With the onset of the 2019 coronavirus pandemic, there was an increase in the number of reported cases of autoimmune manifestations in the midst of the disease and new cases of autoimmunity after infection.
ObjectiveTo correlate the rate of positive tests of SARS-CoV-2 with the rate of new diagnoses of autoimmune disease in paediatric patients of the department of Huila (Colombia), between March 2020 and September 2021.
MethodsThe socio-demographic characterization of the subjects diagnosed with autoimmune disease and of the cases of SARS-CoV-2 was performed; an analysis of the monthly trend of events was performed and a correlation model was applied between the rate of SARS-CoV-2 diagnosis and the rate of autoimmune diseases during the study period.
Results94 cases of autoimmune disease were diagnosed, with an average age of 8.3 years, most were girls and belonged to the urban area and the subsidized regimen, and immune thrombocytopenic purpura was the most common diagnosis. In addition, 4303 cases of SARS-CoV-2 were detected, mostly adolescents, from urban areas and the contributory system. Finally, it was evidenced that the highest rate of autoimmune disease was reported just after the highest peak of SARS-CoV-2.
ConclusionsThis study did not show a statistical correlation between the rate of SARS-CoV-2 and the rate of diagnoses of autoimmune disease; however, it showed an enormous peak of autoimmunity after the highest peak of infection in paediatric patients of the department during the first eighteen months of the pandemic.
Con el inició de la pandemia por coronavirus 2019, se empezó a notar un aumento en el número de casos reportados de manifestaciones autoinmunes en medio de la enfermedad y casos nuevos de autoinmunidad tras la infección.
ObjetivoCorrelacionar la tasa de pruebas positivas de SARS-CoV-2 con la tasa de diagnósticos nuevos de enfermedad autoinmune en pacientes pediátricos del departamento del Huila (Colombia) entre marzo del 2020 y septiembre del 2021.
Materiales y métodosSe llevó a cabo la caracterización sociodemográfica de los sujetos con diagnóstico de enfermedad autoinmune y de los casos de SARS-CoV-2; se hizo un análisis de la tendencia mensual de los eventos y se aplicó un modelo de correlación entre la tasa de diagnóstico de SARS-CoV-2 y la tasa de enfermedades autoinmunes durante el periodo del estudio.
ResultadosSe diagnosticaron 94 casos de enfermedad autoinmune, con una edad promedio de 8,3 años, la mayoría fueron niñas y pertenecían a la zona urbana y al régimen subsidiado, y la púrpura trombocitopénica inmune fue el diagnóstico más común. Adicionalmente, se detectaron 4.303 casos de SARS-CoV-2, en su mayoría adolescentes, del área urbana y del régimen contributivo. Finalmente, se evidenció que la mayor tasa de enfermedad autoinmune se reportó justo después del pico más alto de SARS-CoV-2.
ConclusionesEste estudio no demostró una correlación estadística entre la tasa de SARS-CoV-2 y la tasa de diagnósticos de enfermedad autoinmune, sin embargo, puso en evidencia un enorme pico de autoinmunidad tras la mayor cúspide de infección, en los pacientes pediátricos del departamento durante los primeros 18 meses de la pandemia.
Coronavirus disease 2019 (COVID-19) began in Wuhan, China, in December 2019 and spread throughout the world, until it was declared a pandemic by the World Health Organization (WHO) on March 11, 2020.1 This disease is caused by a strain of coronavirus associated with severe acute respiratory syndrome (SARS) that was called SARS-CoV-2, evoking the SARS-CoV that caused the SARS epidemic in 2002.2
SARS-CoV-2 belongs to the family of coronaviruses, which are spherical enveloped viruses with a single-stranded RNA genome; the virus has spike glycoproteins on its surface, which give it the characteristic structure similar to a corona radiata, from which it receives its name. These proteins allow it to bind to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell and invade it.3 Most of the time, infected subjects present flu-like symptoms,4 but they can also exhibit systemic manifestations that affect multiple organs through immune-mediated mechanisms.5
A disease is defined as autoimmune when an adaptive response to autoantigens causes tissue damage,6 which can be triggered by genetic, hormonal and environmental factors that lead to a state of hyperstimulation. Among the environmental factors, viruses such as Epstein-Barr (EBV), hepatitis C, hepatitis B, Zika, parvovirus B19, herpesvirus, human immunodeficiency virus (HIV), cytomegalovirus and enterovirus, among others, have been widely studied.7
Likewise, with the beginning of the COVID-19 pandemic there has also been developing evidence that in the same way as other viruses, SARS-CoV-2 is capable of triggering autoimmunity, mainly through the mechanism of molecular mimicry,8,9 by which high levels of autoantibodies, cytokines and other inflammatory markers are generated, with secondary tissue damage.5,10 This fact became evident because since the emergence of the new SARS-CoV-2,11 several countries reported autoimmune manifestations in the midst of the disease, such as arthritis,12,13 antiphospholipid antibody syndrome (APS),14,15 vasculitis,16–19 hemophagocytic lymphohistiocytosis (HLH),20,21, immune thrombocytopenic purpura (ITP), autoimmune hemolytic anemia (AHA),22 skin lesions,23,24 neurological25,26 and renal27 manifestations, systemic lupus erythematosus (SLE),28,29 myositis30 and multisystem inflammatory syndrome temporally associated with SARS-CoV-2/COVID-19 infection (PIMS-TS),31 among many others.
On the other hand, the majority of the epidemiological data on autoimmune diseases come from developed countries and from surveys carried out on adults, which is why the data on the pediatric population are not completely known; juvenile idiopathic arthritis (JIA), which is the most common rheumatic disease in children,32 occurs with an incidence of 1.6–23 cases per 100,000.33 In Colombia, the Center for the Study of Autoimmune Diseases (CREA, for its acronym in Spanish, Centro de Estudios de Enfermedades Autoinmunes)34 reported a prevalence of autoimmune diseases of 5% and an incidence of 1–20 per 100,000 inhabitants, based on studies in adults35,36; in his degree thesis for the Universidad Javeriana, G. Espitia reported a prevalence for JIA of 40/100,000 inhabitants and for SLE of 25/100,000 inhabitants under 19 years of age.37 As for SARS-CoV-2, in the first year of the pandemic this virus caused around 210 million cases and 5 million deaths worldwide38; in Colombia, 4.5 million infections and 120,000 deaths39; and in the department of Huila, 54,000 cases and 1,600 deaths.40
Despite the fact that almost three years have elapsed since the beginning of the COVID-19 pandemic, and although research has been developing in this regard, it has not yet been completely established how the human body responds to SARS-CoV-2 infection, and it has also not been clarified whether the virus is actually capable of generating autoimmunity in infected subjects. This led us to conduct this work, which aims to correlate the rate of positive SARS-CoV-2 tests with the rate of new diagnoses of autoimmune disease in pediatric patients in Huila during the first 18 months of the pandemic, to clarify a possible temporal relationship between the events.
MethodsType of studyA retrospective analytical observational and longitudinal study was conducted, with a level of relational research and ecological design.
Population and sampleThe population of interest was the pediatric population of Huila under 15 years of age, which is made up of around 250,000 individuals.39,40 The study population was made up of patients under 15 years of age with a new diagnosis of autoimmune disease, established in the three largest pediatric care centers in the department of Huila, and by subjects under 15 years of age who were tested for SARS-CoV-2 during the study period.
Inclusion criteriaSARS-CoV-2 infectionSubjects under 15 years of age with a positive result in one of the SARS-CoV-2 diagnostic tests (antigen detection by chromatographic immunoassay or polymerase chain reaction technique) between March 2020 and September 2021.
Multiple case: subjects with a second positive result after 90 days of a first examination.
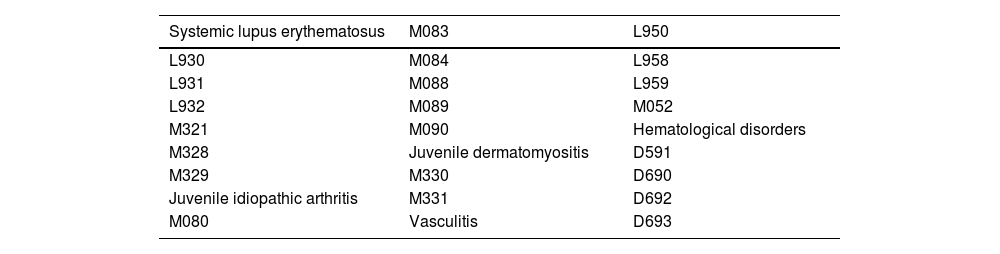
Autoimmune diseasePatients under 15 years of age with a new diagnosis of autoimmune disease (according to the most frequent codes of the International Classification of Diseases [ICD-10] in pediatrics, specified in Table 1), made by a specialist in pediatric rheumatology, between September 2018 and September 2021 in three referral centers in Huila.
ICD-10 codes used.
| Systemic lupus erythematosus | M083 | L950 |
|---|---|---|
| L930 | M084 | L958 |
| L931 | M088 | L959 |
| L932 | M089 | M052 |
| M321 | M090 | Hematological disorders |
| M328 | Juvenile dermatomyositis | D591 |
| M329 | M330 | D690 |
| Juvenile idiopathic arthritis | M331 | D692 |
| M080 | Vasculitis | D693 |
International Classification of Diseases: ICD-10.
Multiple diagnosis: individuals with more than one diagnosis of autoimmune disease in the study period.
Exclusion criteriaSARS-CoV-2 infectionSubjects with a second positive result in the first 90 days after a previous infection.
Autoimmune diseasePatients treated with the same diagnosis in more than one of the reference centers included in the work.
Collection techniques and instrumentsData collection was carried out by the main researcher; the information was obtained through the application of an instrument with the variables of interest; the method of collecting data from subjects with autoimmune diseases was the review of hospital databases, using the ICD-10 codes described in Table 1, for which the data bank of a previous work (Uscopedia, Universidad Surcolombiana) was used as information source. On the other hand, the cases of SARS-CoV-2 infection were taken from the databases of the Public Health Laboratory of Huila (Laboratorio de Salud Pública del Huila) and the National Institute of Health (Instituto Nacional de Salud).39 Finallly, for the calculation of the rates used in the work, population data from de National Administrative Department of statistics (DANE, Departamento Administrativo Nacional de Estadística) were used.41
Analysis of the informationThe data were processed in the statistical software IBM SPSS Statistics 22, Standard license by the Universidad de Caldas. Initially, a sociodemographic description of the cases was carried out, for which the quantitative variable age and the qualitative variables month of diagnosis, municipality and area of origin, sex, type of social security and socioeconomic stratum were analyzed. Mean, standard deviation, minimum and maximum were analyzed for age, and absolute values and percentages were applied for qualitative variables. Then, the monthly rates of occurrence of events: positive SARS-CoV-2 tests and incidence of autoimmune disease were calculated, using the pediatric population of Huila under 15 years of age as a common denominator; the monthly percentage of positivity of SARS-CoV-2 was also calculated. The events were distributed into 36 periods for autoimmune diseases (to visualize the variations in incidence before and after the beginning of the pandemic), and into 18 analysis periods for positive SARS-CoV-2 tests and percentage of positivity; the rates were represented graphically in line diagrams, and the curves obtained were analyzed. Finally, the Kolmogorov-Smirnov statistic was applied to determine the normal distribution of the variables, and the Pearson correlation model was used between the detection rate of SARS-CoV-2 and the rate of new diagnoses of autoimmune disease during the period of interest.
Ethical considerationsThe research complied with current regulations on bioethical research and was endorsed by the Ethics Committee of the University of Caldas in Act of approval 017 of 2021; it was carried out in accordance with the fundamental guidelines for research in humans and applying the principles of medical ethics. It is classified in risk category A, “risk-free research”.
ResultsSociodemographic characterizationAutoimmune diseaseUsing 22 ICD-10 diagnostic codes of the most frequent autoimmune diseases in pediatrics, it was found that between September 2018 and September 2021, 94 new cases of autoimmune diseases were diagnosed in children under 15 years of age in the department of Huila. It was found that 58.5% of the subjects were female (n = 55) and that their ages ranged between one month and 14.9 years (X = 8.3 years; SD = 4.9 years); the majority corresponded to the five-year period of 10−14 years (47.9%), followed by the groups of 0−4 years (33%) and 5−9 years (19,1%). The majority of patients (79.8%) came from the urban area (n = 75) and 68.1% belonged to the subsidized health regime (n = 64).
The diagnoses most frequently found were idiopathic thrombocytopenic purpura (45.7%, n = 43), juvenile idiopathic arthritis (16%, n = 15) and systemic lupus erythematosus (14.9%, n = 14). The majority of cases were more frequent in women, such as hemolytic anemia (60% vs. 40%), vasculitis (80 vs. 20%), juvenile idiopathic arthritis (66.7% vs. 33.3%) and systemic lupus erythematosus (85.7% vs. 14.3%); however, ITP (46.5% vs. 53.5%) and other purpuras (40% vs. 60%) were more frequent in men.
SARS-CoV-2 infectionIn September 2021, 4,303 cases of SARS-CoV-2 had been diagnosed in children under 15 years of age in the department of Huila, of which 74.4% were detected by PCR and 25.6% by antigen test. The mean age at diagnosis was 7.9 years, with ages ranging between 0.1 and 14 years (SD = 4.5 years); most patients belonged to the group of 10-14 years (44.2%), followed by the quinquennia of 5−9 years (28.6%) and 0−4 years (27.2%); the distribution by sex was similar in the population (male 50.6% vs. female 49.4%), and the majority of subjects came from urban areas (90.9%) and belonged to contributory (59.8%) and subsidized (26.4%) health regimes.
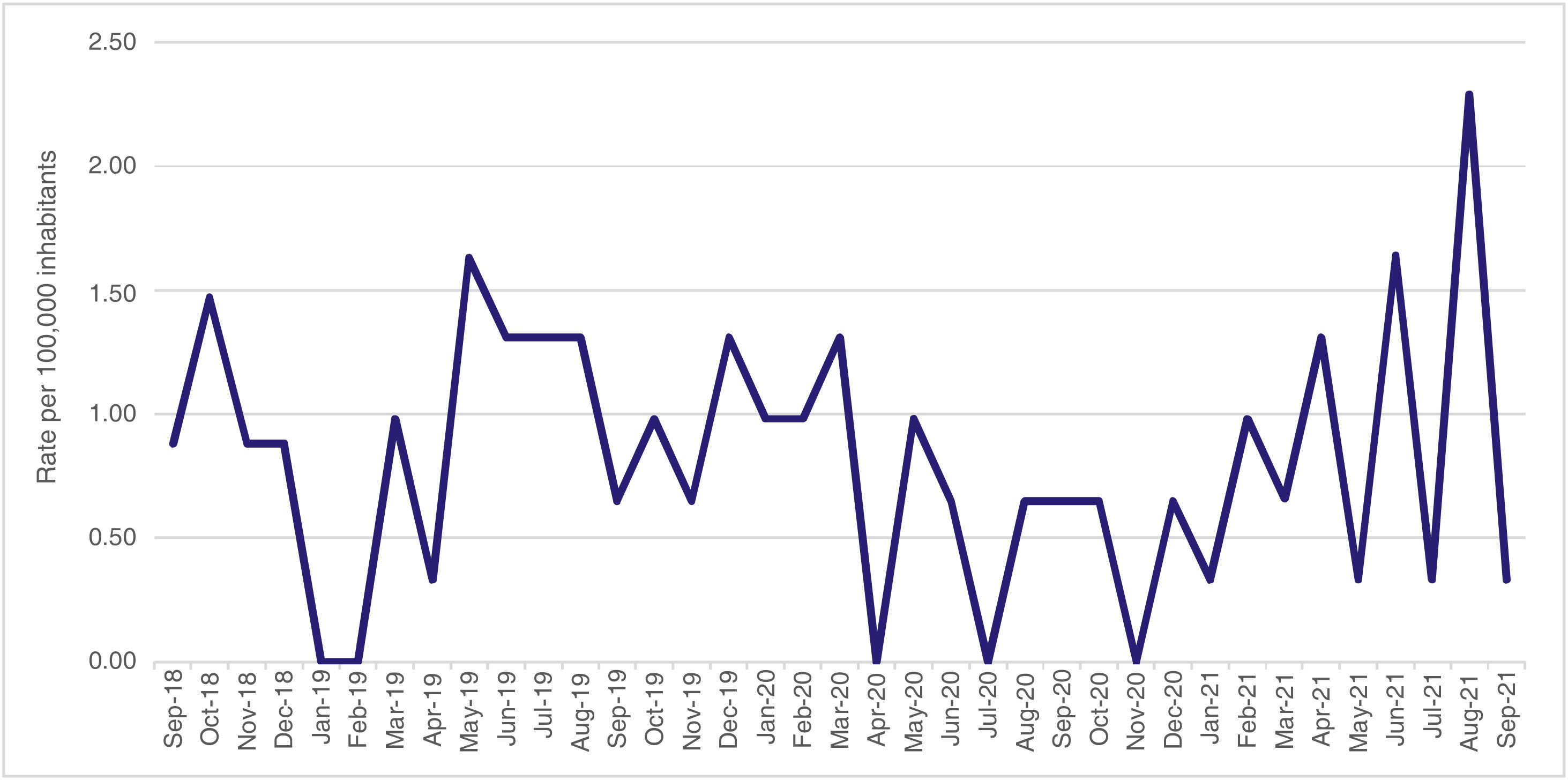
Trend of the rate of diagnoses of autoimmune diseasesThe incidence of autoimmune diseases studied in children under 15 years of age in the department of Huila was analyzed over a period of 36 months, and it was evidenced that the average monthly incidence rate was 0.81 cases per 100,000 inhabitants, with values that ranged between 0 and 2.29 cases during the study period (SD = 0.53 cases per 100,000 inhabitants); no clear trend line was observed; however, in the last year, a clear bimonthly rise in diagnosed cases was evident, up to the highest reported value, which was in August 2021. The highest rates were reported in October 2018, May 2019, June 2021, and August 2021 (Fig. 1).
The highest incidence rates reported during the study period were for ITP (1.30 cases per 100,000 inhabitants in December 2019), SLE (0.88 cases per 100,000 inhabitants in October 2018), JIA (0.65 cases per 100,000 inhabitants in June 2019) and vasculitis (0.65 cases per 100,000 inhabitants in August 2021). No cases of this last event had been reported during the study period before the pandemic.
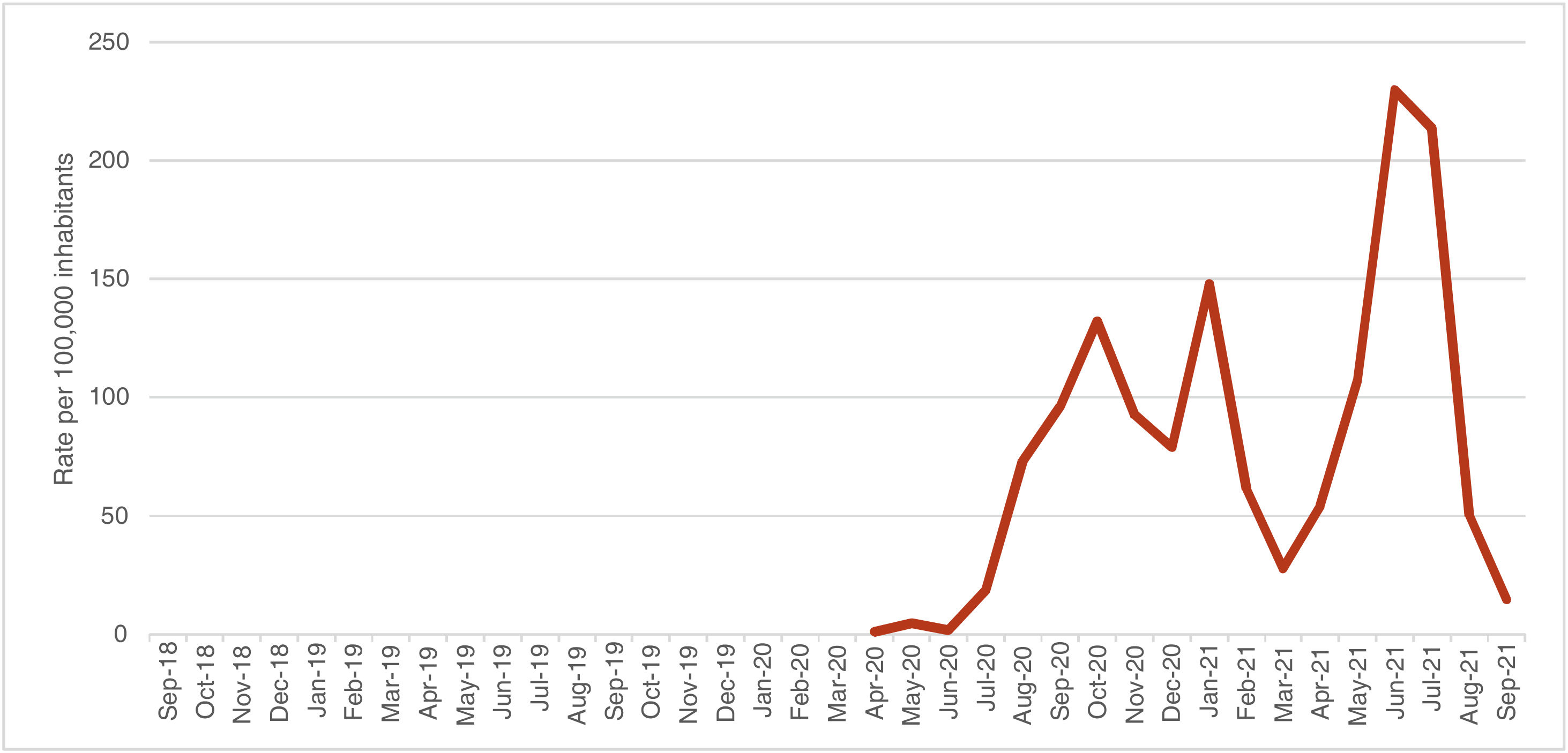
Trend of the SARS-CoV-2 incidence rateThe incidence rate of SARS-CoV-2 in children under 15 years of age in Huila was analyzed over a period of 18 months, and it was found an average monthly rate of 78.22 cases per 100,000 inhabitants, with a minimum value of 1.31 and a maximum of 229.96 cases per 100,000 habitants (SD = 68.29 cases per 100.000 de inhabitants); 4 peaks of incidence were evidenced: October 2020, January 2021, June 2021 and July 2021 (Fig. 2).
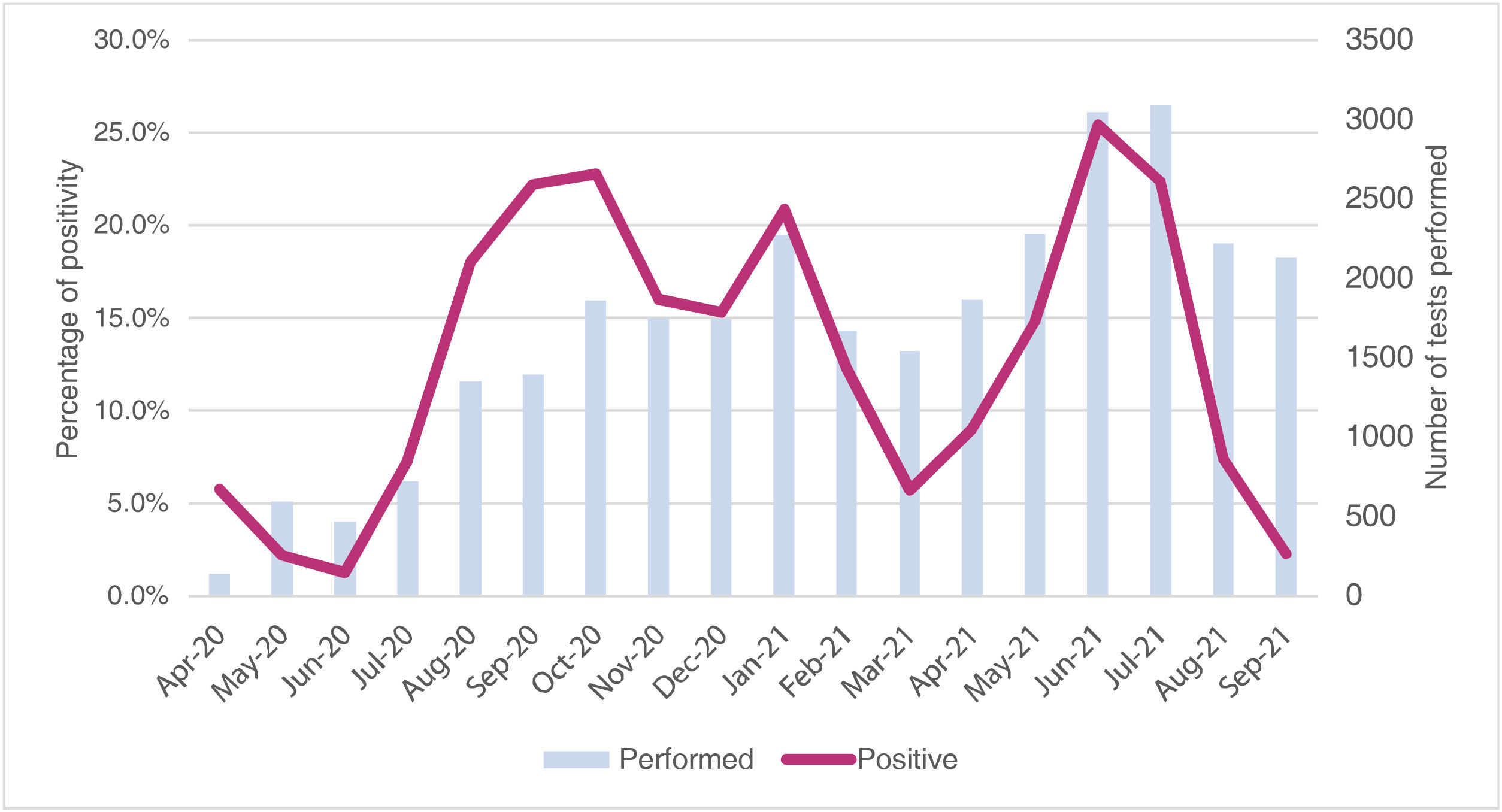
Trend of the percentage of positivity of the SARS-CoV-2 testsBetween April 2020 and September 2021, 30,142 SARS-CoV-2 tests were performed in the department of Huila in children under 15 years of age. They were mostly analyzed by PCR (64,4%), and in second place by antigen test (35.6%); they were carried out similarly in men and in women (52.1% vs. 47.7%), and were performed at an average age of 6.9 years (0.1 and 14.9 years; SD = 4.63 years). The overall positivity percentage was 15.1%, and it was higher with the PCR test than with the antigen test (19.8 vs. 6.7%); it was similar in men and women (14.7 vs. 15.6%) and it was higher in the five-year period from 10 to 14 years (20.5%) than in the groups of 5−9 years (15%) and 0−4 years (10.7%). The monthly positivity trend of the SARS-CoV-2 tests was analyzed during 18 months, and it was found an average monthly positivity percentage of 12.8%, with monthly values that ranged between 1.3% and 25.5% (SD = 7.98%) (Fig. 3).
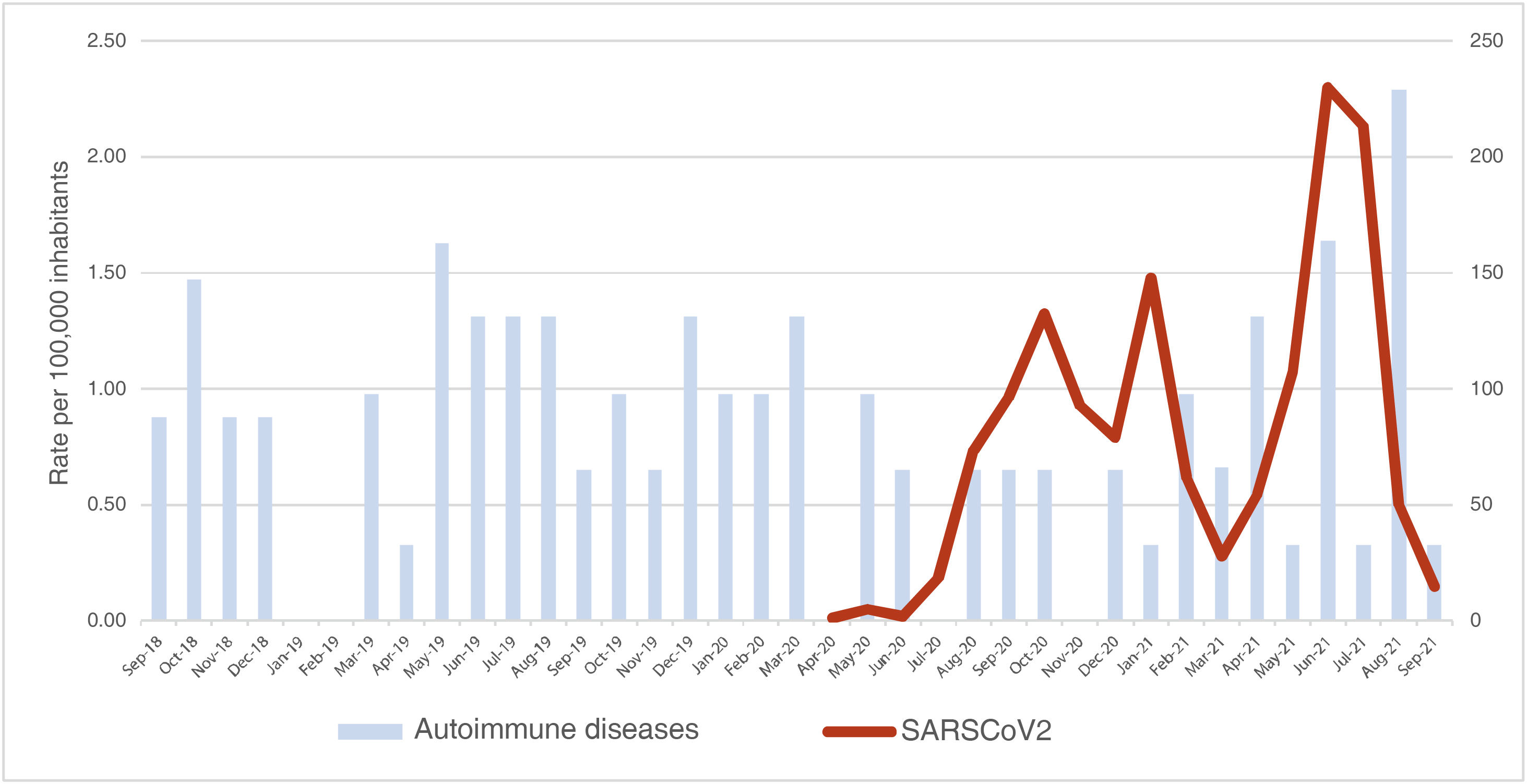
Correlation between the rate of new diagnoses of autoimmune disease and the incidence rate of SARS-CoV-2A correlation analysis was carried out between the monthly rate of new diagnoses of autoimmune diseases and the monthly incidence rate of cases of SARS-CoV-2 in children under 15 years of age in Huila between March 2020 and September 2021, that is, during the 18 months in which there was a concurrence of the two phenomena. The monthly rate of SARS-CoV-2 behaved like a normal variable (p = 0.200) and the monthly rate of new diagnoses of autoimmune diseases did not meet the requirement (p = 0.005); there was no correlation between the rate of diagnoses of autoimmune diseases and the rate of cases of SARS-CoV-2 in children under 15 years of age in Huila during the study period (p = 0.634) (Fig. 4).
DiscussionThis project was developed with the objective of describing the sociodemographic characteristics and the trend in the rates of occurrence of the events: autoimmune disease and SARS-CoV-2 infection in children under 15 years of age in the department of Huila, as well as to clarify the possible temporal correlation between the diagnoses studied according to several hypotheses published in this regard.
In relation to autoimmune diseases, the study demonstrated that the most frequently diagnosed in children under 15 years of age from the department were idiopathic thrombocytopenic purpura, juvenile idiopathic arthritis and systemic lupus erythematosus; in several publications JIA was the most frequent rheumatological entity in children; however, these works did not include ITP.42,43
The incidences reported for these pathologies are between 1 and 6.4 cases/100,000 children for ITP,44 between 1.6 and 23 cases/100,000 children for JIA,33 and between 0.3 and 0.9 cases/100,000 children for SLE.45 In Huila, between September 2018 and September 2021, the rate of new diagnoses of ITP was 14.08 cases/100,000, of JIA it was 4.91 cases/100,000 and of SLE it was 4.58 cases/100,000 children under 15 years of age.
In this population, the autoimmune diseases described were more frequent in women, which is consistent with theories that suggest that physiological variations, sex chromosomes and hormonal changes increase the frequency of these disorders in the female gender.46,47 Conversely, it was identified that ITP was more common in boys, which coincides with the case series of children with ITP from the Intercontinental Childhood ITP Study Group registry, which included more than 2,000 children between 3 months and 16 years of age. This work demonstrated that the men/women ratio is high in infants, and that it decreases as age increases, being a more frequent entity in women during adolescence and early adulthood.48
The majority of the autoimmune disorders found were diagnosed in the age group between 10 and 14 years of age, an event that fits with Voskuhl's theory,47 which suggests that hormonal changes that occur during puberty could explain the increase or decrease in occurrence of rheumatological diseases in adolescents.
With respect to the diagnosis of SARS-CoV-2, 4,303 cases were diagnosed in children under 15 years of age in Huila during the study period, which represented approximately 8.7% of the total cases for the department in that period.49 Some countries, such as China (2%)50 and the United States (5.2%)51 also reported low frequencies in children at the beginning of the pandemic; however, recently, the American Academy of Pediatrics noted that pediatric cases represent approximately 19% of the reported total,52 a difference that could be explained by the fact that, apparently, fewer screening tests were carried out in the pediatric population at the beginning of the pandemic.
In the pediatric population of Huila, the mean age at diagnosis of SARS-CoV-2 was 7.9 years, data that is consistent with several publications in China (7 years),53 Mexico (6.5 years)54 and Australia (6.3 years)55; in this group, men accounted for 50.6% of the cases, and this finding was also demonstrated by the same authors.53,54
Regarding the positivity of SARS-CoV-2 tests, the study allowed us to demonstrate that the positivity was higher for PCR than for the antigen test, as explained by the greater sensitivity and specificity of the first technique56; that it increased proportionally with the age group of the patient, as was evidenced in the United States57; and finally, that it was temporally related to the rate of diagnoses of SARS-CoV-2, as was also demonstrated by publications in the United States58 and in Italy.59
Another point is the role of viruses as triggers of autoimmunity, a fact that began to become evident with the seasonal variation in the appearance of some autoimmune diseases. For example, in previous research such as that of Lindsley,60 in which subjects with JIA at a pediatric arthritis clinic in Kansas were followed-up for 10 years, the researchers demonstrated a clear seasonal variation in the onset of the disease, with a predominance of diagnoses in spring and summer and absence of cases in winter, which corresponded to the peaks of enteroviruses in the United States.
Likewise, there is evidence that manifestations of autoimmunity could emerge long after exposure to the environmental agent, and this could explain why, despite the evidence of the possible correlation between SARS-CoV-2 and autoimmune diseases,61–65 no findings with statistical significance were made in this work. For example, in 1988 Pritchard et al.,66 based on the H2N2 influenza epidemic of 1963, analyzed 41 subjects with JIA in the United Kingdom, and evidenced that 14 of them had been born in 1963. These subjects had high values of influenza antibodies and developed the manifestations of JIA in 1977, just after another influenza epidemic. In this way, they suggested the role of an exaggerated immune response in individuals sensitized in utero, which would lead to considering the probability of the appearance of late-onset autoimmune disorders after the long COVID-19 epidemic.
In Huila, to analyze the trend in the appearance of autoimmune disorders, curve smoothing trials were carried out using moving averages of amplitude 3 and 6 and the Holt’s model; however, these trials showed that the event did not have predictive capacity during the study period, due to the lack of sufficient seasons and the low number of cases detected. Despite this, two events with clinical significance were graphically highlighted, namely: the first is that cases of vasculitis that had not been diagnosed before the pandemic began to be registered just after the start thereof, with a maximum peak between August and September 2021; and the second event corresponds to the graphic evidence that the highest peak of autoimmunity recorded during the months of the study (August 2021) occurred just after the highest curve of SARS-CoV-2 since the beginning of the pandemic (June 2021). Even though this could correspond to new cases of autoimmune diseases triggered by the infection, other possible explanations should also be considered; for example, that these were old undetected cases, whose symptoms exacerbated by the infection led to the first diagnosis of the disease; or, secondly, that confinement led to a decrease in consultations at the beginning of the pandemic, with a subsequent increase in cases due to accumulated diagnoses.
As an additional point, in relation to vasculitis associated with SARS-CoV-2, it has been widely described in patients of all ages, taking into account the state of inflammation, endothelial dysfunction and apoptosis caused by the virus67; however, in the five patients from Huila it was not possible to establish this association, because only one individual had a PCR positive for the virus, antibodies were tested in only two of them, which were negative, and none of them had a related epidemiological nexus.
This is the first work conducted in the country in the line of research on SARS-CoV-2 and development of autoimmunity in children, and perhaps one of the first in this line of work worldwide. It stands out for being a simple, low-cost and easy-to-apply work, relevant for dealing with an event of interest in public health, which included a significant sample of data and allowed us to reach firm results that are comparable with the literature. Among the limitations we should mention: the lack of consensus in the current classification of autoimmune diseases; some difficulties implicit in the study design, such as ecological fallacy and aggregation bias, and, finally, a possible underestimation of cases because of those few patients diagnosed outside the department.
ConclusionsIn the pediatric population of Huila, autoimmune diseases are not infrequent, the most common being ITP, JIA and SLE, which occur predominantly in school-age girls, except ITP and other purpuras, which are more frequent in boys.
In this work, no trend was found for the rate of occurrence of the autoimmune diseases studied, nor for the detection rate of SARS-CoV-2 in children in the department of Huila; in addition, due to the duration of the study and the lack of sufficient seasons, seasonality could not be calculated either; however, graphically it was evident that the highest number of autoimmunity cases occurred just after the highest peak of SARS-CoV-2 in the department.
The positivity of SARS-CoV-2 tests in the pediatric population of Huila is around 15%, is higher with PCR, increases proportionally to the age of the patient and is temporally related to the highest numbers of SARS-CoV -2 in the region.
No statistical correlation was demonstrated between the rate of positive SARS-CoV-2 tests and the rate of new diagnoses of autoimmune disease in pediatric patients in Huila during the first 18 months of the COVID-19 pandemic.
FundingNone.
Conflict of interestNone.