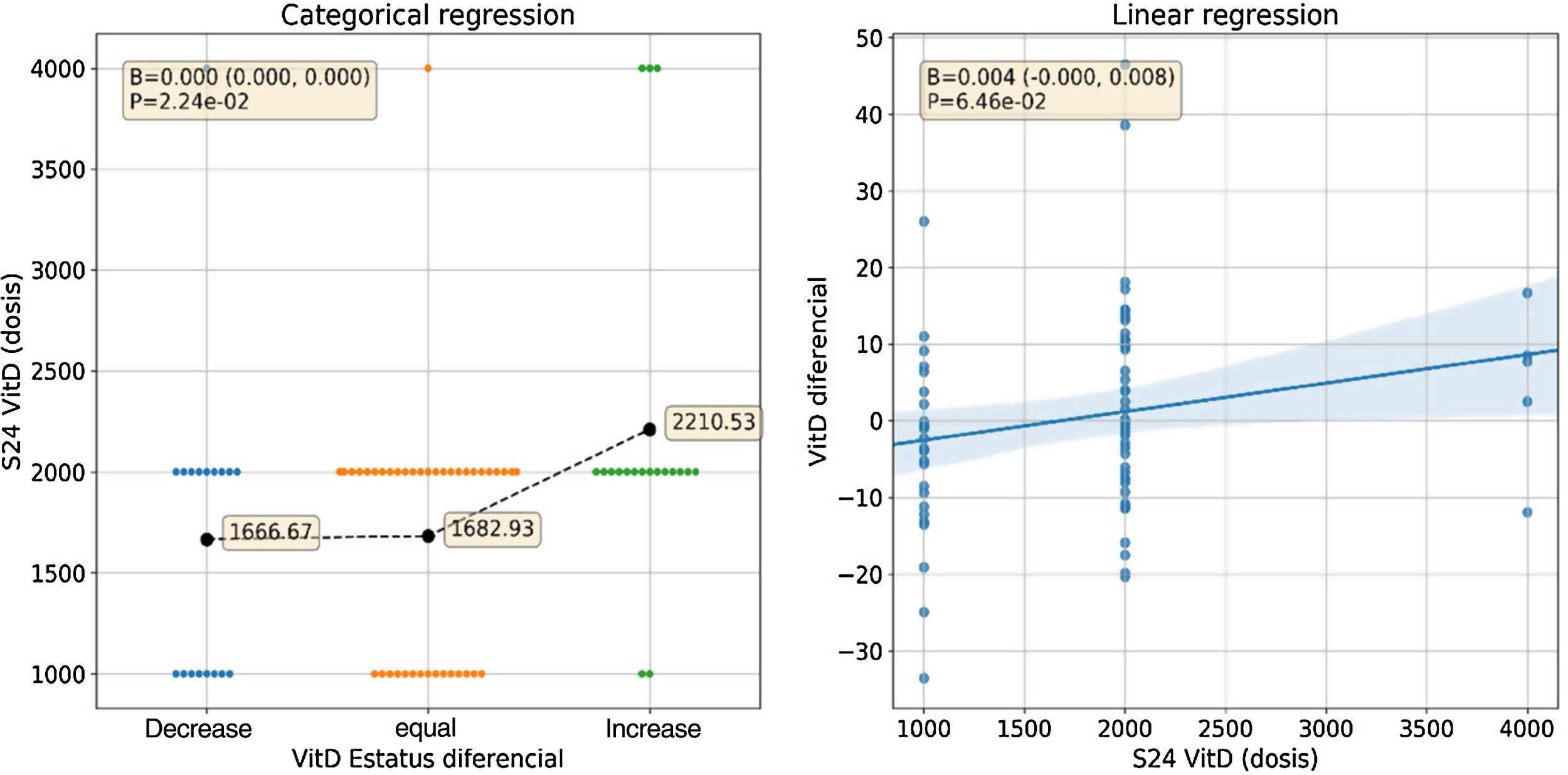
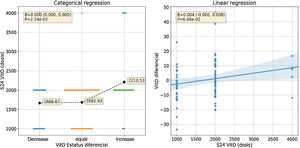
The variation of VD concentration from baseline to week 24, it was observed that 52.6% of the patients showed a decrease VD concentration, while the other 46.2% showed an increase.
Taking into account the categorized VD phenotype, n=19 patients (24.4%) presented a decrease in VD (from normal concentration to insufficiency/deficiency or from insufficiency to deficiency) despite supplementation; n=41 patients (52.6%) remained unchanged and n=18 patients (23.1%) presented an increase in VD. Within the same period, n=22 patients (24.7%) decreased their disease activity, n=58 (65.2%) maintained the same activity and n=9 (10.1%) presented an increase in activity.
In relation to the supplementation doses, it was observed that a daily dose equal to or higher than 2000IU/day was associated with an increase in VD concentration, as shown in Fig. 2.
DiscussionMuller et al. described for the first time the link between low levels of vitamin D and lupus in 1995.20 Since then, numerous studies confirmed a higher prevalence of vitamin D deficiency in patients with SLE, compared with the general population, often observing a correlation with severity of disease as well.11,21–24 The World Health Organization (WHO), the Institute of Medicine (IOM) and the Endocrine Society (ES), take into account different desirable levels of circulation of 25(OH)D3. The ES sets the threshold at 30ng/mL, while the IM sets it at 20ng/mL, a discrepancy partially justified by the different target population considered. In our study, we used the Endocrine Society's cutoff values for vitamin D deficiency and insufficiency. However, both measures seem unrepresentative for nonwhite ethnicities.25,26 Our country is characterized by a mixture of races, especially of European, indigenous (Guaraní) and Asian origin, and at the time of writing this study, we do not have evidence of studies in our population that determine the “more appropriate levels” of vitamin D.
Almost half of the participants had suboptimal levels of VD, with a prevalence of insufficiency and deficiency of 34% and 13% respectively. These values are markedly lower than those found in other studies, with suboptimal levels of 25(OH)D in SLE of 67% in the United States,27 75–85% in Spain,10,28 73.3% in Egyptian patients,29 and 96% in China.30 In Latin America, we found suboptimal levels of VD in 72.6% of SLE patients in Colombia,31 45.9–95% in Brazil32,33 and 89% in Mexico.34
These observations could be related to a high number of patients with low disease activity or remission in our cohort. Only about half of the patients were on immunosupressants and even though it was not registered among the variables, glucocorticoid use was probably low as well. Higher disease activity makes it more difficult for patients to sustain a job and increases health care costs. Given that the economic burden for SLE patients without health coverage is high, lower disease activity provides an opportunity for better adherence to adjuvant medication such as calcium and vitamin D supplements, use of sunscreen and healthier lifestyles with regular exercise and weight control. It should also be noted that 89% of patients were receiving 400IU of VD with their daily 500mg calcium and 89% were on hydroxychloroquine at baseline. Both were previously found to predict higher levels of 25(OH)D.
A study conducted in healthy, young university students in our country found a prevalence of suboptimal levels of 25(OH)D of 75.5%. Vitamin D deficiency was significantly associated with female sex, BMI≥25, lack of sun exposure and sedentary lifestyle.35 Possible explanations for the contradictory lower prevalence of VD deficiency in SLE Paraguayan patients could be attributed to differences in socioeconomical status and lifestyle. Our SLE patients, from a public hospital, are largely employed outdoors in manual labor and commute to work on foot or public transportation. As a consequence, they are more exposed to sunlight, as opposed to university students who spend most of their time indoors. Moreover, adherence to sunscreen is limited by high costs and cultural beliefs.
Several studies have shown that there is a connection between SLE and hypovitaminosis D, that most patients with SLE have insufficient levels of VD and that there is a connection between the activity of SLE and the status of VD. For example, lower levels of 25(OH)D were found in 123 cases with newly diagnosed SLE compared to 240 healthy controls in a statistically significant way.36 In a multicenter study conducted in France, they described deficiency (25(OH)D<10ng/mL) in 15.9% and insufficiency (25(OH)D between 10 and 30ng/mL) in 65.9% of 170 patients with SLE.37 Similarly, we found approximately half of the patients had a vitamin D value below 30ng/mL, and a decrease of 0.54 SLEDAI activity points is observed for each increase of 10ng/mL in VD concentration.
Attar et al. found in a group of 95 SLE patients in Saudi Arabia, significantly lower levels of 25(OH)D in patients with active disease (n=41; 43%) than in those with inactive disease (n=54; 57%); p=0.04).38 In Latin America (LA), the Mexican group headed by García-Carrasco et al.34 described a group of 105 patients with SLE where 86% had insufficiency and 4.6% deficiency of 25(OH)D. In Brazil, de Souza et al.39 found 55% of VD insufficiency in patients with SLE and nephritis. Other studies also demonstrated that VD deficiency increases the risk of SLE activity, especially nephritis.38,40–42 In this study, we also found a relationship between hypovitaminosis D and SLE activity.
Another interesting finding comes from evaluating the status of vitamin D in patients with SLE without prior treatment: hypovitaminosis D is prevalent in patients with SLE who have not yet started therapy, compared to healthy controls (38.6% versus 4.8%). Hypovitaminosis D is associated with a higher ANA titer and with elevated serum levels of pro-inflammatory cytokines IL-17 and IL-23.43
Vitamin D supplementation is indicated for both prevention and treatment of osteoporosis in patients with SLE. Edens and Robinson44 recommended a daily oral dose of cholecalciferol between 800 and 2000IU to maintain serum levels above the objective: 30ng/mL. In a randomized, double-blind, placebocontrolled, 24 week trial, 40 patients with juvenile onset SLE were randomized (1:1) to receive oral cholecalciferol 50,000IU/week (SLE-VD) or placebo (SLE-PL). After 24 weeks, the mean level of 25(OH)VD was higher in the SLE-VD group than in the SLE-PL group (p<0.001), with significant improvement in SLEDAI (p=0.010) and in ECLAM (p=0.006).33 Our study showed good results with a supplementation daily dose of 2000IU with respect to a lower dose.
Dall’Ara et al.42 recommends that, since cases of hypercalcemia and hyperphosphatemia have been reported only with very high doses of vitamin D (more than 50,000IU per day), patients with SLE can receive more generous doses compared with current practice, aiming to take advantage of the immunomodulatory properties of VD. It would be interesting to dose serum 25(OH)VD to adjust our supplementation accordingly. Nevertheless, the availability of serum 25(OH)VD in Latin America is limited, due to numerous factors, mainly economic ones. According to response in a survey conducted among pediatric rheumatologists,45 only 83% answered they had the test available to order.
The variation in PTH levels is explained by vitamin D status, with inverse relationship between them. 25-hydroxyvitamin D levels should be evaluated simultaneously when defining the reference PTH values. However, the standardization of PTH assays is lacking, so comparison studies of PTH immunoassay methods from different kits show differences between them.46,47 In our work, this may be the reason why the values between vitamin D and PTH are not properly related to each other as expected.
The fundamental limitation of our study is the small number of patients. However, it provides relevant data from our country and will enable future studies that include a larger number of patients, with a randomized, double-blind, placebo-controlled design to learn the optimal dose for vitamin D supplementation in patients with SLE.
ConclusionSerum VD levels equal to or below 30ng/mL are associated with higher disease activity measured by SELENA-SLEDAI. In our cohort, SLE patients required a supplementation dose equal to or greater than 2000IU/day to increase their serum VD.
FundingThis project 14-INV-466 was co-funded by the Consejo Nacional de Ciencia y Tecnología (CONACYT) with support from FEEI.
Conflicts of interestThe authors declare no potential conflict of interest regarding research, authorship and/or publication of this article.