Cryptococcus neoformans is the leading causal agent of invasive fungal infections in patients with systemic lupus erythematosus, frequently compromising the central nervous system and the lung. The infection develops during the first two years after diagnosis in patients with active disease, and the main risk factors are glucocorticoids, especially the cumulative dose, and lymphopenia. Mortality is high, exceeding 50%. We present the case of a man with active systemic lupus erythematosus who was admitted due to fever, arthritis, tenosynovitis, and purpura in whom disseminated C. neoformans infection was documented by initial isolation in blood and synovial fluid. Subsequently, he developed central nervous system symptoms like headache and nuchal rigidity that responded to induction treatment with amphotericin and flucytosine, and the manifestations resolved. Although joint and periarticular involvement by C. neoformans is infrequent, these are foci to consider in the approach to patients with lupus and suspected invasive fungal infection.
El Cryptococcus neoformans es el principal agente causal de infecciones fúngicas invasivas en pacientes con lupus eritematoso sistémico que con frecuencia comprometen el sistema nervioso central y el pulmón. La infección se desarrolla durante los 2 primeros años tras el diagnóstico en pacientes con enfermedad activa, y los principales factores de riesgo son los glucocorticoides, especialmente la dosis acumulada, y la linfopenia. La mortalidad es alta, superior al 50%. Presentamos el caso de un hombre con lupus eritematoso sistémico activo que ingresó por fiebre, artritis, tenosinovitis y púrpura, en quien se documentó infección diseminada por C. neoformans mediante aislamiento inicial en sangre y líquido sinovial. Posteriormente, desarrolló síntomas del sistema nervioso central como cefalea y rigidez de nuca que respondieron al tratamiento de inducción con anfotericina y flucitosina, y las manifestaciones se resolvieron completamente. Aunque la afectación articular y periarticular por C. neoformans es infrecuente, estos son focos para considerar en el abordaje de pacientes con lupus y sospecha de infección fúngica invasiva.
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with an alternating relapse-remission course. Although men represent a minority of cases (4%–22%), they develop a more serious disease, with a higher frequency of renal, serous and immunological involvement.1 The main risk factors associated with relapses of SLE are infections (OR 31.3) and discontinuation of maintenance therapy (OR 10.4).2 Infections are responsible for 20%–55% of mortality, mainly during the first year of the disease. While fungi are an unusual etiologic agent, fungal infections have serious prognostic implications.3 In Colombia, 7% of mycotic infections in patients with SLE correspond to Cryptococcus neoformans infections.4 The main tools for the diagnosis of cryptococcosis include direct visualization in cerebrospinal fluid with Indian ink, detection of capsular antigen in peripheral blood by latex agglutination, or isolation of the fungus in cultures of compromised tissues or blood cultures.5 We present the case of a man with a severe relapse of SLE and disseminated cryptococcosis with joint involvement.
Case presentationA 34-year-old male patient, with a history of exposure to birds and a diagnosis of SLE in July 2018 due to the presence of positive antinuclear antibodies, C3 and C4 hypocomplementemia, and the following clinical manifestations: Evans syndrome, Libman Sacks endocarditis, polyarthritis and nephrotic syndrome. The treatment of SLE consisted of prednisolone, chloroquine, induction therapy with cyclophosphamide and maintenance therapy with azathioprine, the latter replaced with mycophenolate when renal involvement was documented. Likewise, he had a history of right renal vein thrombosis with negative antiphospholipid antibodies, secondary syphilis, anogenital condylomatosis, and asymptomatic SARS-CoV-2 infection.
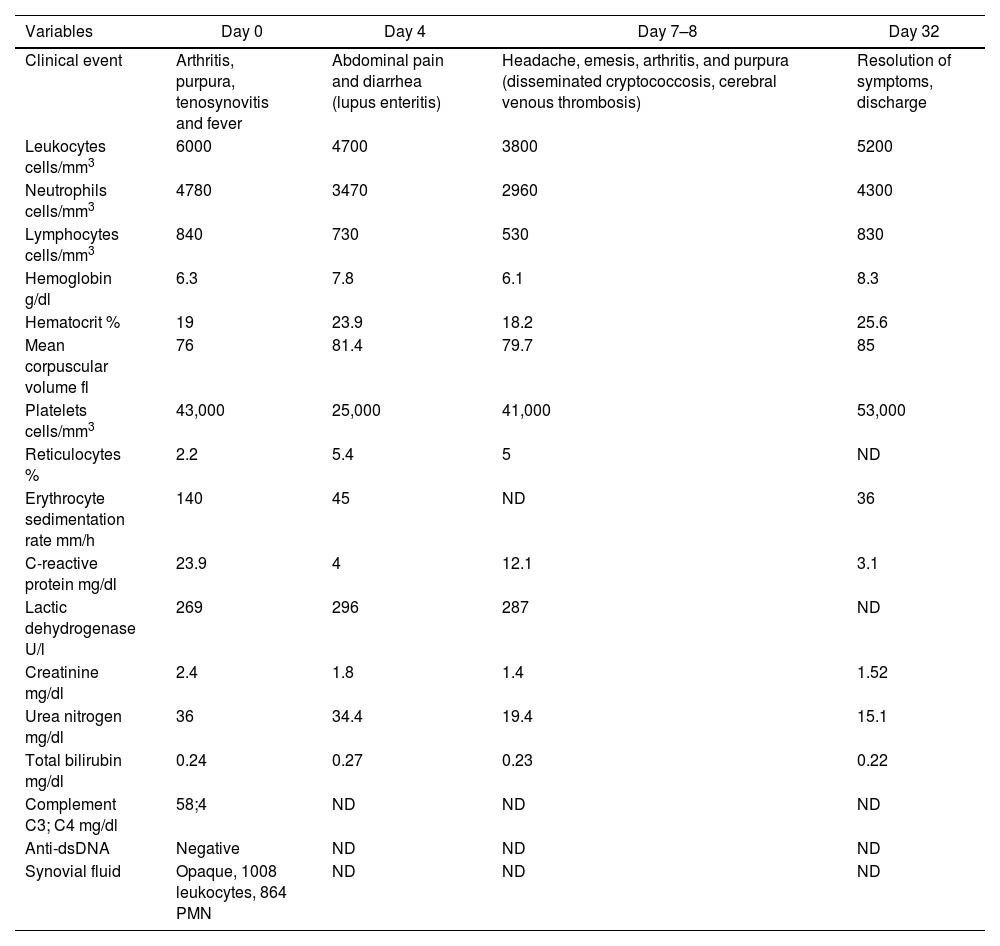
In July 2021, the patient consulted the emergency department for fever, chills, diaphoresis and polyarthritis of one week of evolution. At that time, he had no adherence to mycophenolate due to gastrointestinal intolerance. Extensor tenosynovitis of the right hand and of the dorsiflexors of the left foot, arthritis of the left knee, and palpable purpura in the lower limbs were found on physical examination. Laboratory tests revealed anemia, lymphopenia, thrombocytopenia, persistence of renal involvement (150 mg of proteins, 23 red blood cells and 24 leukocytes per high-power field in urinalysis), hypocomplementemia, elevation of acute phase reactants (Table 1) and negative enzyme-linked immunosorbent assay (ELISA) for the detection of human immunodeficiency virus (HIV). Initially, disseminated gonococcal infection was suspected due to a history of sexually transmitted diseases, arthritis, tenosynovitis, and purpura. Consequently, the dose of prednisolone was reduced to 20 mg/day and arthrocentesis of the left knee for microbiological studies of the synovial fluid and blood cultures were performed. In addition, empiric treatment was initiated with intravenous ceftriaxone 1 g/day and a single dose of azithromycin 1 g orally.
Laboratory tests results.
| Variables | Day 0 | Day 4 | Day 7–8 | Day 32 |
|---|---|---|---|---|
| Clinical event | Arthritis, purpura, tenosynovitis and fever | Abdominal pain and diarrhea (lupus enteritis) | Headache, emesis, arthritis, and purpura (disseminated cryptococcosis, cerebral venous thrombosis) | Resolution of symptoms, discharge |
| Leukocytes cells/mm3 | 6000 | 4700 | 3800 | 5200 |
| Neutrophils cells/mm3 | 4780 | 3470 | 2960 | 4300 |
| Lymphocytes cells/mm3 | 840 | 730 | 530 | 830 |
| Hemoglobin g/dl | 6.3 | 7.8 | 6.1 | 8.3 |
| Hematocrit % | 19 | 23.9 | 18.2 | 25.6 |
| Mean corpuscular volume fl | 76 | 81.4 | 79.7 | 85 |
| Platelets cells/mm3 | 43,000 | 25,000 | 41,000 | 53,000 |
| Reticulocytes % | 2.2 | 5.4 | 5 | ND |
| Erythrocyte sedimentation rate mm/h | 140 | 45 | ND | 36 |
| C-reactive protein mg/dl | 23.9 | 4 | 12.1 | 3.1 |
| Lactic dehydrogenase U/l | 269 | 296 | 287 | ND |
| Creatinine mg/dl | 2.4 | 1.8 | 1.4 | 1.52 |
| Urea nitrogen mg/dl | 36 | 34.4 | 19.4 | 15.1 |
| Total bilirubin mg/dl | 0.24 | 0.27 | 0.23 | 0.22 |
| Complement C3; C4 mg/dl | 58;4 | ND | ND | ND |
| Anti-dsDNA | Negative | ND | ND | ND |
| Synovial fluid | Opaque, 1008 leukocytes, 864 PMN | ND | ND | ND |
Anti-dsDNA: anti-double stranded DNA, PMN: polymorphonuclear; ND: no data.
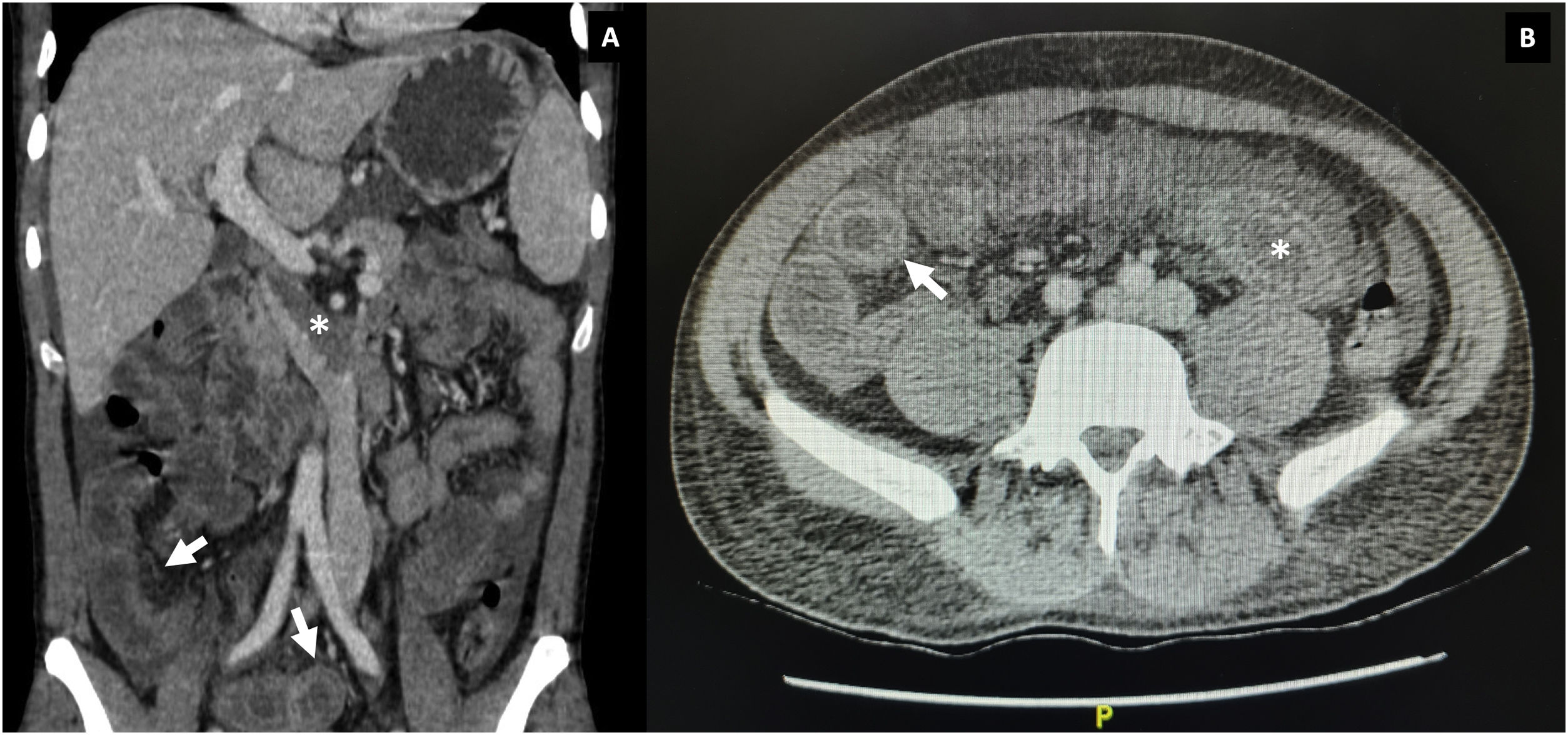
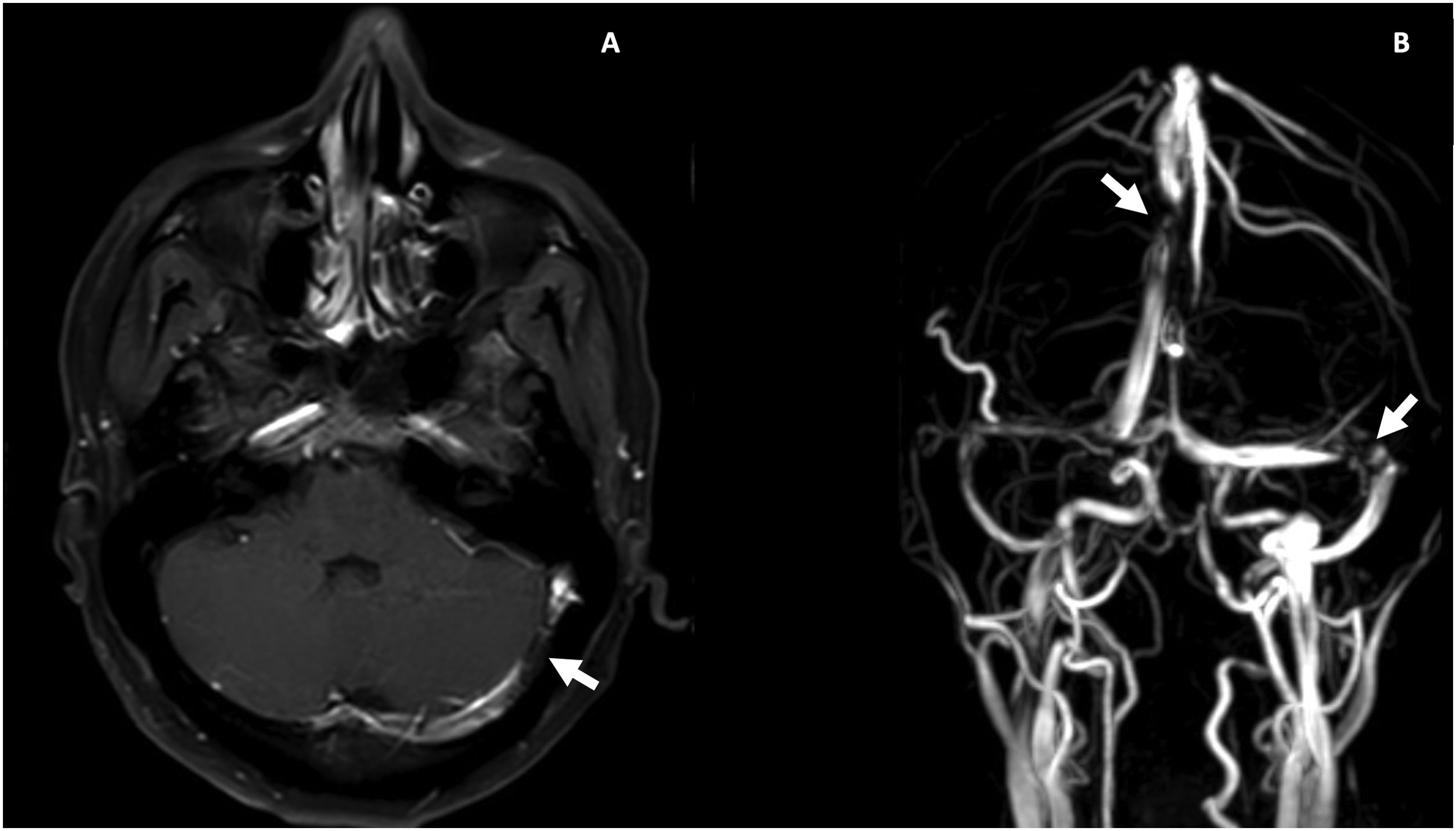
During hospitalization, lupus enteritis was diagnosed on day 4 due to the presence of abdominal pain, diarrhea and a contrast-enhanced CT scan with a target sign in the distal ileum (Fig. 1). In addition, due to an episode of thunderclap headache (day 7) a brain angioresonance was performed, which detected thrombosis at the confluence of the transverse, left sigmoid and superior sagittal venous sinuses (Fig. 2; Table 1). Due to these findings, immunoglobulin infusion and anticoagulation were started.
Contrast enhanced magnetic resonance imaging of the brain. A) Postgadolinium T1, partially recanalized venous thrombosis at the confluence of the left transverse and sigmoid sinuses (white arrow). B) Venous resonance, central flow defect at the junction of the left transverse and sigmoid sinuses and in the posterior aspect of the superior sagittal sinus (white arrows).
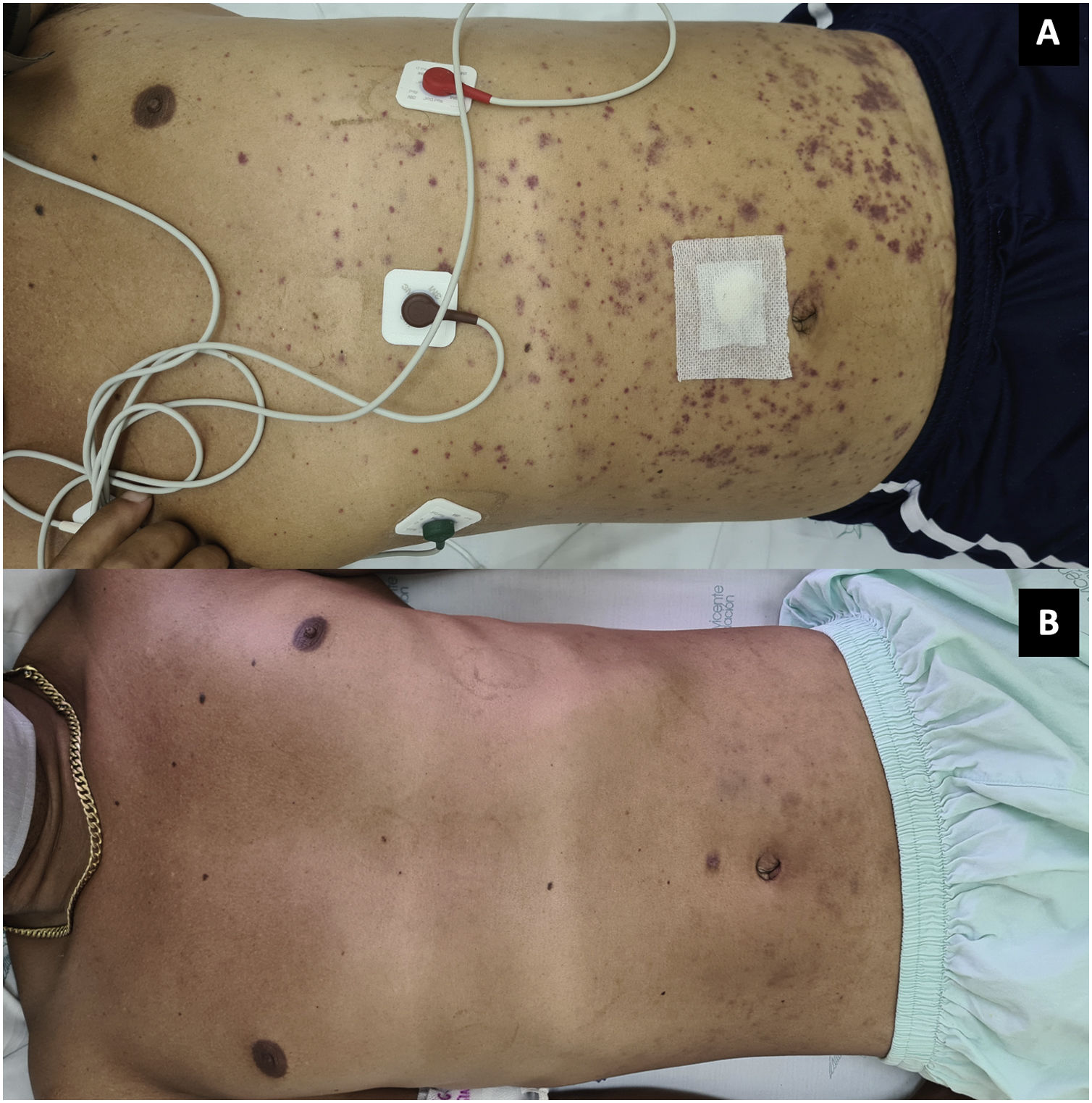
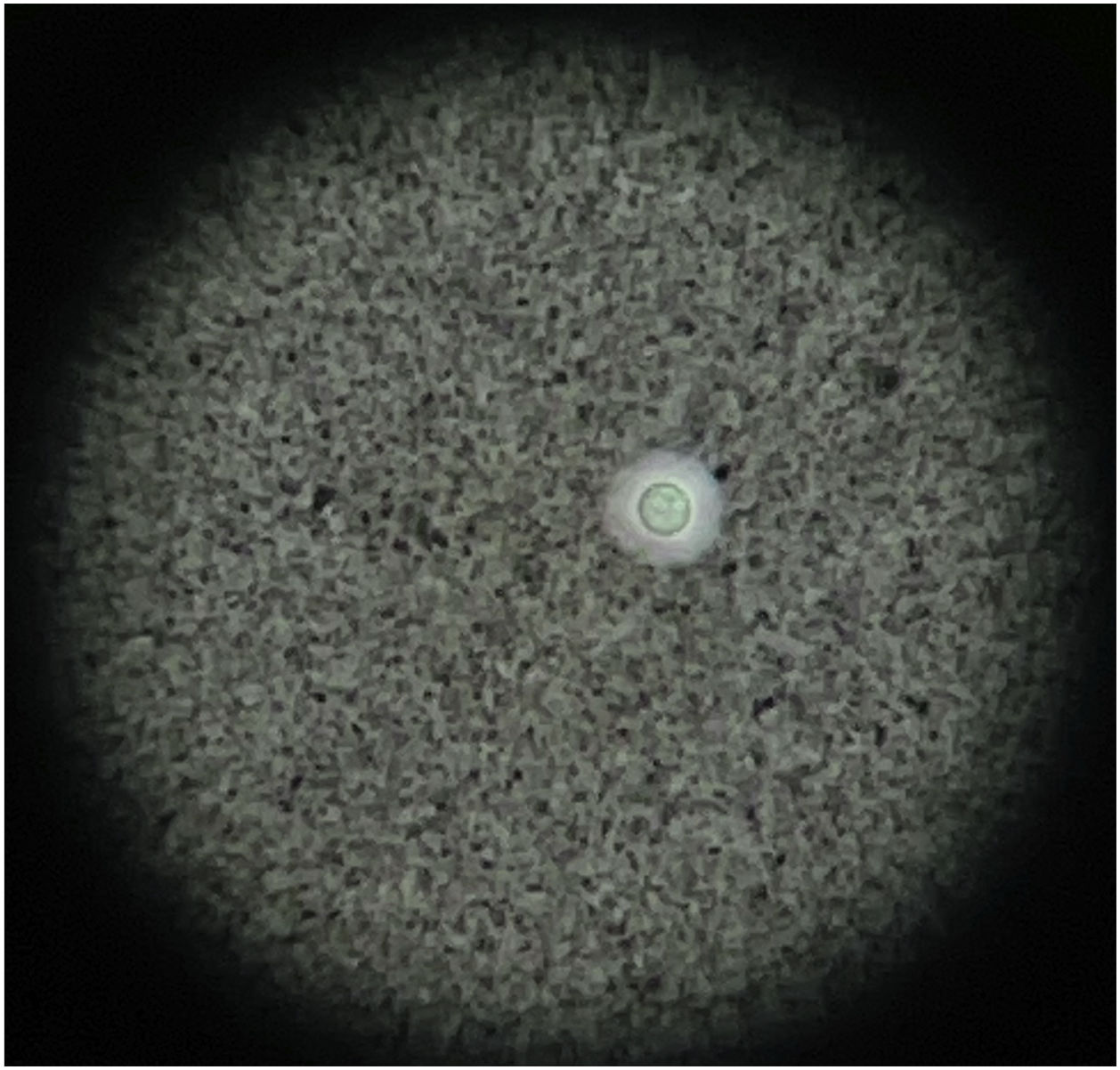
On the next day the symptoms worsened, with arthritis of the knees and extension of the palpable purpura to the trunk and extremities (Fig. 3; Table 1), despite having completed 7 days of antibiotic therapy, which is why the gonococcal infection was questioned. Finally, the synovial fluid culture and the blood cultures reported growth of C. neoformans. Taking into account the neurotropism of this agent, the persistence of the headache and the finding of nuchal rigidity on physical examination, the studies were extended. An opening pressure (OP) of 47 cmH2O was identified in the lumbar puncture, and proteins 60 mg/dl, glucose 31 mg/dl, leukocytes 65 cells/mm3 (90% polymorphonuclear), blastoconidia in KOH, encapsulated yeasts in Indian ink (Fig. 4) and fungal culture positive for C. neoformans were found in the cerebrospinal fluid (CSF). The foregoing confirmed the disseminated cryptococcal infection, for which liposomal amphotericin B 300 mg/day intravenously every 6 h plus flucytosine 2 g orally every 6 h was started as induction treatment. A skin biopsy was performed; however, methenamine silver, PAS and mucicarmine stains were negative. The response to antifungal treatment was rapid, achieving resolution of headache, purpura and arthritis. After 17 days of induction treatment, management was switched to consolidation therapy with fluconazole 800 mg orally every day, after confirming that the control fungal culture in the cerebrospinal fluid was negative and that the OP had been normalized. Given the improvement and the absence of adverse reactions to the treatment, the patient was discharged with prescription of fluconazole and outpatient follow-up by rheumatology and infectious diseases.
One of the great challenges in the management of patients with SLE is to differentiate the disease activity from the infection, which is even more complex when both situations occur in the same patient and therapeutic decisions must be made. The induction of lupus activity by infectious processes is secondary to several pathogenic mechanisms, including molecular similarity, dissemination of epitopes, production of superantigens, altered apoptosis and deficient clearance of debris. Likewise, there is an increased risk of infection in SLE due to decreased phagocytosis, lower production of cytokines (IL2, IL8, IL12), defective chemotaxis and complement deficiency.6 The initial approach should be focused on ruling out infection due to the serious implications of immunosuppression, for which acute-phase reactants may be useful. The elevation of CRP > 60 mg/l is a moderately specific parameter for infection (84%), as is the ESR/CRP ratio lower than 2.7 However, these findings may be insufficient, especially when there are manifestations of SLE that elevate acute-phase reactants, such as chronic synovitis and serositis.8
In SLE, bacterial infections are the most frequent, followed by viral infections, and fungal infections to a lesser extent.3,9 Invasive fungal infection (IFI) is an uncommon condition (0.6%–3%),10 usually underdiagnosed (30%), which influences fatal outcomes. A systematic review of 461 IFIs in 393 patients with SLE found that the main etiologic agent was Cryptococcus sp. (35%), followed by Aspergillus.10 However, in Colombia the epidemiology is different; in a study of 200 patients with SLE, IFI occurred in a higher percentage of patients (7.5%), being Candida sp. the main agent (60%) and C. neoformans the least isolated.4Cryptococcus sp. is an encapsulated fungus, acquired by inhalation of aerosols, that causes infection in immunocompromised individuals (with or without HIV infection) in whom hematogenous dissemination to organs other than the lung, especially to the central nervous system (CNS), is frequent.11 In SLE it is common for infection to occur within the 2 first years of evolution of the disease, mainly in those subjects with hematological and renal activity, and who are under treatment with glucocorticoids (89%). The sites of more isolation in this population are the CNS (73%) and the blood (26%), while the skin and the joints are poorly described foci.10
Articular cryptococcosis is unusual; there are currently 28 cases reported in the literature, of which 57% were men, 65% with monoarthritis, predominantly in the knee followed by the ankle, and 75% with isolation in additional sites (skin, CNS, bone or blood). The synovial fluid reported inflammatory or non-inflammatory characteristics (leukocytes 200–19,700 cells/mm3), with a predominance of monocytes. The main associated condition was solid organ transplantation, and only one patient had a diagnosis of SLE. In terms of immunosuppression, one-third received glucocorticoids and the most commonly used additional immunosuppressant agent was azathioprine. 25% required surgical interventions and healing was obtained in 71% of cases.12–17 Although exceptional, periarticular soft tissues are also a potential focus of infection with Cryptococcus sp. with tenosynovitis predominantly in the extensors of the hand.12
Primary infection of the skin is rare, it is usually a consequence of hematogenous dissemination and is observed in 15% of non-HIV patients with cryptococcosis, of whom 37% have rheumatological diseases, among which SLE stands out (30%).18 The characteristic lesions are papules ulcerated in the center, with an acneiform or molluscum contagiosum appearance, but they also appear in the form of ulcers, cellulitis, subcutaneous nodules and purpura.5,19 The diagnosis requires identification with special stains such as PAS, silver methenamine, mucicarmine, culture, or histological evidence.18 In the case presented, although the skin biopsy stains were negative, the absence of response to immunoglobulin and the complete resolution of the purpura with the initiation of the antifungal agent suggested that the skin lesions were secondary to cryptococcosis.
The risk of opportunistic infections in SLE comes from intrinsic factors such as disruption of cellular immunity, deficiency of mannose-binding lectin, deficit of complement receptors or of the FcγIII receptor.20 Furthermore, several extrinsic factors have been associated with IFIs in SLE, being 2 the most relevant. First, the use of glucocorticoids, due to their effects on cellular immunity, with greater risk in intermediate or high cumulative doses (OR 1.58; p = 0.01). The second factor is lymphopenia, a frequent finding in SLE (56%) due to disease activity or to glucocorticoids, with a 2.65-fold increased risk.9 Specifically, quantitative and qualitative defects in T lymphocytes are more closely related to this type of infection. Quin et al.21 identified that patients with lupus and opportunistic infection had CD4+ T lymphocyte counts lower than those without infection (173 vs. 485 cells/mm3). Likewise, in a study with murine models of SLE, it was demonstrated that T lymphocytes have an intrinsic defect that leads to a decrease in the production of interferon y, which affects the ability to control intracellular pathogens.22 In the IFI by Cryptococcus sp. the induction treatment scheme consists of amphotericin and flucytosine, followed by consolidation treatment with fluconazole, similar of that for patients with HIV infection.23 Finally, the mortality of patients with SLE and cryptococcosis is high (53%–86%),3,10 and among the poor prognostic factors, the use of glucocorticoids with equivalent doses of prednisolone ≥30 mg/d has demonstrated a 10-fold increased risk of death (OR 9.68).24
The main strength of this case report is to highlight the tropism of C. neoformans for various tissues as a finding to be taken into account in in the diagnostic approach of IFI by this agent in patients with SLE. The limitations include the absence of data on the outpatient follow-up and the non-detection of the etiological agent in the cutaneous lesions, despite resolution with the established treatment.
ConclusionOpportunistic infections in patients with SLE imply greater morbidity and mortality, despite they are infrequent. Cryptococcus is the main associated fungal agent and the main risk factors are SLE activity, lymphopenia and the cumulative dose of steroids. Articular and periarticular involvement are also possible foci of infection that should not be forgotten. It is important to have a high index of suspicion and to initiate treatment in a timely manner to avoid fatal outcomes, which commonly occur in more than half of cases.
Ethical considerationsThe research complies with the current regulations for bioethical research. Given that this is a case report, authorization from the ethics committee of the institution was not considered necessary. For the report of this case, authorization was obtained from the patient for the disclosure of his clinical history and images by signing the informed consent, guaranteeing his anonymity throughout the article.
Conflict of interestNone of the authors declares a conflict of interest for the preparation of this article.