HTLV-1 is a retrovirus that has an impact on human health due to its T-lymphocyte tropism. It occurs worldwide, but is more prevalent in tropical regions. Although most infected individuals will remain asymptomatic, the infection may manifest with complications such as uveitis, myelopathy, and leukemia, among others. The diagnosis is achieved by the detection of anti-HTLV antibodies and a confirmatory test (Western Blot or proviral load). Although there is no specific treatment, medical treatments are aimed towards the management of secondary diseases. Three cases are described of pediatric patients diagnosed with HTLV-1 infection and associated autoimmune manifestations.
El HTLV-1 es un retrovirus que causa impacto en la salud del ser humano, debido al tropismo para infectar a linfocitos T. Está distribuido mundialmente, pero es más prevalente en regiones tropicales. La mayoría de las personas afectadas permanecen asintomáticas, sin embargo, al manifestarse puede causar complicaciones como uveítis, mielopatía, leucemia, entre otras. Su diagnóstico se hace mediante la determinación de anticuerpos anti-HTLV y prueba confirmatoria (Western Blot o carga proviral). No tiene tratamiento específico, las medidas están dirigidas a la prevención y el manejo de las patologías secundarias. Se describen tres pacientes en edad pediátrica con diagnóstico de infección por HTLV-1 y manifestaciones autoinmunes.
Human T-cell lymphotropic virus type I (HTLV-1), is a retrovirus that belongs to the Retroviridae family and to the Oncoviridae subfamily, which has the particularity of infecting human beings.1 One of the first cases described occurred in 1980. In that occasion, the case of a man with cutaneous T-cell lymphoma, associated with the presence of HTLV-1 particles, was documented in the Oncology Unit of the Veterans Health Administration of the National Cancer Institute of the United States.2
There are also currently three other types of human T-cell lymphotropic viruses, known as HTLV types II, III and IV, however, there is no clear evidence indicating a relationship of pathogenicity in humans.3 This retrovirus is known to be associated with the development of malignant lymphoproliferative neoplasms such as leukemia or adult T-cell lymphoma (ATLL), as well as with the development of HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP). HTVL-1 is directly associated with dermatological infectious problems, uveitis and other inflammatory-type disorders.4 However, not all people infected with the virus develop some type of pathological manifestation. In fact, 90% of these carriers remain asymptomatic throughout their lives.1
It is estimated that HTLV-1 is present in approximately five to ten million people around the world. There are currently many endemic areas, including: southwestern Japan, sub-Saharan Africa, the Caribbean, the Middle East, Australomelanesia, and a large part of South America. Epidemiologically, this virus has a local endemic behavior, that is, it has been accentuated in small populations and remains there with a high transmission rate. Despite the large number of infected people around the world, the true prevalence of HTLV-1 remains poorly understood, due to factors such as isolated areas in which the infection has not been identified, serological tests with low specificity, those who are identified are not part of the general population and the HTLV-1 endemic areas are not distributed homogeneously.5
By not having a real prevalence of the virus, it is not possible to know the scope of the consequences, despite the fact that it is described that the majority of carriers remain asymptomatic. The prevalence varies according to the age, gender and socioeconomic level of the population, in most of the areas considered endemic. Very few nucleotide substitutions have been observed in such areas, which makes the virus genome very stable (unlike the genome of the human immunodeficiency virus, or HIV). Therefore, four subtypes have been described geographically: the Cosmopolitan subtype A, the Central African subtype B, the Central African subtype D/pygmies and the Australomelanesian subtype C. Of all of them, the one that has geographically represented the greatest severe commitment is the Cosmopolitan subtype A.5
In Europe, the country in which more research has been done on the subject is the United Kingdom, reporting that at least 80% of people infected with HTLV-1 are children or descendants of immigrants from an endemic area. This is consistent with data from Spain and France, but in this case the infected population is related to the high percentage of Latin Americans who immigrate to these two countries annually. On the other hand, in South America, Brazil and Peru are the countries that currently have the largest endemic areas.5 In Peru, in a study published in 2010, it was reported that the highest percentage of people seropositive for HTLV-1 corresponded to sex workers, a group followed by pregnant women. Of the entire population studied, one third had a close relative with seropositive screening.1
In Colombia, cases are described in the Pacific area, specifically in the region of Tumaco in the department of Nariño, an area well known for the high prevalence of HAM/TSP. The overall prevalence rate of HTLV-1 was 2.8%.5 On the other hand, in a study published in the city of Cali they found that between the years 2008 and 2014, of 77,117 records of donors in the Fundación Valle del Lili, 0.24% presented seroprevalence, and of this cumulate, 39% were men, while 61% corresponded to people of the female gender. In Bogota, 0.07% of positive results were recorded, which could be explained because eight blood banks were included.6
PathogenesisThe genomic organization of the HTLV is similar to that of other retroviruses. However, stands out the presence of a single region called pX, which codes for a number of regulatory proteins involved in viral pathogenesis, due to activating and suppressor actions, respectively, which results in the transient expression of viral gene products and the consequent evasion of the host immune response.7
It is noteworthy that HTLV-1 infects CD4 T lymphocytes and HTLV-2 infects CD8 T lymphocytes. However, the main entry of the virus into the cell occurs by direct cell-to-cell contact. In addition, it is remarkable that the margin of infection of these viruses extends to other types of cells: dendritic cells, monocytes, macrophages, fibroblasts and even B lymphocytes.8
In HTLV-1, important characteristics stand out with respect to replicative mechanisms and pathogenesis, therefore its impact on the development of diseases in humans, such as: low viral replication rate (which translates into a relatively low viral load and a conservation of genetic stability, thus evading the immune response), induction of proliferation and transformation of T cells without leading them to death.9
Antibodies play a role in the pathogenesis of the disease, taking into account that high levels may be linked with greater susceptibility to diseases associated with HTLV-1 infection, such as HAM/TSP. In addition, the count of HTLV-specific cytotoxic T lymphocytes is also related to a high frequency of diseases linked with this virus, even in asymptomatic carriers, which allows a more reliable evaluation of the viral control of the disease.8
TransmissionThe routes of transmission of HTLV-1 include:
- •
Sexual transmission: is a frequent route of transmission, but the exact percentage of transmission has not yet been estimated. However, in a summary of studies about the frequency of HTLV-1 in the population of sex workers in Peru, a percentage of seropositivity of 21.8% was estimated in the Departments of Callao and Loreto,1
- •
Breastfeeding: there are HTLV-1 antigens in breast milk whose transmission is due to the presence of T lymphocytes. These antigens have an estimated concentration between 16% and 30% that increases directly proportionally to the duration of the lactation.3
- •
Blood transfusions: through infected cells, but not by plasma, with a possibility of seroconversion of 40%–60%. However, the seroprevalence of HTLV-1 in blood donors is much lower than in the general population.4
In the pediatric population, the systematic review of HTLV-1 makes it possible to describe cases in which is reported the development of diseases that occur in adults, such as infectious dermatitis, the juvenile form of HAM/TSP, and even leukemia or T-cell lymphoma. The difficulty arises because the description of the development of the pathogenesis is in the long-term, that is, over the years, in addition to the fact that pediatric patients also remain asymptomatic.10
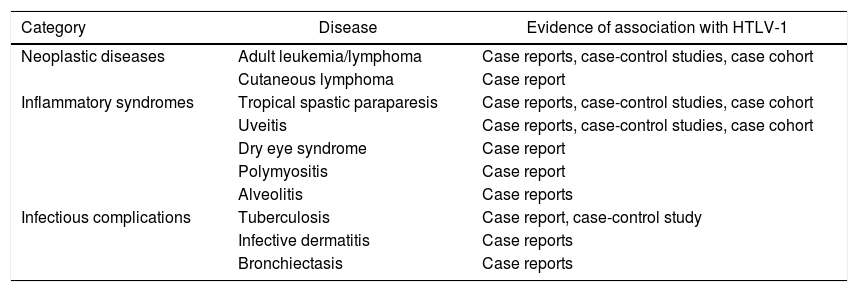
In an article published in Lima, Peru, in the year 2010,1 it was described a classification of the clinical spectrum of the infection with the virus into three important groups that are represented in Table 1.
Diseases associated with HTLV-1.
| Category | Disease | Evidence of association with HTLV-1 |
|---|---|---|
| Neoplastic diseases | Adult leukemia/lymphoma | Case reports, case-control studies, case cohort |
| Cutaneous lymphoma | Case report | |
| Inflammatory syndromes | Tropical spastic paraparesis | Case reports, case-control studies, case cohort |
| Uveitis | Case reports, case-control studies, case cohort | |
| Dry eye syndrome | Case report | |
| Polymyositis | Case report | |
| Alveolitis | Case reports | |
| Infectious complications | Tuberculosis | Case report, case-control study |
| Infective dermatitis | Case reports | |
| Bronchiectasis | Case reports |
Table adapted from Gotuzzo Herencia et al.1.
The diagnosis is established with the detection of anti-HTLV 1 and 2 antibodies in plasma, using screening techniques such as Elisa, gelatin particle agglutination, and chemiluminescence. Reactive tests must be confirmed with an additional specific technique such as Western Blot (WB), while in indeterminate cases or in HTLV not typified by WB, a nested polymerase chain reaction (n-PCR) should be performed to confirm the infection. Recently, it has been implemented another type of diagnostic tool, such as the quantification of the proviral load (CPV) of HTLV 1–2 from cells of infected patients, using the real-time PCR (RT-PCR) technique, in order to establish an indication about the course of the disease and assess the tendency to develop pathologies associated with the infection by this virus, mainly with HTLV-1. However, this diagnostic tool has its predominant effectiveness in the biological monitoring of the efficacy of chemotherapeutic or antiretroviral treatments.11
Description of case 1A six-year-old Afro-descendant male patient from Tumaco, Nariño, who attended the pediatric gastroenterology outpatient service with a prolonged clinical picture of diarrheal stools Bristol 6–7, with mucus, without blood, associated with intermittent fever and anal lesions with purulent discharge. About the dietary history, he received exclusive breastfeeding until the first year of life. Regarding his family history, a paternal aunt suffers from systemic lupus erythematosus. Likewise, the patient has a history of multiple hospitalizations for chronic diarrhea, with a report of elevated calprotectin (1948 μg/g; normal value <50 μg/g), hypoalbuminemia, vitamin D deficiency, rectosigmoidoscopy with ulcerative and erosive lesions in the mucosa and a colon biopsy that reported acute focal erosive colitis. Therefore, he was diagnosed with inflammatory bowel disease suggestive of Crohn's disease. Due to the relief of the symptoms, he was discharged with sulfasalazine; however, gastrointestinal symptoms persisted.
In the initial physical examination, the following pathological data were found: a state of chronic global malnutrition, a fistula with serous secretion in the anal region and marked xeroderma in the skin. Given the suspicion of bowel inflammatory disease, it was requested an automated detection by nested multiplex polymerase chain reaction using a FilmArray® Gastrointestinal panel (Biomérieux, FilmArray™ 2.0, Marcy-L’étoile, France) system, a method of amplification, detection and analysis of nucleic acids in a closed system, by which enterotoxigenic E. coli was identified. In addition, the patient presented a coproscopic test positive for E. histolytica, for which he was managed with ceftriaxone and metronidazole.
By gastroenterology, treatment with the biological infliximab was indicated. Subsequently, an upper digestive tract endoscopy was performed, which reported superficial chronic gastritis of the body and the fundus, as well as a colonoscopy, which reported severe ileopancolitis.
Among the studies performed in the intentional search for immunodeficiencies, positive HTLV-1 antibodies were reported. The confirmatory test was carried out by means of WB, which was also positive. The patient was evaluated by ophthalmology, and uveitis was ruled out.
Description of case 2A 12-year-old female patient, Afro-descendant, from Cali, Colombia, who was admitted due to a clinical picture of nine days of evolution consisting of fever, intense otalgia, rhinorrhea, conjunctival injection and general malaise. The physical examination on admission did not show infectious clinical signs that could explain the fever, and for this reason it was decided to hospitalize her.
Hematological, immunological and imaging studies were indicated, which identified: blood count with normal report; elevated C-reactive protein (31.49 mg/L; reference values: 0.00 mg/L–5.00 mg/L); negative procalcitonin; non-infectious urinalysis; negative influenza A and B antigen tests; negative tests for dengue, leptospira, toxoplasma, and herpes simplex virus type I and II; AP chest X-ray without alterations and tomography of the paranasal sinuses without significant pathological findings. It is noteworthy that the patient was later admitted in an afebrile period, but manifesting a sudden decrease in visual acuity to the point of presenting amaurosis.
The area of pediatric neurology assessed the patient and found an alteration in the neurological exam due to bilateral loss of vision, predominantly central and of color. For this reason, a lumbar puncture was performed, identifying in the cerebrospinal fluid (CSF) cytochemical test pleocytosis at the expense of the lymphocytes, proteinorrhachia, hypoglycorrhachia and a Gram staining in which no germs were observed. A FilmArray® Meningitis/Encephalitis panel test was performed, in which no microorganisms were detected.
Due to the lack of clinical signs that explain the decrease in visual acuity, the patient was assessed by pediatric ophthalmology and retinology, finding signs compatible with bilateral posterior uveitis, and therefore, management with atropine, ophthalmic prednisolone and immunomodulation therapy with cyclophosphamide was started. Within the extension paraclinical tests, antibodies against HTLV types 1 and 2 were taken, which yielded positive results, with a confirmatory test through positive WB for HTLV-1, and consequently, an infection with this virus was diagnosed.
Description of case 3A 15-month-old male patient from Chachajo, San Juan de Buenaventura, Department of Chocó, Colombia, belonging to the Wounaan indigenous community, was referred for admission with a previous clinical history of hospitalization for three months in another institution due to persistent thrombocytopenia without improvement. The patient was managed with platelet transfusion and intravenous immunoglobulin G (only one dose prior to arrival at our institution) and suspicion of infection with HTLV-1 and 2 viruses due to the report of positive antibodies for this microorganisms. In addition, he had a history of repetitive respiratory infections.
On physical examination, a chronically ill appearance was observed, with global malnutrition and neurodevelopmental delay. As additional pathological findings, clubbing fingers were observed, while hepatosplenomegaly was found on abdominal palpation, which was confirmed by abdominal ultrasound.
The patient was assessed by the pediatric hematology area, which considered that he was suffering from thrombocytopenia secondary to hepatosplenomegaly and HTLV-1 virus infection, so it was decided to perform a bone marrow aspiration and biopsy and start intravenous immunoglobulin G. A flow cytometry report negative for leukemia was obtained, as well as a normocellular myelogram without leukemic infiltration, with marked thrombocytopenia and a bone marrow biopsy negative for neoplastic infiltration. However, the patient presented a partial response to the management prescribed with intravenous immunoglobulin G.
Due to the pulmonary involvement, a fibrobronchoscopy and obtention of bronchoalveolar lavage was performed, finding signs of HTLV-1 and 2-associated pneumopathy. Subsequently a confirmatory Western Blot test report for positive HTLV-1 was obtained. Due to the persistence of severe thrombocytopenia, it was decided, in conjunction with the departments of infectious diseases, hematology and pediatric rheumatology, to start pulses of methylprednisolone (15 mg/kg/day for two days), with subsequent change to prednisolone (2 mg/kg/day).
DiscussionHTLV-1 infection is common in Latin America.5 Its clinical manifestations and associated diseases have been mostly reported in adult populations. Given the natural history of the disease, in those patients who develop symptoms, they usually appear decades after the infection. In this case report we contribute to the knowledge of the autoimmune manifestations associated with this disease in three pediatric patients.
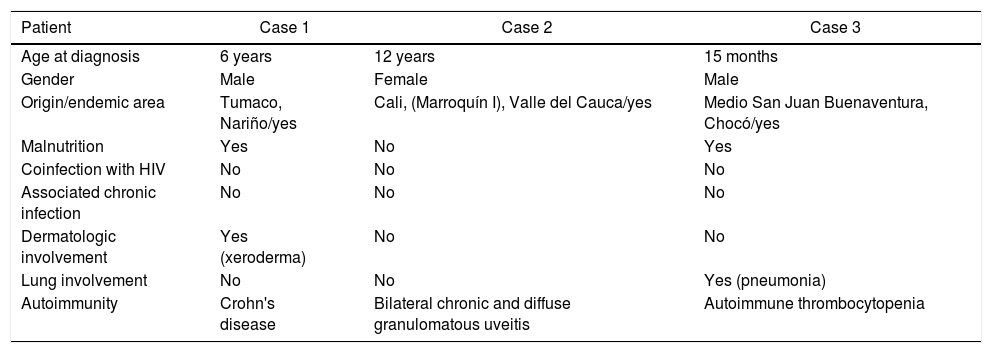
There are risk factors that increase susceptibility to HTLV-1 infection during pregnancy or breastfeeding, which can be related to the patients evaluated. These factors include the place of birth, race, low educational and socioeconomic level, family history of leukemia or lymphoma, antecedents of transfusions both in the mother and in the newborn, among others.12 The pertinent data of the three patients presented are shown in Table 2.
Description of clinical cases.
| Patient | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age at diagnosis | 6 years | 12 years | 15 months |
| Gender | Male | Female | Male |
| Origin/endemic area | Tumaco, Nariño/yes | Cali, (Marroquín I), Valle del Cauca/yes | Medio San Juan Buenaventura, Chocó/yes |
| Malnutrition | Yes | No | Yes |
| Coinfection with HIV | No | No | No |
| Associated chronic infection | No | No | No |
| Dermatologic involvement | Yes (xeroderma) | No | No |
| Lung involvement | No | No | Yes (pneumonia) |
| Autoimmunity | Crohn's disease | Bilateral chronic and diffuse granulomatous uveitis | Autoimmune thrombocytopenia |
Own elaboration.
The three patients come from the Colombian Pacific zone, identified as an endemic area for HTLV-1, two Afro-descendants and one indigenous patient.
The three patients had parents with a low level of education and socioeconomic status. Case 1 comes from Tumaco, Nariño, where studies estimate a prevalence of 3% by serological tests.13 The case 2 comes from Cali and the third patient belongs to the indigenous ethnic group in the department of Choco, where a reactivity rate twenty times higher than the national average is reported.14 It has been reported an increased prevalence in Afro-descendant and Indigenous populations, associated with low economic levels throughout Latin America.15 In the case of the reported patients, some of their demographic characteristics are described.
According to the information compiled, cases 1 and 3 were breastfeed for more than six months, and even received exclusive breastfeeding for at least twelve months, which increases the risk of infection. The infection in these three patients is considered to be most likely associated with transmission through breastfeeding.
The most effective measure that has been established in developed countries, such as Japan, to prevent vertical transmission from mother to child through breastfeeding is to suspend its administration and start feeding with formula. However, the sociocultural, epidemiological and economic settings of Colombia and other developing countries make this measure potentially counterproductive.16
Two of the patients presented nutritional alterations, with weight and height below the 5th percentile for age, in a state of malnutrition (cases 1 and 3). Montano et al., in a study about early neurodevelopment in children with HTLV-1, reported an index of chronic malnutrition of 30% in seropositive patients, compared to 8% in the seronegative group.17 Studies on the nutritional status of children with HTLV-1 are limited. In adults, it has been found an increased prevalence of nutritional disorders, both malnutrition and obesity, as well as uncertainty in terms of access to food in adult patients with HTLV-1.18
It should be kept in mind that the patients of cases 1 and 3, both with nutritional problems, live in rural areas, far from urban zones, with limited economic, human, medical, and educational resources, which aggravates their condition.
In the cases reported in this study, it was found that the patients presented diverse involvements. In case 1, the patient presented dermatological manifestations of marked xeroderma. The patient of case 3 presented crusted lesions and hypo- and hyperpigmented macules on the trunk and extremities associated with pruritus. The patient of case 2 did not present dermatological lesions. Schierhout et al. described the relationship between HTLV-1 infection and associated diseases, they found a high relative risk for inflammatory diseases such as seborrheic dermatitis in children and adults, eczema in children, Sjögren's syndrome, bronchiectasis, bronchitis or bronchiolitis, asthma, fibromyalgia, rheumatoid arthritis; infectious conditions such as tuberculosis, community-acquired pneumonia, Strongyloides stercoralis hyperinfection syndrome; cancerous conditions such as cervical cancer, liver cancer and lymphomas.16 It was found a protective factor against the development of gastric cancer.19
Maloney et al. identified hyperreflexia in children as an early manifestation.21 On the other hand, they reported similar hemoglobin average values in infected and uninfected patients with an increased relative risk of developing severe anemia in infected children.21 In the patients reported here, it was found that cases 1 and 3 had low volume anemia that required transfusion of red blood cells.
Cases of association between HTLV-1 infection and the development of autoimmune diseases such as myelopathy or tropical spastic paraparesis (immunological dysfunctions that include the spontaneous proliferation of CD4+ T lymphocytes infected with HTLV-1 and increased production of proinflammatory cytokines) and autoimmune diseases such as Sjögren's syndrome, arthropathies, and uveitis.22 This case report focuses on the autoimmune manifestations associated with HTLV infection and raises awareness of their variety, but its added value lies in the fact that the population in which they are reported is pediatric.
Several theories attempt to explain the process by which autoimmunity against infection by some pathogen develops. In general, viruses can induce autoimmunity by activating T cell autorreactivity through upregulation of Th-1-type cytokines or other selective molecules; the preferential destruction or infection of a subset of CD4+ T cells: the production of viral superantigens; de novo by autoepitopes released secondary to T cell-mediated damage, specifically by viruses, and cross-reactivity with viral epitopes.
In the case of the viruses, three main theories are proposed. First, molecular mimicry, in which similar structures that are shared by molecules from different genes, hosts, and viruses are found.23 In this way, it is possible to have segments of similar amino acid alignments, from each species, and they can be different proteins. Once an immune response against the shared determinant of both the host and the virus is created, it can be evoked a tissue-specific immune response that can lead to destruction of the cells and the underlying tissues.23,24 The two most likely mechanisms that lead to tissue damage are the creation of antibodies that recognize specific determinants on target cells or the cytotoxic cross-reactivity of effector lymphocytes.23
The second theory is the bystander activation, which occurs after the activation of immature, autorreactive T cells by an activated antigen-presenting cell, which can lead to autoimmunity.24
Finally, the third theory is a persistent viral infection that provokes a persistent immune response.24 It has also been recorded that the infection of CD4+ T lymphocytes has caused alterations in the transcription factors and signaling cascades that alter their cellular function, which ends in a deregulation that favors a Th1-mediated immune response (which has been associated with the development of rheumatoid arthritis), while deregulation that favors the expression of Th2 has been associated with the development of systemic lupus erythematosus.22 It is noteworthy that the changes previously described at the immunological level occurred in apparently asymptomatic patients.25
The reviews of HTLV in pediatric populations have focused primarily on the methods of transmission and the instauration of protocols that prevent the infection through breastfeeding. Autoimmune diseases secondary to HTLV-1 and 2 infections have been mostly reported in adult populations, which is possibly due to the fact that symptoms do not manifest until decades after the infection. Pediatric patients are usually asymptomatic. However, the most common affections are infective dermatitis, immune dysfunction with superimposed bacterial infections of the skin, chronic bronchiectasis, Strongyloides stercolaris infestation, hypergammaglobulinemia and T cell activation with elevated CD4 counts.
In studies and case reports, it has been found an association between HTLV infection and the development of neurological manifestations and cognitive dysfunctions.26,27 The most frequently reported autoimmune disorders in children are HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP), adult T-cell leukemia/lymphoma, and uveitis.10,28,29 Other reported autoimmune disorders include Sjögren's syndrome, rheumatoid arthritis, lupus erythematosus, autoimmune thyroiditis, and polymyositis, among others.13,30–32
Paradoxically, according to the literature review, some studies report that in patients with underlying autoimmune diseases such as lupus erythematosus, polymyositis and other connective tissue diseases, in which it was intended to determine the prevalence of HTLV infection, it was not possible to find an active infection by the retrovirus.33,34 In the study that included patients with connective tissue diseases, it was concluded that rheumatological diseases are associated with HTLV-1 geographically and not etiologically. Other studies have demonstrated that the polymerase chain reaction does not identify HTLV-1 as the causative agent in rheumatic diseases, although a minority of these patients have antibodies that have weak cross-reactions with retroviral antigens.35
It was found that each of the three patients reported in this study developed a different autoimmune manifestation, two of them with manifestations rarely reported, even less in this age group. The first patient presented chronic diarrheal clinical pictures during the last five years. Coinfection with Strongyloides stercolaris has been widely reported in the literature19; however, this was not identified in this patient, who was diagnosed with HTLV-1-associated Crohn’s disease.
Gastrointestinal manifestations associated with HTLV-1 infection, such as ulcerative colitis and Crohn's disease, have been described in the literature in endemic areas.36 However, most of these have been diagnosed as ulcerative colitis associated or not with Strongyloides stercolaris infection in adult populations. This would be the first case in the literature, to our knowledge, in which this association is reported in any population. The extent of the lesions is noteworthy, since it led to the recommendation of management with total colectomy, which describes a highly aggressive process. Cases of lymphomas and lymphosarcomas of the colon that mimic ulcerative colitis and Crohn's disease have been previously reported, all of them in adult patients.37,38 However, due to the aggressive clinical evolution seen in this patient, long-term follow-up should be considered.
The second patient presented uveitis. An association between HTLV-1 infection and this entity has been reported, more frequently in women. This condition manifests itself with blurred vision and affects bilaterally almost half of the people who suffer from it. Some of the ocular signs that may be present are iritis, vitreous opacities, retinal vasculitis, retinal hemorrhages and exudates. It has been seen a good therapeutic response to the use of topical or systemic corticosteroids.18 A case report by Kihara et al.29 describes the cases of five children who developed uveitis secondary to HTLV-1, with classic symptoms of uveitis in adults; however, all patients had unilateral uveitis. The patient in this report evolved until developing bilateral amaurosis, which agrees with most of the literature, she responded favorably to the administration of corticosteroids, but with the described sequelae.
The third patient had idiopathic thrombocytopenic purpura (ITP). In the study conducted by Matsushita et al. it was found a prevalence of HTVL-1 in patients with ITP higher than in healthy patients, in addition, the patients with ITP and HTLV infection had a poor response to the therapy with prednisolone. Although some patients obtained a transient increase in the platelet count, very few achieved complete or partial responses. The authors concluded that splenectomy should be considered in these individuals if there is no response to conventional therapy.20 This was evidenced in patient 3, who had a history of ITP with poor response to steroid therapy, and for this reason, splenectomy was considered.
In this way, it becomes evident that pediatric patients can present different symptoms associated with HTLV-1, which requires meticulous medical care. From the epidemiological point of view, it is possible to establish that the most severe manifestations of HTLV 1 or 2 infections tend to be expressed decades after infection. However, although the literature is scarce, we must be aware that pediatric patients who are positive for HTLV 1 and 2 can present a wide variety of clinical manifestations, with associated coinfections in many cases. Regarding this report, the apparent easiness with which a secondary autoimmune response is formed must be taken into account, regardless of which theory can explain it, because the consequences in the short, medium and long term are serious, going from continuous and prolonged hospitalizations to highly invasive surgeries or possible physical disabilities. Given the young age of the patients, the aggressiveness of the clinical manifestations and the associated long-term complications, strict medical follow-up is required.
Since the study population is underage, without antecedents of transfusions, transplants, use of intravenous drugs, or having initiated social behavior, it is possible to infer that the route of contagion in these patients corresponds, with high probability, to breastfeeding, in relation with the data provided in the clinical history and mentioned above, in addition to the literature review conducted.
ConclusionsThe academic literature on autoimmune manifestations in pediatric patients is scarce.
The most frequently reported autoimmune diseases secondary to HTLV-1 in children are HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP), adult T-cell leukemia/lymphoma, and uveitis. However, manifestations such as thrombocytopenia, idiopathic purpura or Crohn's disease can occur.
To our knowledge, this is the first case report in the literature, in which a pediatric patient with a diagnosis of Crohn’s disease associated with HTLV-1 is presented.
Rheumatic diseases are associated with HTLV-1 infection in endemic areas.
It is important to be particularly careful with gastrointestinal manifestations such as ulcerative colitis or Crohn's disease due to the risk of imitating lymphomas or lymphosarcomas.
The patient who presented uveitis secondary to HTLV-1 infection responded favorably to the administration of corticosteroids. However, this is not yet a clear indication for treatment; each case must be individualized.
A strict clinical follow-up should be done in HTLV-1 positive patients, especially if they develop neurological or autoimmune manifestations.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Restrepo Figueroa LI, Basto Escobar XA, García Muñoz CA, Malagón Caicedo JP, Jojoa Rios JD, Rojas Hernández JP. Manifestaciones autoinmunes en pacientes pediátricos con infección por virus linfotrópico humano de células T tipo I (HTLV-1). Rev Colomb Reumatol. 2022;29:137–144.