Antiphospholipid syndrome diagnosis requires abnormal results of lupus anticoagulant and high titles of anticardiolipin (aCL) and β2glycoprotein I (anti-β2GPI) antibodies. The latter immunological tests lack a standard threshold in clinical practice.
ObjectiveTo determine the 99th percentile of aCL and anti-β2GPI in healthy volunteers.
Materials and methodsThis cross-sectional study reviewed antibody titles of anticardiolipin and β2glycoprotein I (IgG and IgM by enzyme-linked immunosorbent assay) in forty-nine healthy blood donors in Medellin, Colombia. Sociodemographic and immunological variables are also assessed. Antibody titles are described in median and interquartile range and the 99th percentile was estimated.
ResultsWe analysed samples from 16 men and 33 women. We found that the upper limits of the reference range (99th percentile) of aCL and anti-β2GPI were: aCL IgM: 18.0, aCL IgG: 16.1, anti-β2GPI IgM: 16.4, and anti- β2GPI IgG: 6.9.
ConclusionsThe upper limits obtained differ greatly from the arbitrary classification values suggested in the international guidelines, taking into account that values greater than 40 international units are usually required, and the values identified in this study are between 6.9 and 18 international units. We suggest conducting additional studies that validate cut-off points according to the percentiles explored in this work.
El diagnóstico del síndrome antifosfolípido requiere resultados anormales de anticoagulante lúpico y títulos elevados de anticuerpos anticardiolipina (aCL) y β2glicoproteína I (anti-β2GPI). Las últimas pruebas inmunológicas carecen de un umbral estándar en la práctica clínica.
ObjetivoDeterminar el punto de referencia (percentil 99) para los aCL y anti-β2GPI en voluntarios sanos.
Materiales y métodosSe llevó a cabo un estudio transversal descriptivo en el que se realizó una medición de los títulos de anticuerpos anticardiolipina y β2glicoproteína I (IgG e IgM por ensayo inmunoabsorbente ligado a enzimas) en 49 donantes de sangre sanos en Medellín, Colombia. Se excluyeron aquellos sujetos que tuvieran antecedentes de trombosis, morbilidad del embarazo, tumores, infecciones o enfermedades autoinmunes y también se evaluaron las variables sociodemográficas e inmunológicas. Los títulos de los anticuerpos se describen en mediana y rango intercuartílico. Se estimó el percentil 99.
ResultadosSe analizaron aCL y anti-β2GPI en 16 hombres y 33 mujeres. Los valores del límite superior del rango de referencia (percentil 99) de aCL y anti-β2GPI fueron: aCL IgM: 18,0, aCL IgG: 16,1, anti-β2GPI IgM: 16,4 y anti-β2GPI IgG: 6,9.
ConclusionesLos límites superiores obtenidos difieren en gran medida de los valores arbitrarios de clasificación sugeridos en las pautas internacionales, tendiendo en cuenta que usualmente se requieren valores superiores a 40 unidades internacionales y los valores identificados en este estudio están entre 6,9 y 18 de estas unidades. Se sugiere hacer estudios adicionales que validen puntos de corte según los percentiles explorados en este estudio.
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by the presence of two groups of clinical manifestations (thrombosis and gestational morbidity) and persistently positive antiphospholipid antibodies (APA). Its incidence is estimated at about 5 new cases per 100,000 individuals per year and its prevalence ranges between 40 and 50 cases per 100,000 individuals.1 APS has a high burden of disease: from a thrombotic perspective, it is estimated that 13% of subjects with cerebrovascular attack (CVA), 11% of individuals with acute myocardial infarction and 9.5% of patients with deep vein thrombosis have APS.2 Women under 50 years of age with CVA and APS have a 201-fold increased risk of suffering a new ischemic event.2 With respect to relevant gestational morbidity (defined as recurrent pregnancy loss, preterm delivery, preeclampsia and intrauterine growth retardation), APS is responsible for up to 6% of these manifestations in the general obstetric population; furthermore, 11% of stillbirths are secondary to this pathology.3
The diagnosis of this entity is established by fulfilling at least one clinical criterion and one laboratory criterion (APA); the latter must be positive in at least two separate measurements, at least 12 weeks apart.4 Regarding the laboratory criterion, there are basically 3 APAs: lupus anticoagulant (LA), anticardiolipin antibodies (aCL) and anti-β2 glycoprotein I (anti-β2GPI). The first test is functional, widely standardized, however, the aCLs have two options for positivity: having more than 40 IU (considered moderate or high titers) by the Elisa technique or a value equal to or greater than the 99th percentile. On the other hand, anti-β2GPI must be higher than the 99th percentile and are expressed in arbitrary units.4 The definition of these values of aCL and anti-β2GPI has problems: the Sidney criteria of 2006 suggest that this 99th percentile should be taken from the cut-off points of the manufacturer of the test; the 99th percentile of this cut-off point ranges, for example, for the aCLs between 15 and 39 IU, which leads to diagnostic imprecision.5 Several reasons have been proposed to explain this variability in the 99th percentile of the APAs: different execution equipment have been used, there are multiple execution techniques, there is a lack of uniformity in the reference material for calibration, and the validation of this percentile has been done in healthy people, but not in patients with APS.
From the clinical point of view, it has been described in observational studies (mainly cohorts) that low titers of aCL (between 20 and 40 IU) appear to be clinically significant when they are higher than 20 IU (present in 17–23%) in patients with both thrombotic and obstetric APS.6,7 Low titers have also been considered for patients with high suspicion of APS, with the aggravating factor that, especially the aCLs of IgM type, tend to give false positive results in this range, particularly in the presence of cryoglobulins, positive rheumatoid factor or infections.8
In relation to anti-β2GPI, these antibodies are not reported in international units like the aCLs, but rather are labeled in arbitrary units, in addition, their exact 99th percentile is unknown, a fact that goes against the recommendations of the Scientific and Standardization Subcommittee of the International Society of Thrombosis and Hemostasis.5
From a technical point of view, it is recommended that the nonparametric cut-off point (that is, the one used when the distribution of the data is unknown and the statistical result comes from an order) of the 99th percentile is more specific than the presence of moderate or high titers of APA in the diagnosis of APS.9 In addition, these cut-off points must be determined in a population of healthy volunteers.10–12 After an exhaustive literature review, it is clear that the 99th percentile of the APAs, or studies that have attempted to define it, that would be very useful since they would allow for greater diagnostic accuracy of this entity, which has a high burden of morbidity and mortality and whose proper diagnosis allows the initiation and maintenance of antithrombotic or anticoagulant therapy that is not exempt of risks, especially bleeding, but that should be established in the indicated population, are unknown in our environment.
It is essential to consider that knowing more clearly the 99th percentile in our environment could increase both the diagnostic precision and validity, since in this way the accuracy and reproducibility of the diagnostic approach of patients can be favored, in addition to increasing the potential of the test to discriminate between sick and healthy people for specific conditions such as APS.
The objective of this study was to determine the reference point (99th percentile) for aCL and anti-β2GPI, according to the sociodemographic characteristics and antibody isotypes in healthy volunteers in an institution of the city of Medellín.
Patients and methodsStudy design and patients’ selectionA descriptive cross-sectional study was conducted in healthy people who were blood donors in an institution in northwestern Colombia, who also met the eligibility criteria and agreed to participate in the study between December 2019 and January 2020.
The specific eligibility criteria were:
Healthy populationColombian men and non-pregnant women over 18 years of age.
Without clinical signs of thrombosis, tumors, or autoimmune diseases.
Without signs of current infection (respiratory, skin and soft tissue, gastrointestinal, genitourinary, nervous system, or in any other organ or system).
Patients with negative results of routine screening tests for infectious diseases (antibodies against HIV I and II, hepatitis B surface antigen, hepatitis B core, antibodies against hepatitis C, syphilis, antibodies against Chagas disease and against HTLV I and II), carried out by protocol in the blood bank.
The number of participants in the study was determined by convenience, due to cost issues of the laboratory tests that had to be performed, which is why it was decided to select a population of 49 subjects who attended a center as voluntary blood donors. This number was considered sufficient, taking into account that to meet the general objective of the study it is recommended to use between 30 and 50 sera from healthy individuals.13 It is essential to highlight that sample size calculations have not been documented in the literature, but rather that this is the analytical and pre-analytical way of conducting this type of studies.
Data collection processOnce the endorsement of the health ethics committee of the participating institution was obtained, authorization was requested from all participants through a written informed consent that was filled out before answering the usual form used for blood donors and the collection of the blood sample. The objective of this form was to identify the patient's personal and family history and lifestyle habits. The person in charge of reviewing this form, in addition to making an assessment of the clinical signs, was a specialist in internal medicine. This assessment was clinical, by means of anamnesis and complete physical examination. The questionnaire asked about the date of the last menstruation and the use of family planning methods in order to determine the pregnancy status of the women. Those subjects in whom no risk conditions were detected in the survey were considered healthy population. Likewise, according to the international protocols for being blood donors, it was necessary to verify the negative results of multiple tests for infectious diseases.
A measurement of aCL and anti-β2GPI IgM and IgG in plasma was done to each participant, using an enzyme-linked immunosorbent assay (Elisa), used in the reference laboratory. This method is the one that laboratories use more often.13,14 Elisa detects all the antibodies reactive with β2GPI and cardiolipin antigen, including non-pathogenic antibodies, independent phospholipids and the low affinity anti-β2GPI. To mimic the binding of β2GPI to negatively charged phospholipids, negatively charged plates (high binding or irradiated with gamma rays) are used, while to increase the density of the antigen after the process of incubation in walls and sequential washing, it is possible to obtain a quantitative result of the APA.15
For the measurement of aCl, Quanta Lite® ACA IgM III e IgG III (Inova Diagnostics, San Diego CA, USA) was used, which has as a normal reference range values lower than 12.5 MPL (standard units of immunoglobulin M) and 15 GPL (standard units of immunoglobulin G), respectively. Regarding the measurement of anti-β2GPI, QUANTA Lite® β2 GPI IgM e IgG (Inova Diagnostics), was used, which has normal reference range values lower than 20 units/mL for both tests. The blood sample used corresponded to the same that was taken for blood donation; consequently, a volume of blood larger than that usually used for this procedure was not required. Once the sample was taken, it was processed in the laboratory.
Sociodemographic (sex and age) and immunological (values of aCL and anti-β2GPI IgG and IgM antibodies in plasma) were collected. Once the processing of the sample and the collection of information were completed, it was exported to an electronic spreadsheet in Microsoft Excel® 2016, in which the variables that required it were recoded and the consistency of the data was explored before their subsequent analysis.
To control the possible biases that could be incurred, the following strategies were applied: a discussion was organized between the researchers of the protocol and of the process of collecting the information; it was attempted to control the information bias by standardization when entering the information into the database, which also had validated fields to avoid errors in the typing of the data and thus increase their reliability; in addition, the quality of the information was assessed through the exploration of values (detection of extreme data, inconsistent values, among others) and in case of doubt, the information was compared with the primary sources.
Statistical analysisQualitative variables were expressed using absolute and relative frequencies and quantitative variables were expressed using mean with standard deviation (SD) or median with interquartile range, according to the distribution of the data assessed by the Shapiro-Wilk test, indicated for an n lower than 50 observations. In order to determine the reference or positivity cut-off point, the 99th percentile of the distribution of the aCL and the anti-β2GPI IgG and IgM values was estimated. These values were also described according to the sociodemographic variables and the isotypes of the antibodies, while the cut-off points obtained were compared with those proposed by the manufacturer in the Elisa technique. The analyses were performed using the software IBM SPSS version 24 (IBM Corp., Armonk, NY, USA).
Ethical considerationsThis research was classified as minimal risk, according to Resolution 8430 of 1993 of the Ministry of Health and Social Protection, and was approved by the health research ethics committee of the participating institutions. All patients voluntarily agreed to participate in the study and signed the informed consent.
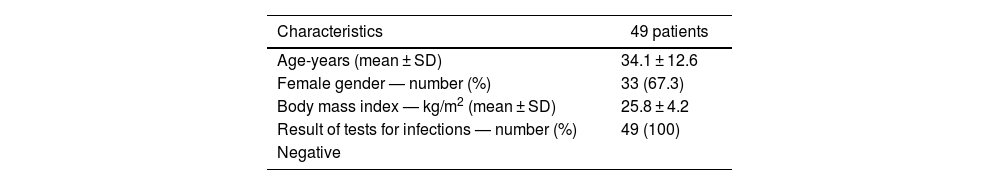
ResultsBlood samples were collected from 49 subjects, 16 men and 33 women, of mixed race, no Afro-Colombians or subjects of other races. All subjects were asymptomatic. The general characteristics of the sample are presented in Table 1.
General characteristics of 49 healthy blood donor subjects.
| Characteristics | 49 patients |
|---|---|
| Age-years (mean ± SD) | 34.1 ± 12.6 |
| Female gender — number (%) | 33 (67.3) |
| Body mass index — kg/m2 (mean ± SD) | 25.8 ± 4.2 |
| Result of tests for infections — number (%) | 49 (100) |
| Negative |
Source: Own elaboration.
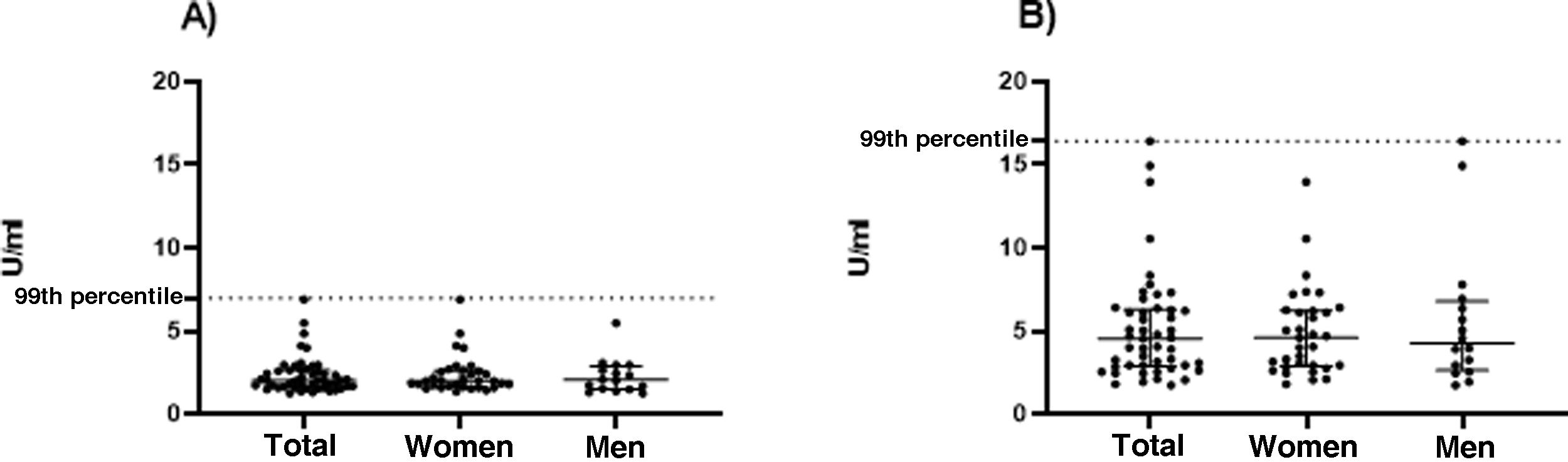
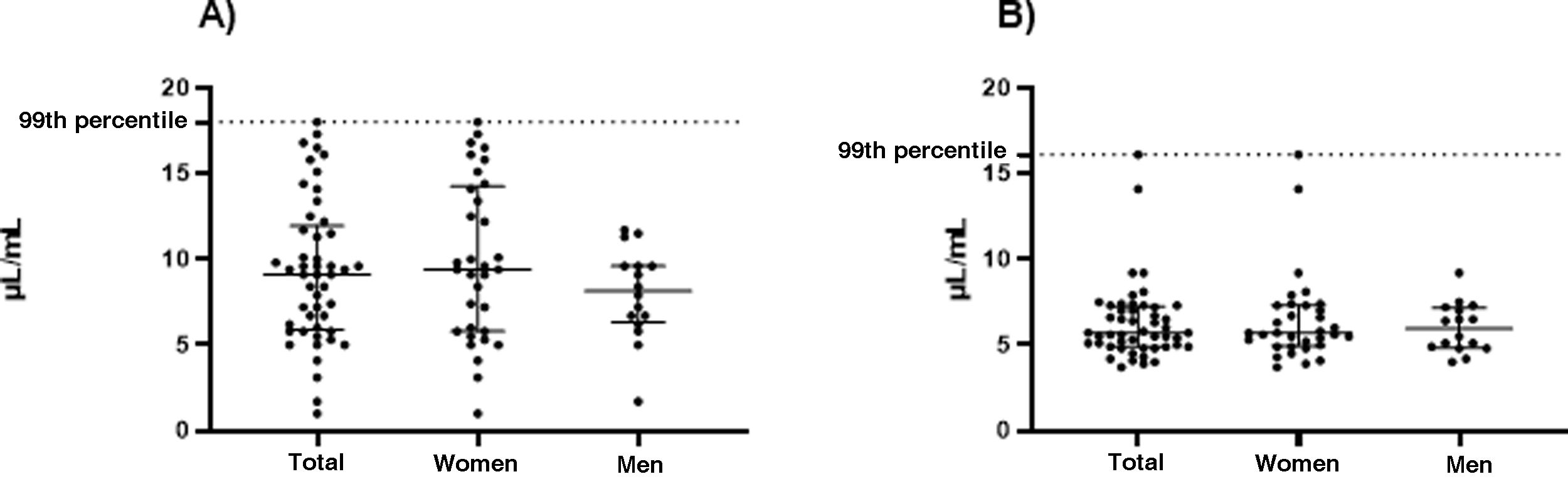
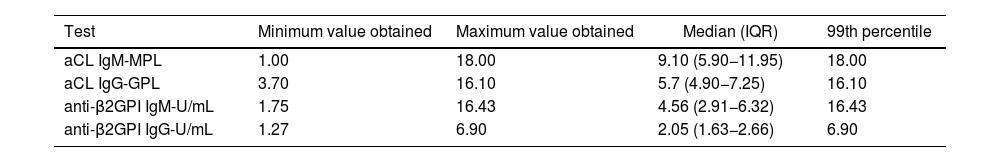
The values of the upper limit of the reference range (99th percentile) of aCL and anti-β2GPI in 49 healthy subjects were: aCL IgM: 18.0, aCL IgG: 16.1, anti-β2GPI IgM: 16.4 and anti-β2GPI IgG: 6.9. This information is shown in more detail in Table 2 and in Figs. 1 and 2.
99th percentile of the different antiphospholipid antibody tests performed on 49 healthy volunteer donors from a blood bank.
| Test | Minimum value obtained | Maximum value obtained | Median (IQR) | 99th percentile |
|---|---|---|---|---|
| aCL IgM-MPL | 1.00 | 18.00 | 9.10 (5.90−11.95) | 18.00 |
| aCL IgG-GPL | 3.70 | 16.10 | 5.7 (4.90−7.25) | 16.10 |
| anti-β2GPI IgM-U/mL | 1.75 | 16.43 | 4.56 (2.91−6.32) | 16.43 |
| anti-β2GPI IgG-U/mL | 1.27 | 6.90 | 2.05 (1.63−2.66) | 6.90 |
aCL: anticardiolipin antibodies; anti-β2GPI: anti-β2 glycoprotein I; SD: standard deviation; IQR: interquartile range.
GPL: 1 mL/mL of IgG aCL; MPL: 1 mL/mL of IgM.
Source: Own elaboration.
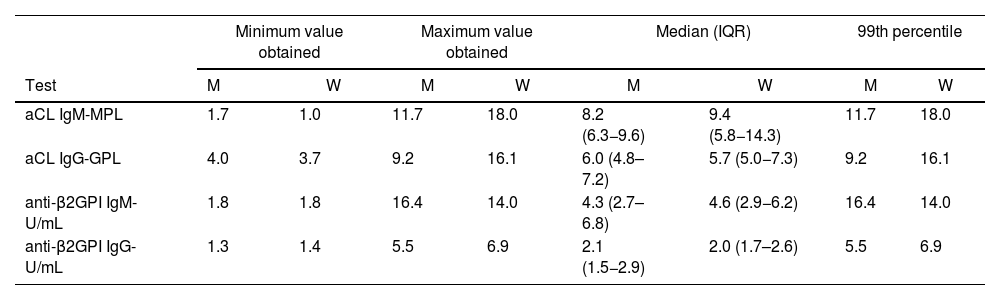
Table 3 presents the results of aCL and anti-β2GPI, according to the sex of the participant. Higher values of the 99th percentile of aCL, both IgG and IgM, are observed in women.
99th percentile of the different antiphospholipid antibody tests according to the sex of the participants.
| Minimum value obtained | Maximum value obtained | Median (IQR) | 99th percentile | |||||
|---|---|---|---|---|---|---|---|---|
| Test | M | W | M | W | M | W | M | W |
| aCL IgM-MPL | 1.7 | 1.0 | 11.7 | 18.0 | 8.2 (6.3−9.6) | 9.4 (5.8−14.3) | 11.7 | 18.0 |
| aCL IgG-GPL | 4.0 | 3.7 | 9.2 | 16.1 | 6.0 (4.8–7.2) | 5.7 (5.0−7.3) | 9.2 | 16.1 |
| anti-β2GPI IgM-U/mL | 1.8 | 1.8 | 16.4 | 14.0 | 4.3 (2.7–6.8) | 4.6 (2.9−6.2) | 16.4 | 14.0 |
| anti-β2GPI IgG-U/mL | 1.3 | 1.4 | 5.5 | 6.9 | 2.1 (1.5−2.9) | 2.0 (1.7–2.6) | 5.5 | 6.9 |
M: men, W: women; aCL: anticardiolipin antibodies; anti-β2GPI: anti-β2 glycoprotein I; SD: standard deviation; IQR: interquartile range.
GPL: 1 mL/mL of IgG aCL; MPL: 1 mL/mL of IgM.
Source: Own elaboration.
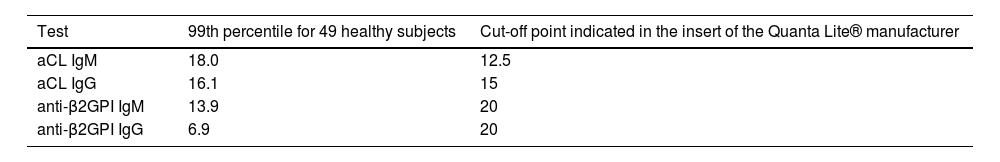
The results of aCl and anti-β2GPI in 49 healthy subjects, as well as the reference values of the manufacturer of the tests are described in Table 4.
99th percentile of the different antiphospholipid antibody tests in 49 healthy subjects and the reference values para the manufacturer of the tests.
| Test | 99th percentile for 49 healthy subjects | Cut-off point indicated in the insert of the Quanta Lite® manufacturer |
|---|---|---|
| aCL IgM | 18.0 | 12.5 |
| aCL IgG | 16.1 | 15 |
| anti-β2GPI IgM | 13.9 | 20 |
| anti-β2GPI IgG | 6.9 | 20 |
aCL: anticardiolipin antibodies; anti-β2GPI: anti-β2 glycoprotein I.
Source: Own elaboration.
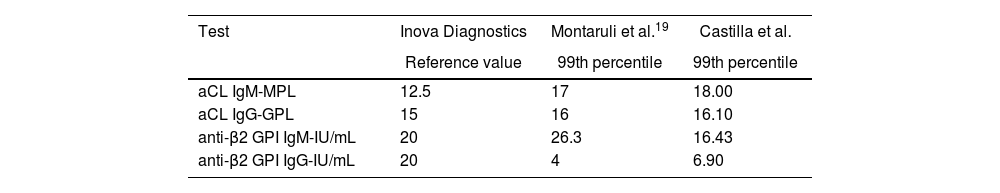
Table 5 compares the different values of the 99th percentile for the manufacturer Inova Diagnostics.
Comparison of different values of the 99th percentile for the manufacturer Inova Diagnostics.
| Test | Inova Diagnostics | Montaruli et al.19 | Castilla et al. |
|---|---|---|---|
| Reference value | 99th percentile | 99th percentile | |
| aCL IgM-MPL | 12.5 | 17 | 18.00 |
| aCL IgG-GPL | 15 | 16 | 16.10 |
| anti-β2 GPI IgM-IU/mL | 20 | 26.3 | 16.43 |
| anti-β2 GPI IgG-IU/mL | 20 | 4 | 6.90 |
GPL: 1 mL/mL of IgG aCL; MPL: 1 mL/mL of IgM.
Source: Own elaboration.
To the best of our knowledge, this is the first study in our environment that evaluates the value of the 99th percentile for aCL and anti-β2GPI in healthy population. It is essential to highlight that the upper limits of the normal reference range of APA found in this study differ from the cut-off classification values selected arbitrarily by the international consensus guidelines on aCL and anti-β2GPI testing,15,16 which were obtained from observational studies and the Elisa cut-off point reported by the manufacturer. It is important to remember that if the clinically significant values of APA are not clearly known, a diagnostic failure can occur in 9.5–29.4% of cases.17 Of the four APAs in which the 99th percentile was identified in the subjects, assessed, only the aCL IgG has a value really close to the cut-off point indicated on the insert of the manufacturer, the others differ to a greater extent.
It is also remarkably that the 99th percentile identified in the sample of patients in this study is also different when comparing these results between men and women, however, as is known, the reference standard does not make distinctions between sexes. In the literature, different technical problems have been reported in the Elisa assays used for the determination of APA, both pre- and post-analytical, in addition to a significant interlaboratory variability with respect to the performance for aCL and anti-β2GPI, which is due to the lack of uniformity in the reference material for calibration.18
The main scientific problem is that the laboratory criterion based on aCL and anti-β2GPI for the diagnosis of APS is not very precise, in addition, variation has been observed in different populations, both in the reference ranges obtained with the commercial kits and in the values set by the classification criteria.19 This could be an explanation for the discordant results found.
The variation in the test results originates from methodological problems in the performance of the assays, differences in calibration, and lack of consensus in the interpretation of the results with respect to the classification as positive or negative.15 The lack of uniformity in the reference material used for calibration, as well as the variety of the calibrators, is one of the main problems, since it generates differences in the titers between the assays.15 Today it is clear that the most common method for detection of aCL and anti-β2GPI is by Elisa, however, as time passes, more automated platforms have been introduced to the market that will require to give more details and additional specifications for the detection and interpretation of their results.20
Regarding the available information about the manufacturer Quanta Lite® (Inova Diagnostics), it is known that the reference points for these laboratory tests were obtained from random samples of normal donors, in which 489 and 488 samples were analyzed to obtain the reference range in the IgM and IgG aCLs, respectively. On the other hand, for anti-β2GPI IgM, this manufacturer reports that a total of 313 normal samples from a group made up of a similar number of men and women aged between 18 and 58 years were tested. Likewise, 256 samples from normal donors were used to obtain the value of anti-β2GPI IgG.
Several attempts have been made in the literature to determine this 99th percentile in healthy volunteers, with different results.
Montaruli et al. defined this percentile in a population of northwestern Italy in 104 healthy subjects; they compared three available Elisa techniques finding substantial differences between the cut-off points reported by the manufacturer (between 7 and 18 IU) most of which corresponded to low titers, distant from the arbitrary reference value of the Sidney criteria.19 It should be highlighted that one of the Elisa techniques used in this study corresponds to the same manufacturer used in this work (Inova Diagnostics). When comparing the results reported by these authors with this particular manufacturer (Table 5), it could be concluded that the IgG and IgM aCLs and the anti-β2GPI IgG have 99th percentile values similar to what is documented in this study; however, the value corresponding to anti-β2GPI IgM is substantially different.
Ruffatti et al. wanted to validate the moderate and high titers of APA in 90 subjects with APS, starting from a 99th percentile in healthy subjects of 17.4 IU, obtained in 100 healthy volunteers, and they found that only 64 subjects had moderate of high titers. Likewise, up to 74% of the patients with gestational morbidity and 16.4% of the patients with thrombosis had low titers of APA, a result that reiterates the higher specificity of the 99th percentile obtained in healthy population when compared with the moderate or high titers of APA.9
It is noteworthy that in the current study, in a similar way to those described previously, really low 99th percentile values for anti-β2GPI are found in healthy population. A crucial aspect in the identification of APA are their diagnostic and prognostic implications, due to the frequency of rethrombosis and recurrent gestational morbidity, in addition to the morbidity and mortality inherent to these clinical complications. Other clear fact is that is more specific to obtain the 99th percentile in healthy volunteers, because it would partially rectify the pre-analytical, analytical and post-analytical errors of the laboratory tests available for the diagnosis of APS.
Our study has several weaknesses, such as the fact that it was conducted only with one type of commercial trial, the population was selected from a single institution, and the inclusion of a small number of patients, all of them of mixed race, which is why it prevents us from extrapolating the results to patients of other ethnicities and countries. For this reason, it is not representative of the total population.
It is very important to remember that the classification criteria and the diagnostic criteria are different, hence, it is probable that this type of studies will not alter the classification criteria, since they have research purposes and, therefore, they will always tend to give value to specificity. However, results with these characteristics do provide fundamental information to focus on patients who are precriteria and in whom the clinician considers the diagnosis but not the classification because this data of population distribution changes the weight of the results found.
The strengths of the study are that it is the first approach related to this topic in our environment. Obtaining a reference value of the 99th percentile for APA is extremely important, since in some way it can increase the diagnostic performance of the tests, thus providing an adequate and adjusted management, in addition to impact the complications associated with the disease, both clinical and those related to bleeding secondary to the treatment. The next step after having obtained these results will be to compare the values of the 99th percentile of APA with other types of commercial trials, in addition, with a population that already has the diagnosis of APS, this in order to validate these results.
Finally, it is necessary to mention that this is a study that opens a field for research, since if these results are not taken with caution, we could incur in an overdiagnosis. For that purpose, it will be necessary to delve deeper into the topic and seek that other groups of researchers corroborate the information in another population.
ConclusionsFor the diagnosis of antiphospholipid syndrome, at least one clinical and one laboratory criterion must be met, which is why it is so important to have clear information related to the value of the antiphospholipid antibodies. In the present study, differences were found when determining the reference point (99th percentile) for the aCls and anti-β2GPI with respect to the international cut-off points and, to a lesser extent, for the reference values of the laboratory, mainly according to the isotype of the antibody, taking into account that values higher than 40 IU are usually required and the values identified in this study are between 6.9 and 18 of these units. This type of studies is important because it helps to characterize the population and allows to reinforce to a greater extent the information known regarding the diagnosis of antiphospholipid syndrome.
FundingThe present research used funds derived from the Colombian Association of Rheumatology; these funds did not have a specific association with the commercial sector and were non-profit.
Conflict of interestThe authors declare that they have no conflict of interest for the preparation of this article.
Thanks to Dr. María Fernanda Álvarez Barreneche for the review of the manuscript and to the blood bank and the laboratory service of the Pablo Tobón Uribe Hospital.