Rheumatoid arthritis (RA) is an inflammatory autoimmune disease characterized by inflammation of multiple joints, leading to destruction of cartilage and juxta articular bone. It eventually leads to deformity, disability, and impaired quality of life. Methotrexate (MTX) has a reported response rate of 33–65%, and this variability may be explained by genetic variations (polymorphisms) in the metabolic pathway of this drug.
To evaluate possible relationships between polymorphisms in the metabolic pathway and response to MTX in patients with RA.
MethodologyA systematic search and review of the literature was conducted. A total of 29 studies that evaluated polymorphisms in the metabolic pathway of MTX were included, due to their full text and methodological quality.
ResultsOf the 29 studies, five were systematic reviews and/or meta-analyses, three of which clinical trials none was triple blind and only one was double-blind, six were cohort, seven were case–control, and eight cross sectional. The polymorphism identified were: methylene tetrahydrofolate reductase, dihydrofolate reductase, thymidylate synthase, 5-amino-imidazole-4-carboxamide ribonucleotide formyl transferase (AICAR formyltransferase), 5-aminoimidazole-4-carboxamide polymorphisms formyltransferase/IMP cyclohydrolase ribonucleotide (ATIC) identified conveyors attached to ATP cassette (ABC ATP-binding cassette), folylpoly-glutamate, glutamyl hydrolase, reduced folate carrier (RFC-SLC10A1). The dihydrofolate reductase and methylene tetrahydrofolate reductase polymorphism were shown to be associated with increased MTX toxicity. RFC and C677T polymorphisms are associated with better efficacy of MTX.
ConclusionsThe polymorphisms of methylene tetrahydrofolate reductase, C677T and RFC1-G80A generate increased efficacy and toxicity in patients treated with MTX. However, for the other polymorphisms, although studies show statistically significant associations, they are not conclusive and some are contradictory. This justifies conducting multicenter studies to assess the presence and association with the effectiveness or toxicity in patients with RA treated with MTX.
La artritis reumatoide (AR) es una enfermedad inflamatoria de origen autoinmune, caracterizada por inflamación de múltiples articulaciones que lleva a destrucción del cartílago y del hueso yuxta articular, con el tiempo genera deformidad, discapacidad y deterioro de la calidad de vida. El metotrexato (MTX), reporta un índice de respuesta del 33 al 65%, esta variabilidad puede ser explicada por las variaciones genéticas (polimorfismos) en la ruta metabólica de este fármaco.
ObjetivoEvaluar las posibles asociaciones entre los polimorfismos de la ruta metabólica del MTX y su respuesta en pacientes con AR.
MetodologíaRevisión sistemática de la literatura cualitativa (integrative review). Se realizó una búsqueda sistemática de la literatura, 29 estudios fueron incluidos por texto completo y por calidad metodológica para alcanzar el objetivo del estudio, estos estudios evaluaron polimorfismos en la ruta metabólica del MTX.
ResultadosDe los 29 estudios, cinco fueron revisiones sistemáticas o metaanálisis, tres ensayos clínicos de los cuales ninguno fue triple ciego y solo uno fue doble ciego, seis fueron cohortes, siete fueron casos y controles y ocho de corte transversal. Se identificaron los polimorfismos de metiltetrahidofolato reductasa, dihidrofolato reductasa, timidilato sintetasa, 5-aminoimidazol-4-carboxamida ribonucleótido formiltransferasa (AICAR formiltransferasa), 5-aminoimidazol-4-carboxamida ribonucleótido formiltransferasa/IMP ciclohidrolasa (ATIC), transportadores de casete unidos a ATP (ABC ATP-binding cassette), folilglutamato sintetasa, glutamil hidrolasa, transportador de folato reducido (RFC-SLC10A1). Los polimorfismos metiltetrahidofolato reductasa y dihidrofolato reductasa demostraron estar asociados con un aumento en la toxicidad del MTX; los polimorfismos RFC y C677T están asociados a una mejor eficacia del MTX.
ConclusionesLos polimorfismos de metiltetrahidofolato reductasa C677T y RFC1 - G80A generan aumento de eficacia y toxicidad en pacientes tratados con MTX. Sin embargo, para los demás polimorfismos, aunque los estudios muestran asociaciones estadísticamente significativas, no son concluyentes y algunos son contradictorios. Lo anterior justifica la realización de estudios de carácter multicéntrico, para evaluar la presencia y asociación, con la eficacia o toxicidad en los pacientes con artritis reumatoide tratados con MTX.
Rheumatoid arthritis (RA) is a chronic inflammatory disease of autoimmune origin, characterized by inflammation of multiple joints. Over time it leads to variable degrees of destruction of the articular cartilage and the juxta-articular bone, generating progressive deformity, and thereby disability, alteration of the quality of life and a decrease in the life expectancy.1 In the treatment are included the nonsteroidal anti-inflammatory drugs (NSAIDs), synthetic and biological disease-modifying antirheumatic drugs (DMARDs), analgesic agents and non-pharmacological measures seeking to alleviate pain, reduce the damage and preserve the joint function.2
DMARDs are by definition drugs associated with the reduction of the articular and bone damage caused by the disease. Among the DMARDs, methotrexate (MTX) has been the most used. This drug was discovered in 1948 and was initially indicated for the treatment of neoplastic diseases. In 1951, it was used for the first time for the treatment of psoriatic arthritis. In 1980 it started to be used for the treatment of RA, showing a good response and an adequate safety profile. Since the 1990s, MTX became the DMARD of first choice.3,4 A significant percentage of patients do not respond clinically to treatment. Because of the limited result of monotherapy with this drug, combinations of MTX with other traditional DMARDs are used to increase the response percentage in the treatment of RA.5
MTX is a folic acid analog, originally designed to inhibit the activity of the enzyme dihydrofolate reductase (DHFR), responsible for converting dihydrofolates into tetrahydrofolates, involved in the transfer of one carbon atom in the intracellular metabolic pathways, such as the de novo synthesis of purines, pyrimidines and polyamines, as well as in the transmethylation of phospholipids and proteins. MTX has additionally immunosuppressive and anti-inflammatory properties.
As for the immunosuppressive activity, it inhibits the proliferation of CD3–CD4 lymphocytes and other immunocompetent cells such as monocytes, macrophages and polymorphonuclear neutrophils. MTX also modulates cytokines such as interleukin 4 (IL–4) and interleukin 10 (IL-10), interferon-alpha and interleukin 2 (IL-2), thus generating anti-inflammatory and immunoregulatory actions.
Once inside the cell, MTX polyglutamates bind competitively and with greater affinity than dihydrofolate to several enzymes and inhibit their function: DHFR, TYMS and AICAR formyltransferase.6 When AICAR formyltransferase is inhibited, intracellular accumulation of AICAR is generated, leading to increased release of adenosine in the blood. This mediator activates the extracellular receptors A2a, A2b and A3 in monocytes and macrophages, inhibits the production of tumor necrosis factor alpha (TNFα), IL-6 and IL-8, promotes the transcription of mRNA for the IL-1 receptor antagonist and increases the secretion of IL-10, a potent anti-inflammatory.7 The activation of adenosine receptors on human endothelial cells inhibits the production of IL-6 and IL-8 and decreases the expression of E-selectin on the cell surface. The foregoing explains the important role of adenosine in the anti-inflammatory response.8,9
MTX enters into the cell through the folate carrier RFC1-SLC19A1. The egress of the drug from the cell is controlled by the members of the family of the ATP-binding cassette transporters (ABC ATP-binding cassette), predominantly ABCC1 and ABCG2, also known as multi-drug resistant proteins.10 Within the cell, by action of the enzyme folyl-glutamate synthetase (FPGS), the MTX is converted into polyglutamate forms known as MTXPG, this process can be reversed by the enzyme γ glutamyl hydrolase (GGH). The MTXPG inhibit the DHFR, enzyme that reduces the dihydrofolate into tetrahydrofolate. The conversion of tetrahydrofolate into a form of 5-methyl occurs thanks to the methionine synthase, and implies the synthesis of an intermediate metabolite called 5,10-methyl-tetrahydrofolate (by the enzyme serine hydroxymethyltransferase (SHMT1). The resulting product 5-methyl-tetrahydrofolate is a biologically important fraction since it acts as a donor of one carbon in cellular reactions such as the conversion of homocysteine into methionine.4
In addition to the inhibition of the folate pathway, the MTXPGs also influence the de novo synthesis of pyrimidines through the inhibition of TYMS, which converts deoxyuridilate into deoxythymidylate. The MTXPGs inhibit the enzyme AICAR transformylase, also known as bifunctional purine biosynthesis protein (PURH), encoded by ATIC, which leads to intracellular accumulation of adenosine aminoimidazole carboxamide ribonucleotide. This product and its metabolites inhibit two enzymes which are important in the metabolism of adenosine, adenosine deaminase and AMP deaminase, causing intracellular accumulation of adenosine nucleotides, that when dephosphorylated, generate extracellular accumulation of adenosine, a powerful anti-inflammatory agent.11
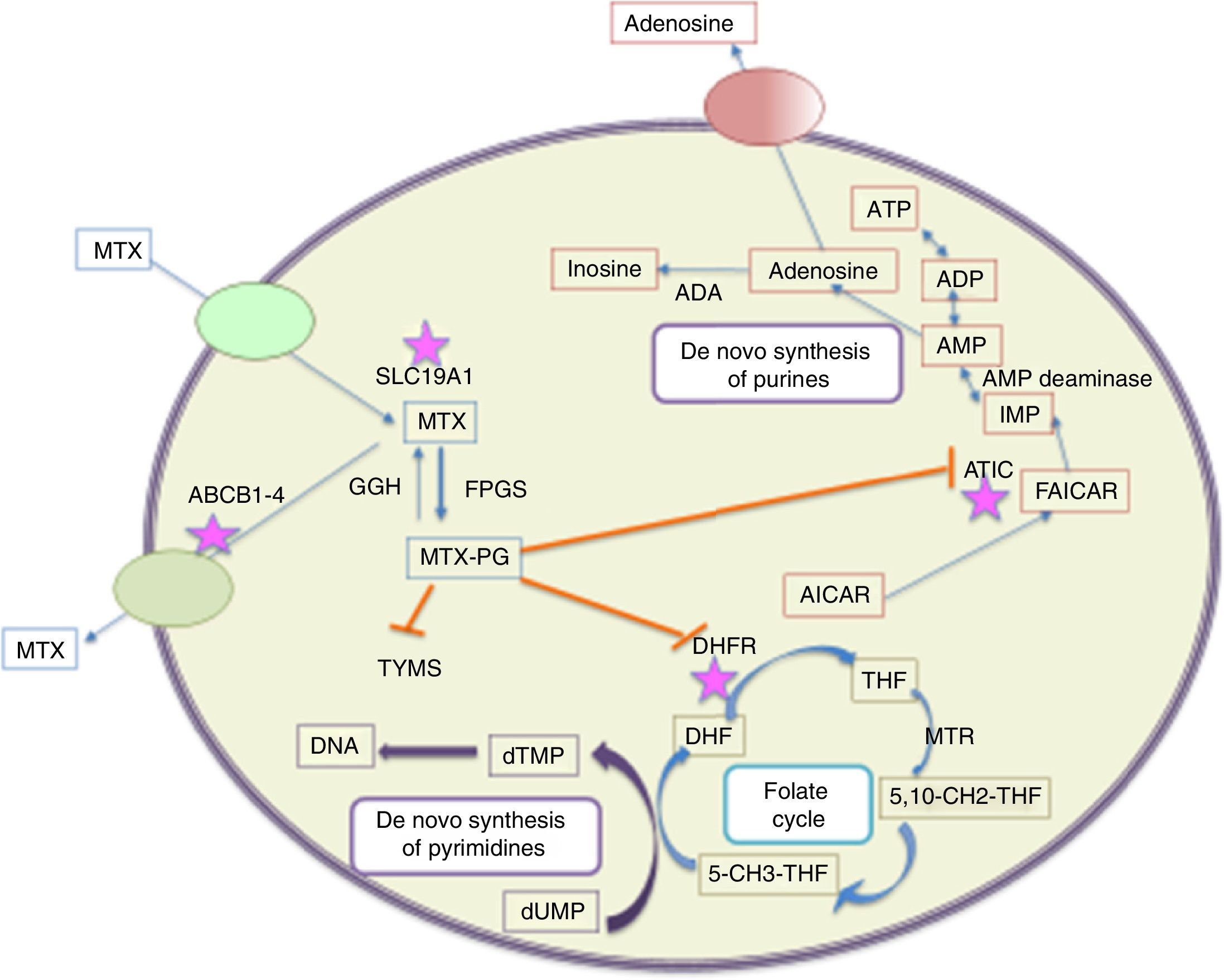
Three metabolic pathways of MTX have been described; the first one, in which the drug is synthesized by intestinal bacteria in 4-amino-deoxy-N10-methyl acid. This metabolite represents less than 5% of the administered dose and its detection in plasma or urine is rare. The second metabolic pathway occurs in the liver, were it is converted into 7-hydroxy MTX,12 considered an inhibitor of dihydrofolate reductase, 10 times less potent than MTX polyglutamates. The third and most important metabolic pathway is the intracellular conversion of MTX into polyglutamates. Its mechanism of action is concentrated in this latter pathway.13 The cells that most convert the MTX into polyglutamyl derivatives (form in which it is stored) include: erythrocytes, fibroblasts, myeloid precursors in the bone marrow, keratinocytes, synovial membrane, cortical bone and trabecular bone. From there are derived its pharmacological effect on the decline in the lymphocyte population and the adverse gastrointestinal, cutaneous and mucosal adverse events14 (Fig. 1).
Metabolic pathways of intracellular metabolism of MTX. MTX enters actively into the cells through SLC19A1, while the efflux occurs across the cell membrane mediated by several transporters (ABC 1–4). Once inside the cell occurs the polyglutamynation (MTX-PG) by the enzyme FPGS, reaction reversed by GGH. MTXPG inhibits several enzymes that are important in the folate metabolism, such as dihydrofolate reductase (DHFR), resulting in depletion of tetrahydrofolate (THF) (a precursor of the folate cofactor 5-CH3-THF). The inhibition of TYMS by MTX-PG, and indirectly through the depletion of THF, leads to the inhibition of the biosynthesis of pyrimidine. The decrease in the synthesis of folate compounds also leads to the inhibition of the biosynthesis of purines. MTX-PG inhibits ATIC, causing intracellular accumulation of AICAR, leading eventually to the generation of adenosine. The asterisks indicate the proteins in which polymorphisms have been more associated with alterations in the response to methotrexate in different populations. ABCB1–4: ATP-ABC transporters from 1 to 4; ADA: adenosine deaminase; AICAR: 5-aminoimidazole-4-carboxamide ribonucleotide; AMPD: adenosine monophosphate deaminase; ATIC: bifunctional purine biosynthesis protein; 5-CH3-THF: 5-methyl-tetrahydrofolate; 5,10-CH2-THF: 5,10-methylenetetrahydrofolate; DHF: dihydrofolate; DHFR: dihydrofolate reductase; dTMP: deoxythymidine monophosphate; FAICAR: 10-formyl-5-aminoimidazole-4-carboxamide; FPGS: folylpolyglutamate synthetase; GGH: gamma-glutamyl hydrolase; IMP: inosine 5-monophosphate; MTHFR: methylenetetrahydrofolate reductase; MTR: methyl tetrahydrofolate reductase; MTRR: methionine sinthase reductase; MTX: methotrexate; MTX-PG: MTX polyglutamate; SLC19A1: solute carrier 19A1; THF: tetrahydrofolate; TYMS: thymidylate synthase.
The adverse events derived from MTX are related to the amount and frequency of the administered dose. The majority of these events can be detected early and are reversible. They can be divided according to the affected system in: gastrointestinal such as vomiting, diarrhea and stomatitis which can lead to dehydration; hematological such as bone marrow depression with aplastic anemia, pancytopenia, leukopenia, neutropenia or thrombocytopenia. Hepatic/biliary/pancreatic: acute and chronic hepatotoxicity. Acute, with elevation of liver enzymes, usually transient and asymptomatic. Chronic toxicity is potentially lethal; after prolonged use, persistent abnormalities in the liver function tests may precede the onset of cirrhosis.
Variability in the response to MTX. Despite being the most commonly used DMARD because of its long-term efficacy and safety, the response to this drug is highly variable among patients. This is attributed to different factors such as: gender, age, duration of the disease, C-reactive protein, levels of rheumatoid factor and genetic variations. Among them, genetic factors are considered of great importance and therefore they have been studied, supported by pharmacogenetics, for the search and analysis of the individual genetic variations (polymorphisms) associated with the therapeutic efficacy and safety of the treatment with MTX.15–18
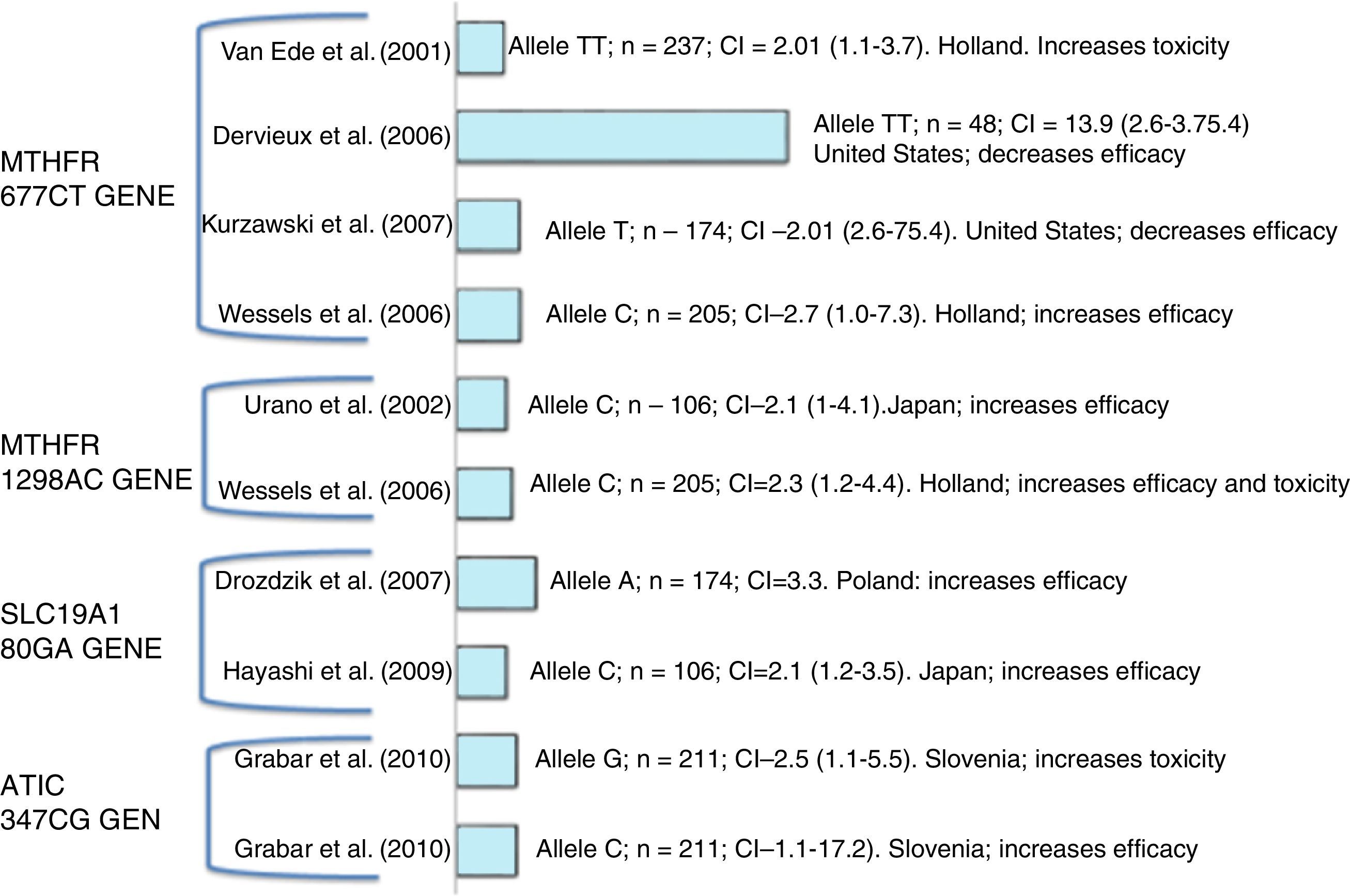
Diverse polymorphisms that affect the response and toxicity can be found in the metabolic pathway of this drug (Fig. 2). ABCs, SLC10A1. ABC (ABC ATP-binding cassette) and RFC (reduced folate carrier), especially SLC19A1/RFC1, are transporters of MTX that influence its cellular absorption and efflux.19
Polymorphisms in enzymes involved in the polyglutamation of MTX: FPGS and GGH. The amount of intracellular MTX polyglutamates depends on the polyglutamation rate, which is determined by the activities of the enzymes FPGS and GGH. The PMF in these enzymes can generate changes in the efficacy or toxicity.
DHFR. Dihydrofolate reductase is one of the therapeutic targets of MTX. The polymorphisms that stand out are rs12517451, rs10072026 and rs1643657 for a greater association with adverse events; SNP DHFR 317AG (rs70991108) is associated with a greater response to MTX, and patients with 317AA genotype with lower response.20,21
TYMS. This enzyme, required for the de novo synthesis of thymidylate, is directly inhibited by MTXPG. The PMFs in the gene that encodes this enzyme include tandem repetition (two or three repetitions of a unit of 28pb) in the enhancer region in the 5′-UTR. Patients homozygous for the triple (3R) repetition of TYMS allele, exhibit an increased TYMS mRNA expression and greater enzyme activity compared with those with the 2R allele.22
ATIC. It is an important gene in the adenosine pathway; this gene encodes the enzyme involved in the release of extracellular adenosine which has anti-inflammatory properties.23
Polymorphisms in the MTHFR. These are of the most widely studied polymorphisms and play an important role in the response to treatment since they affect the enzyme activity and the metabolism of MTX, among these PMF are found; C677T (rs1801133) and A1298C (rs1801131).24,25
Materials and methodsA qualitative systematic review (integrative review) of the literature on experimental studies (randomized clinical trials, clinical trials), analytical observational (cohort, case–controls) and cross-sectional studies was conducted in order to evaluate the effect of MTHFR, ATIC, DHFR, ATYM, FPGS, GGH SLC10A1 and ABC polymorphisms on the effectiveness and therapeutic safety of MTX in the management of RA. A search of primary studies, systematic reviews and meta-analyses was carried out in the most important scientific databases using MeSH terms and all fields terms for each component of the PICOT (population, intervention, control, outcome and type of study) question.
Types of studiesRandomized controlled clinical trials, cohort, case–control and cross-sectional studies, published and unpublished, which study the relationship between the presence of genetic polymorphisms and the clinical effectiveness of MTX in the management of RA were included. In all cases the number of patients studied was greater than 10. For experimental studies, those that contemplated randomization were taken into account. Within the analytical studies were included all those who contemplated the exposure, the target population and bias control strategies.
Type of participantsAdults (older than 18 years) with a diagnosis of RA and treatment with MTX.
Type of interventionPolymorphisms in MTHFR, ATIC, DHFR, ATYM, FPGS, GGH, SLC10A1 and ABC.
Types of outcomesEfficacy of MTX on the progression of the disease by ACR 20% response criteria. Safety of MTX estimating the number of adverse effects in terms of frequency and severity of such events.
Electronic searchIn order to identify the studies to be included in this review, a search in the databases was conducted using the same strategy. The search contemplated a combination of keywords and the recommended filters of PubMed Central. Manual search was carried out in related references and key authors. The search was limited to English and Spanish languages.
Search termsFilter: (“arthritis, rheumatoid” [MeSH Terms] OR (“arthritis” [All Fields] AND “rheumatoid” [All Fields]) OR “rheumatoid arthritis” [All Fields] OR (“rheumatoid” [All Fields] AND “arthritis” [All Fields])) AND (“pharmacogenetics” [MeSH Terms] OR “pharmacogenetics” [All Fields]) AND (“methotrexate” [MeSH Terms] OR “methotrexate” [All Fields]) AND (“Polymorphism”) [MeSH Terms].
Databases- •
Cochrane Library.
- •
Cochrane Controlled Trials Register: Cochrane Library.
- •
Science Citation Index (1981–2015).
- •
MEDLINE (1966–2015).
- •
CINAHL (1982–2015).
- •
SIGLE (1980–2015).
- •
LILACS (1982–2015).
- •
Scielo (2005–2015).
- •
Center of Reviews and Dissemination of United Kingdom.
- •
Clinical Trials.gov.
The abstracts of the articles found in the search were reviewed in order to eliminate the irrelevant articles. Subsequently, the relevant articles were reviewed by three independent persons to verify that they met the inclusion criteria. Differences were resolved by consensus.
Data extractionThe studies that met the inclusion criteria were analyzed for data extraction. Data were extracted independently by two persons (rheumatologist and clinical pharmacologist) experts in methodology and the results were re-evaluated (PhD-clinical epidemiologist) for consistency based on the format of data collection designed and validated for that purpose. The data included were: number of patients, population, age, gender, dose of MTX, evaluated polymorphism, outcome (efficacy-toxicity), number of events, measures of frequency and association, confidence intervals and statistical significance.
Measures of effectAnalysis of meta-analysis type was not carried out due to the high heterogeneity of the studies. Therefore, it was decided to make a narrative analysis of the results of the studies. Data were collected in an evidence table.
Missing dataSome studies did not reported measures of frequency or association, and therefore the statistical significance was taken and compared with the direct results of the study.
Publication biasAn exhaustive literature search was conducted in all relevant databases. Local information was manually sought in the Colombian Journal of Rheumatology, Memories of the Global Arthritis Research Network and of the Meeting and Bio-Rheumatology International Congress.
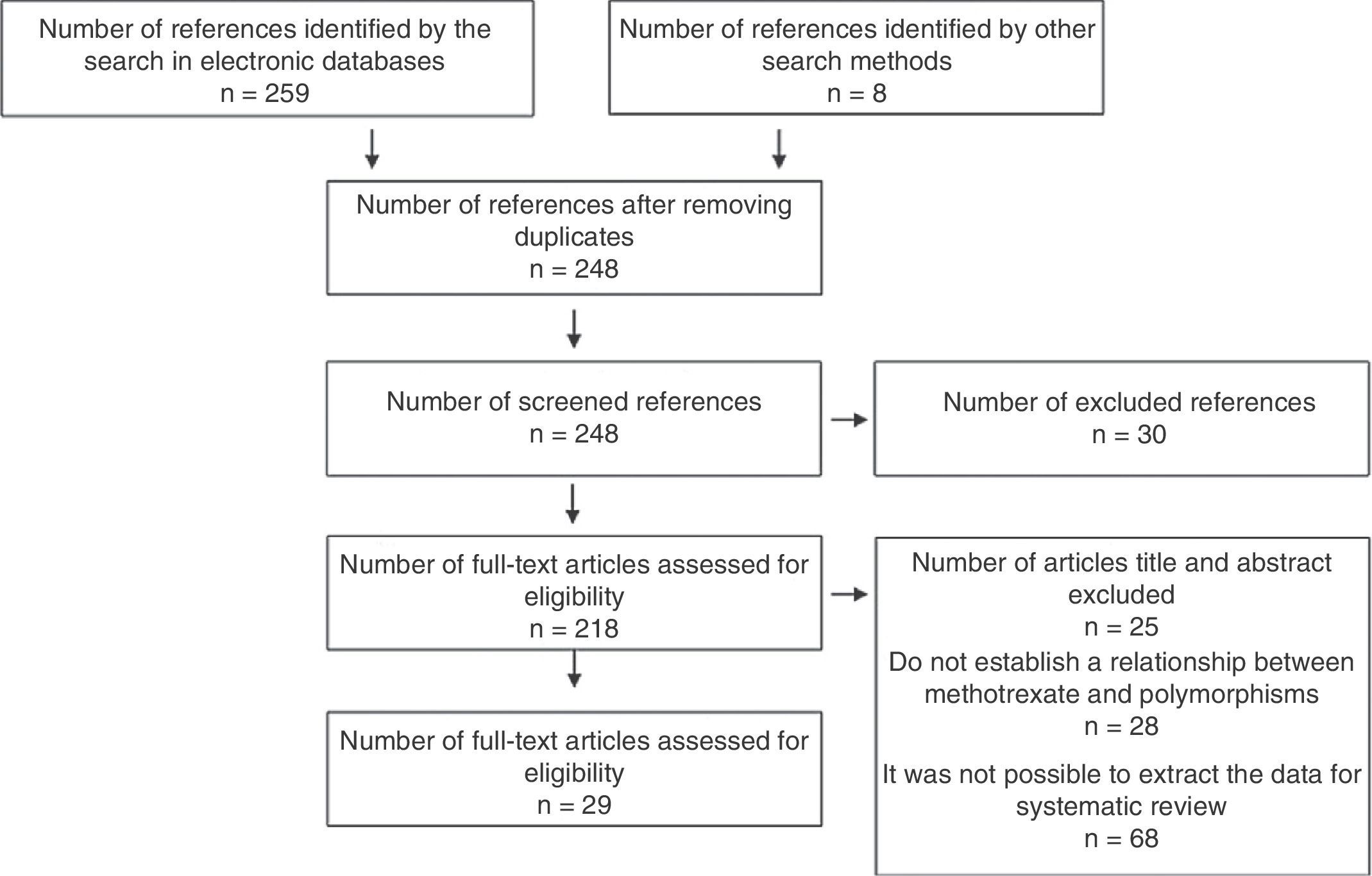
ResultsA total of 267 references were identified with the search terms. Of these, 215 were excluded after applying the eligibility criteria of their titles or abstracts, with exclusion of duplicates. Of the remaining 52 studies, 23 were discarded due to a lack of a control group, of randomization, and of clarity in the method of evaluation of the results. A total of 29 studies were finally included. All eligible studies were in English (Figs. 3 and 4).
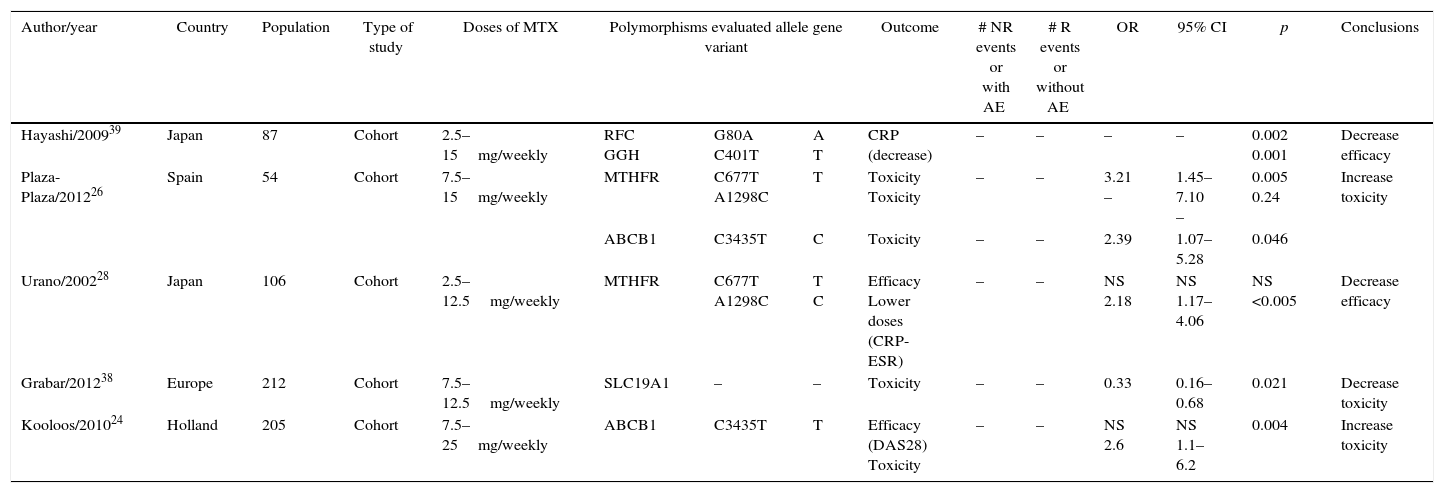
Of the 29 studies, five were systematic reviews or meta-analyses, three were clinical trials, none of which was triple-blind and only one was double-blind, six were cohort, seven were case–control, and eight were cross-sectional (Tables 1 and 2).
Cohort studies.
| Author/year | Country | Population | Type of study | Doses of MTX | Polymorphisms evaluated allele gene variant | Outcome | # NR events or with AE | # R events or without AE | OR | 95% CI | p | Conclusions | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hayashi/200939 | Japan | 87 | Cohort | 2.5–15mg/weekly | RFC GGH | G80A C401T | A T | CRP (decrease) | – | – | – | – | 0.002 0.001 | Decrease efficacy |
| Plaza-Plaza/201226 | Spain | 54 | Cohort | 7.5–15mg/weekly | MTHFR | C677T A1298C | T | Toxicity Toxicity | – | – | 3.21 – | 1.45–7.10 – | 0.005 0.24 | Increase toxicity |
| ABCB1 | C3435T | C | Toxicity | – | – | 2.39 | 1.07–5.28 | 0.046 | ||||||
| Urano/200228 | Japan | 106 | Cohort | 2.5–12.5mg/weekly | MTHFR | C677T A1298C | T C | Efficacy Lower doses (CRP-ESR) | – | – | NS 2.18 | NS 1.17–4.06 | NS <0.005 | Decrease efficacy |
| Grabar/201238 | Europe | 212 | Cohort | 7.5–12.5mg/weekly | SLC19A1 | – | – | Toxicity | – | – | 0.33 | 0.16–0.68 | 0.021 | Decrease toxicity |
| Kooloos/201024 | Holland | 205 | Cohort | 7.5–25mg/weekly | ABCB1 | C3435T | T | Efficacy (DAS28) Toxicity | – | – | NS 2.6 | NS 1.1–6.2 | 0.004 | Increase toxicity |
| Case–control studies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author/year | Country | Population | Type of study | Doses of MTX | Polymorphisms evaluated allele gene variant | Outcome | # NR events or with AE | # R events or without AE | OR | 95% CI | p | Conclusions | ||
| Mena/201129 | Mexico | 70 | Case–control | 7.5–15mg/weekly | MTHFR | C677T A1298C | C T A C | Increase in transaminases | – | – | 1.1 0. 0.36 2.5 | 0.46–2.61 0.38–2.14 0.14–0.89 1.11–6.75 | 0.821 0.821 0.023 0.023 | Increase toxicity |
| Jekic/201322 | Belgrade | 184 | Case–control | 10–12.5mg/weekly | TYMS GGH | 3RG/3RC G354T | 3RG/3RG GG | DAS 28 (decrease) Myelosuppression | NR 38 MS 8 | R 146 176 | 5.4 – | 1.4–21.1 – | 0.002 0.003 | Decrease efficacy |
| Aggarwal/200630 | India | 150 | Case–control | 7.5–15mg/weekly | MTHFR | C677T | T | Efficacy (DAS28) | 64 | – | No differences | No relationship | ||
| Takatori/200627 | Japan | 124 | Case–control | 6mg/weekly | ABCB1 | C3435T | C | Efficacy (CA, VSG,PCR) Toxicity | – | – | 8.78 NS | 1.13–68.5 NS | 0.038 NS | Increase toxicity |
| ATIC RFC1 TYMS | C347G G80A | – | – | – | NS | NS | NS | No relationship | ||||||
| Hayashi/201343 | Japan | 170 | Case–control | 2–12mg/weekly | RFC1 | G80A | AA | Efficacy | – | – | 2.27 | 1.35–3.84 | 0.0018 | Decrease efficacy |
| Pawlik/200731 | Poland | 50 | Case–control | 7.5–15mg/weekly | MTHFR | C677T A1298C | T C | Efficacy | – | – | 2.3 1.15 | 0.76–7.01 0.45–2.91 | NS | No relationship |
| Cross-sectional studies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author/year | Country | Population | Type of study | Doses of MTX | Polymorphisms evaluated allele gene variant | Outcome | # NR events or with AE | # R events or without AE | OR | 95% CI | p | Conclusions | ||
| Dervieux/200440 | USA | 226 | Cross-sectional | 5–25mg/weekly | RFC GGH | G80A C401T | A T | Increase in concentration of MTX | – | – | 4.8 3.4 | 1.8–13 1.4–8.4 | 0.002 0.007 | Increase efficacy |
| Owen/201320 | UK | 309 | Cross-sectional | 5–15mg/weekly | ATIC SLC19A1 | C347G | – | Efficacy | – | – | 1.65 1.86 | 1.13–2.42 1.26–2.74 | 0.01 0.001 | Decrease efficacy |
| Weisman/200632 | USA | 214 | Cross-sectional | 7.5–15mg/weekly | MTHFR TSER ATIC SHMT1 | C677T G347G C1420C | – | Toxicity | – | – | 3.3 5.38 2.97 2.38 | 1.72–6.45 3.05–9.49 1.64–5.36 1.19–4.77 | 0.01 <0.01 <0.01 <0.05 | Increase toxicity |
| Drozdzik/200741 | USA | 174 | Cross-sectional | 7.5–15mg/weekly | RFC1 | G80A | AA | Increase in MTX, efficacy and transaminases | – | – | 3.32 | 1.26–8.79 | 0.021 | Increase efficacy |
| Berkun/200435 | – | 93 | Cross-sectional | 7.5–15mg/weekly | MTHFR | A1298C | AA | Reduction of adverse events | – | – | 5.24 | 1.38–20 | 0.03 | Decrease AE and efficacy |
| Kim/200633 | Korea | 385 | Cross-sectional | 7.5–15mg/weekly | MTHFR | C677T | T | Toxicity | – | – | 3.8 | 2.29–6.33 | <0.05 | Increase toxicity |
| Kurzawski/200744 | Poland | 174 | Cross-sectional | 7.5–15mg/weekly | MTHFR | C677T A1298C | T C | Efficacy Toxicity | – | – | 2.61 1.93 | 1.35–5.04 1.19–3.77 | – | Increase the frequency of remission |
| Taniguchi/200734 | Tokyo | 208 | Cross-sectional | 6mg/weekly | MTHFR | C677T | T | Increase in transaminases | – | – | 2.4 | 1.29–4.55 | 0.003 | Increase toxicity |
| Controlled clinical trials | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author/year | Country | Population | Type of study | Doses of MTX | Polymorphisms evaluated allele gene variant | Outcome | # NR events or with AE | # R events or without AE | OR | 95% CI | p | Conclusions | ||
| van Ede/200136 | USA | 236 | Clinical trial | 7.5–25mg/weekly | MTHFR | C677T | T | Increase in transaminases | – | – | 2.27 | 1.06–5.34 | – | Increase toxicity |
| Wessels/200637 | USA | 247 | Clinical trial | 7.5–15mg/weekly | MTHFR | C677T A1298C | T C | Efficacy | – | – | 2.23 | 1.18–4.41 | 0.014 | Increase efficacy |
| MTHFR | A1298C | C | Toxicity | – | – | 2.5 | 1.32–4.72 | 0.005 | Increase toxicity | |||||
| Wessels/200645 | USA | 247 | Clinical trial | 7.5–15mg/weekly | AMPD1 ATIC ITP4 | C34T C347G C94A | – | Efficacy (DAS) | – | – | 2.1 2.5 2.7 | 1–4.5 1.3–4.7 1.1–8.1 | NS <0.05 NS | Increase efficacy |
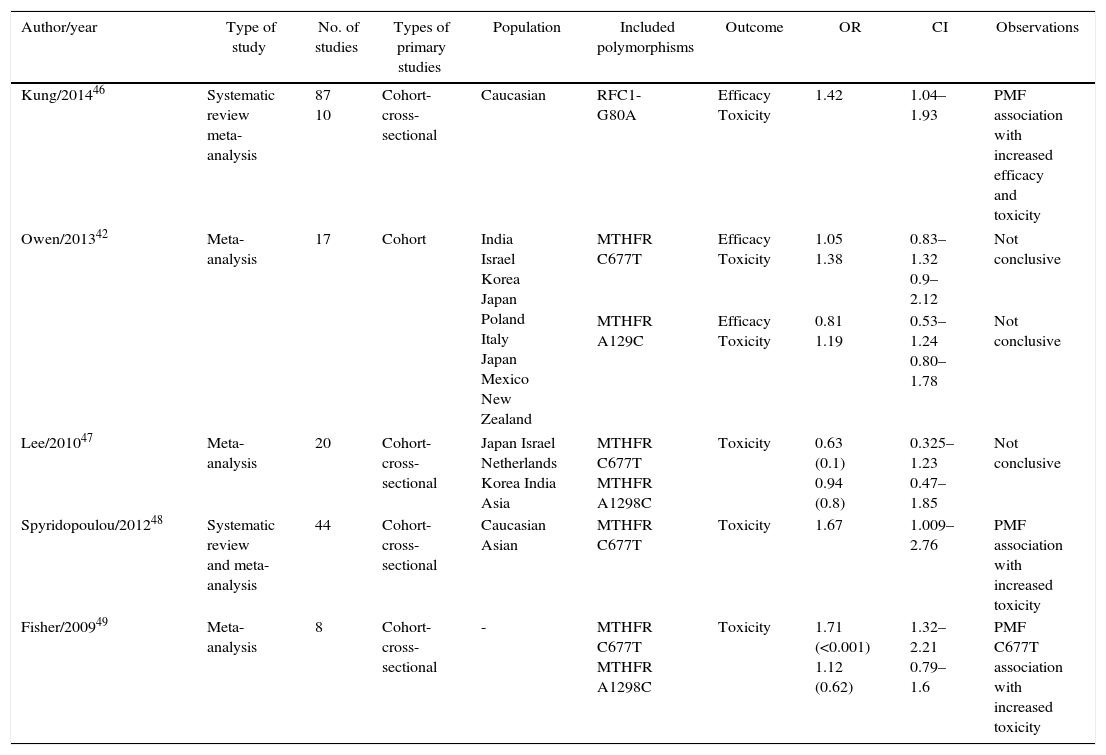
Systematic reviews and meta-analyses.
| Author/year | Type of study | No. of studies | Types of primary studies | Population | Included polymorphisms | Outcome | OR | CI | Observations |
|---|---|---|---|---|---|---|---|---|---|
| Kung/201446 | Systematic review meta-analysis | 87 10 | Cohort-cross-sectional | Caucasian | RFC1-G80A | Efficacy Toxicity | 1.42 | 1.04–1.93 | PMF association with increased efficacy and toxicity |
| Owen/201342 | Meta-analysis | 17 | Cohort | India Israel Korea Japan Poland Italy Japan Mexico New Zealand | MTHFR C677T | Efficacy Toxicity | 1.05 1.38 | 0.83–1.32 0.9–2.12 | Not conclusive |
| MTHFR A129C | Efficacy Toxicity | 0.81 1.19 | 0.53–1.24 0.80–1.78 | Not conclusive | |||||
| Lee/201047 | Meta-analysis | 20 | Cohort-cross-sectional | Japan Israel Netherlands Korea India Asia | MTHFR C677T MTHFR A1298C | Toxicity | 0.63 (0.1) 0.94 (0.8) | 0.325–1.23 0.47–1.85 | Not conclusive |
| Spyridopoulou/201248 | Systematic review and meta-analysis | 44 | Cohort-cross-sectional | Caucasian Asian | MTHFR C677T | Toxicity | 1.67 | 1.009–2.76 | PMF association with increased toxicity |
| Fisher/200949 | Meta-analysis | 8 | Cohort-cross-sectional | - | MTHFR C677T MTHFR A1298C | Toxicity | 1.71 (<0.001) 1.12 (0.62) | 1.32–2.21 0.79–1.6 | PMF C677T association with increased toxicity |
Three studies evaluated the presence of ABCB1 polymorphism, two cohort studies and one case–control study. The studies of Plaza-Plaza et al. and Kooloos et al., evidence a clear association between the presence of ABCB1 polymorphism and the increase in MTX toxicity with OR 2.39 95% CI (1.07–5.28) and OR 2.6 95% CI (1.1–6.2) respectively.24,26 The case–control study published by Takatori et al., 2006, also reported a statistically significant association between the presence of the polymorphism and the increased toxicity of MTX, OR 8.78 95% CI (1.13–68.5).27
MTHFR polymorphismTwelve studies were identified for the evaluation of MTHFR polymorphism. The study designs were 2 cohort studies, 3 control-case studies, 5 cross-sectional studies and 2 controlled clinical trials.
Cohort studies. In the cohort studies was established a relationship between the polymorphism and the decrease in the effectiveness of MTX, the study by Plaza-Plaza et al.26 established that it exhibits an association of risk of toxicity with MTX, OR 3.21 95% CI (1.45–7.10), in the study of Urano et al.28 was established a relationship for the decrease of the effectiveness of MTX for the control of the disease, OR 2.18 95% CI (1.17–4.06). In the case–control studies the association is not consistent. In the study published by Mena et al.29 was established the relationship between the presence of MTHFR polymorphism and the increased toxicity of MTX for all C677T and A1298C evaluated alleles, however, only for the A1298C allele this relationship was statistically significant for the increased toxicity with MTX, OR 2.75 95% CI (1.11–6.75). The studies of Aggarwal et al.30 and Pawlik et al.31 could not establish a clear association between the presence of the polymorphism and the therapeutic behavior of MTX.
In the cross-sectional studies the evidence is not consistent. The studies of Weisman et al.32, Kim et al.33 and Taniguchi et al.34 reported for the polymorphism with the C677T allele, an association with the increase of MTX toxicity, OR 3.3 95% CI (1.72–6.45); OR 3.8 95% CI (2.29–6.33) and OR 2.4 95% CI (1.29–4.55) respectively. The study of Berkun et al.35 reported a statistically significant relationship between the presence of this polymorphism and the decrease in the number of adverse events, OR 5.24 95% CI (1.38–20) (Table 1). Two controlled clinical trials reported the association between the presence of the C677T gene polymorphism for two opposing outcomes. The study of van Ede et al.36 established a risk association between the presence of the polymorphism and the increased toxicity with MTX, OR 2.27 95% CI (1.06–5.34) and the study of Wessels et al.37 which established an association with improved efficacy of MTX in individuals with presence of this polymorphism, OR 2.23 95% CI (1.18–4.41).
SLC19A1 polymorphismOnly one cohort study, which assessed the association between this polymorphism and the behavior of MTX was included in this systematic review. The study of Grabar et al.38 established that the presence of this polymorphism reduces the toxicity of MTX, OR 0.33 95% CI (0.16–0.68).
RFC polymorphismFive studies evaluated the presence of this polymorphism and the therapeutic behavior of MTX. One cohort study, two case–control studies and two cross-sectional studies were included in this systematic review for the evaluation of this polymorphism. In the cohort study conducted by Hayashi et al.39 was evidenced an association between the decrease in the efficacy of MTX and the presence of this polymorphism; the same author published in 2013 a case–control study which evaluated the association between the presence of RFC1 polymorphism and the effectiveness of MTX, where it was evidenced that this association reduces the effectiveness of MTX, OR 2.27 95% CI (1.35–3.84). Another case–control study, published by Takatori et al.27 did not established a relationship between the presence of this polymorphism and the effectiveness and toxicity of MTX. In two cross-sectional studies, Dervieux et al.40 and Drozdzik et al.41 established an association between the presence of the polymorphism and the increased efficacy of MTX, OR 4.8 95% CI (1.8–13) and OR 3.32 95% CI (1.26–8.79) respectively.
Other polymorphismsGGH, ATIC, and TSER polymorphisms have been explored by different authors; in this review the study conducted by Dervieux et al.40 establishes that the presence of GGH polymorphism increases the efficacy of MTX, OR 3.4 95% CI (1.4–8.4). Owen et al.20,42 found and association between the presence of the ATIC polymorphism and the reduction of the effectiveness of MTX, OR 1.65 95% CI 1.13–2.42; another author, Weisman et al. evidenced for the same polymorphism an association with the increase of toxicity OR 2.97 95% CI (1.64–5.36). The same author explored the relationship between the increase in the MTX toxicity and the presence of the TSER polymorphism establishing an OR of 5.38 95% IC (3.05–9.49).32
Population characteristics, polymorphism and genotypesThe effect of the genetic variation of the metabolic pathway of MTX is given by polymorphisms in enzymes such as MTHFR, some transporters of MTX across the cell membrane and enzymes that influence the metabolic pathway. Different SNPs have been linked to the adverse effects or the therapeutic response to this drug C677T and A1298C studied in Caucasian and Japanese populations.28,50 Likewise, polymorphisms related with the reduced folate carrier RFC1-G80A and ATIC-C347G have been studied in Caucasian, Asian, African and Mexican populations.10,51
A study conducted in mestizo population of Mexico reported different PMF, finding that the risk genotype MTHFR C677T exhibited a high prevalence of 33% genotype TT, the risk genotype AA MTHFD1 G1958A in the mestizo was 34% and for the Amerindians 58%, unlike the populations of Asian and African descent where the frequency of AA was low (4%), demonstrating that there is a differential geographic distribution of the risk variants in the metabolic pathway of folate/homocysteine in relation with the ethnic origin.51,52
There have been described ABC B1 polymorphisms, such as the C3435T (rs1045642) which is positively associated with the response to treatment for RA with MTX and in some cases with increased toxicity. Another PMF is the rs4793665 of ABC C3 with positive association regarding treatment effectiveness.26,53 In RFC/SLC19A1, patients who carry the allele G in G80A (rs1051266) have a lower intracellular uptake of MTX compared with those who carry the allele A, which would lead to lower efficacy.17,43. A recent study conducted in Portuguese population suggests an increased early gastrointestinal toxicity in the carriers of the G allele, which is significantly reduced with the supplement of folic acid.54 In a study carried out with population of the Treatment of Early Aggressive Rheumatoid Arthritis it was found an association between variants in the SLC22A2 gene and a better response to MTX.55
In RA patients with AA or AG of FPGS 2572CT (rs1544105) genotypes it has been found a poor response to MTX; and in patients with SNP 63942342CT (rs12681874) of GGH genotype it has been found an association with greater efficacy.56 The non-TT genotype of GGH T16C (rs1800909) was associated with increased risk of liver dysfunction and 354T GGH-G polymorphism was related with a higher risk of myelosuppression.20,22
In studies with the TYMS gene it has been found that the 3G/3G genotype of the 3R allele is associated with a poor response to MTX in patients with RA.22 An association with inadequate response to treatment with MTX was found in patients with the ATIC 26293TC SNP (rs12995526); and the rs3821353, rs7563206 and rs16853834 variants have been associated with higher efficacy.23
Negative associations regarding toxicity were reported for the MTHFR C677T polymorphism.24,25 However, in RA patients with CT or TT genotypes it has been found a higher risk of adverse events than in those with the CC genotype,57 these studies have suggested and association between C677T and toxicity of MTX in Caucasians and Asians. It was not found any positive association between C677T polymorphism and therapeutic effectiveness.58,59
As for the PMF A1298C of MTHFR, there are studies that report an association with effectiveness and toxicity and others that do not find it significant. The rs1801131C variant prevails in the group that responds to MTX, patients with genotypes CC or CA have a greater clinical response than those with genotype AA; in addition, A1298C has been associated with elevation of transaminases.29
In the systematic reviews and meta-analyses (Table 2) is evident that the RFC1-G80A polymorphism is associated with increased efficacy and toxicity and the PMF of the MTHFR C677T gene is associated with toxicity in patients treated with MTX.
Discussion and conclusionsThere is no doubt about the importance of MTX in the treatment of patients with RA. All guidelines published by international organizations agree that it should be the first-line drug. This is justified by its low cost and its good safety profile. MTX is currently the most commonly used drug for the treatment of RA worldwide,60 and has proven to be more effective than other DMARDS.61 However, the therapeutic response measured by the construct ACR 20% is achieved on average by 45% of patients.62 The considerable number of patients with therapeutic failure is due to the limited efficacy and the toxicity of monotherapy, represented in frequent adverse effects, product of the individual variability in the response.14 So far, many genetic variants have been studied and the results are contradictory. The majority of authors agree on the potential of pharmacogenetics in rheumatology, but they emphasize that with the data obtained in the different studies conducted to date, there is no enough robust information to recommend the use of any of these genetic tests in clinical practice.
This study has limitations inherent to the design. There is a possible publication bias because information was not sought in specific rheumatology databases, only memories of congresses and in the Colombian Journal of Rheumatology. Likewise, the search was limited only to the Spanish and English languages. It was not possible to conduct meta-analysis due to the differential report of the different clinical outcomes. The results of this systematic review should be considered with caution.
In this review is clear that the meta-analyses have demonstrated that there are two polymorphisms that increase the efficacy and toxicity of MTX: in the RFC1-G80A gene and in the MTHFR C677T gene. However, other genetic variants have shown association in individual studies. The study of these variants in the Colombian population is important to achieve a better understanding of the inter-individual and racial variability. The final objective for the physician and the patient will be to establish a personalized therapy that leads to a better and prompt response in RA patients with the corresponding reduction of adverse events.
According to the review, in Colombia and South America there are no studies that estimate the prevalence of these polymorphisms in patients with RA who receive treatment with MTX, especially taking into account the prevalence and the impact that this disease has on the population and the health care system.51,63,64 This makes it necessary to determine in the Colombian mestizo population, a mixture of the white European with the black African and the pre-Colombian indigenous, the presence of genetic variations that condition the response to treatment with MTX.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Restrepo LF, Giraldo R, Londoño J, Pinzón C, Cortes A, Ballesteros G, et al. Farmacogenética del metotrexato en artritis reumatoide. Revisión sistemática. Rev Colomb Reumatol. 2016;23:102–114.