Random somatic recombination of T cell receptors facilitates the variability of response to foreign antigens. Then, central tolerance occurs, a mechanism designed to avoid exit of autoreactive T cells from the thymus. However, failures in this process can induce the appearance of autoimmunity.
Materials and methodsThis narrative review was conducted through a literature search focused on describing relevant and recent concepts of central tolerance of T cells.
ResultsMultiple cell groups are part of the thymic microenvironment, among them, thymic epithelial cells are responsible for directing development of thymocytes, especially medullary thymic epithelial cells that direct the negative selection process.
ConclusionsFactors that affect thymocytes, the thymic microenvironment, or interaction between them, can lead to development of immunodeficiencies and/or autoimmunity.
La recombinación somática aleatoria de los receptores de células T facilita la variabilidad de respuesta frente a antígenos exógenos. Posteriormente a ella se da la tolerancia central, mecanismo dirigido a evitar la salida del timo de células T autorreactivas. Sin embargo, fallos en este proceso pueden inducir la aparición de autoinmunidad.
Materiales y métodosEsta revisión narrativa se llevó a cabo mediante una búsqueda de la literatura enfocada en describir conceptos relevantes y recientes de la tolerancia central de las células T.
ResultadosMúltiples grupos celulares hacen parte del microambiente tímico, entre los cuales las células epiteliales tímicas son las encargadas de dirigir el desarrollo de los timocitos, específicamente las células epiteliales tímicas medulares que dirigen el proceso de selección negativa.
ConclusionesLos factores que afecten a los timocitos, el microambiente tímico o la interacción entre estos pueden llevar al desarrollo de inmunodeficiencias o autoinmunidad.
The main function of the immune system is the protection of the own against the danger of the foreign. For that purpose, through a process of random somatic recombination of DNA segments, a wide diversity of T cell receptors (TCR)s and B cell receptors is obtained, which allows them to have reactivity against an unlimited number of exogenous antigens. However, the recombination of these receptors can lead to the formation of autoreactive lymphocytes and cause lesions due to autoimmunity.1,2
One of the mechanisms to reduce the risk of lesions due to autoreactivity is the establishment of immunological tolerance, defined as the absence of response to an antigen, induced by exposure to it. Central or thymic tolerance aims to ensure that mature T lymphocytes that leave the thymus are unable to respond to autoantigens.3 The mechanisms for establishing tolerance are not infallible, which leads to the presence of systemic or organ-specific autoimmune diseases in one in every 15 people in the United States.4
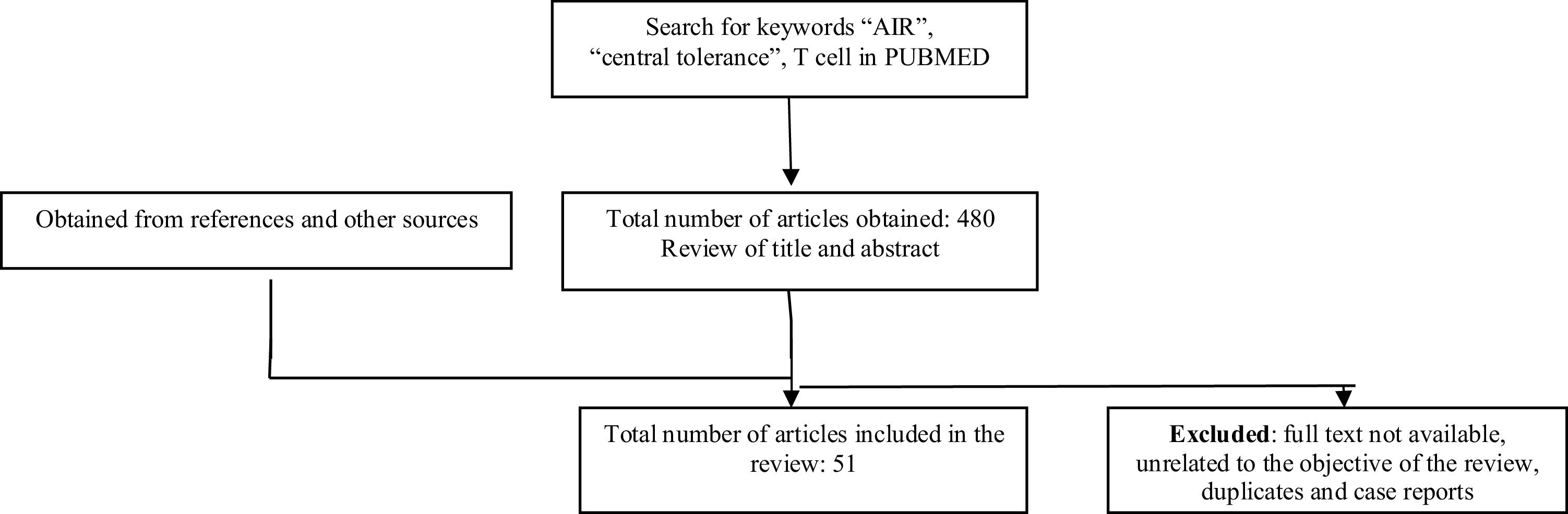
MethodologyA literature search was carried out in the PubMed database, limited to the last 5 years, in which articles in English and Spanish were taken into account. The keywords used were: “AIRE”, “central tolerance” and “T cell”, which were combined with the Boolean term “AND”. The search yielded a total of 480 articles, which included the keywords in their main title or abstract. By reviewing the abstracts, 51 articles were selected. Articles that did not have the full text available or were unrelated to the objective of this review, duplicate studies and case reports were excluded. Some bibliographic references of the selected articles were reviewed (Fig. 1). This narrative review aims to describe relevant and recent concepts of central tolerance of T cells and was carried out following the ethical principles for conducting research in Colombia, as stated in Resolution 8430 of 1993.
ResultsCentral tolerance is a phenomenon inherent to the development of T lymphocytes and takes place in the thymus, which, together with the bone marrow, is considered a primary lymphoid organ. The thymus supports the development and differentiation of several cell types, such as CD4+ T lymphocytes, CD8+ T-lymphocytes, regulatory T cells, γδ cells, and invariant natural killer T cells. Fundamental to this are the stromal niches of the thymic microenvironment, composed predominantly of thymic epithelial cells (TEC) and other groups of stromal cells such as fibroblasts, mesenchymal cells, pericytes and endothelial cells. Among them, the TEC predominantly and fundamentally control the development of thymocytes, since their entry until they leave the thymus as mature T cells,5,6 this being the primary objective of the thymic function. A description of the cellular and functional components involved in the central tolerance mechanism is provided below.
ThymocytesT-cell precursors face a differentiation process that takes weeks, which begins in the bone marrow, where lymphoid specificity is expressed early, and ends in the thymus, where they commit to the T cell lineage. These lymphoid progenitors that originate from the hematopoietic stem cell undergo a progressive process of differentiation upon entering the thymus at a stage known as early thymic progenitor. The lymphotoxin (LTα1β2/LTβR axis) produced by single positive (SP) thymocytes induces the expression of adhesion molecules such as P-selectin, ICAM-1 and VCAM-1 in the endothelial cells of the thymic portal, and in the TECs, the production of the chemokines CCL19, CCL21, CCL25 and CXCL12, which facilitate the thymic homing of lymphoid progenitor cells.7 The subsequent differentiation of these progenitor cells is mediated by the ability to express and respond to Notch signaling.8
The T cell precursors enter the thymic corticomedullary junction and progressively migrate towards the subcapsular region, guided by a complex network of chemokines, among which CCL25 (which is recognized by the CCR9 receptor) stands out, which allow interaction with the thymic stromal cells and the progressive development of thymocytes. During this phase of development, immature double-negative thymocytes (CD4– CD8–) receive survival signals from cytokines such as interleukin -7, stem cell-stimulating factor (SCF) and delta-like ligand 4 (DLL4), produced by cortical TECs (cTECs).8,9 Upon reaching the subcapsular region, the double-negative thymocytes are in stage 3 (Linlo CD44lo, Kitlo, CD25+), in which they begin the rearrangement of the TCR loci and achieve differentiation into double-positive (DP) cells (CD4+ CD8+ ) of αβ lineage. These are the most abundant cells in the thymus8 and undergo positive selection and restriction from the major histocompatibility complex (MHC) (which is mediated by cTEC) to subsequently reach the SP T cell state (CD4+ or CD8+).9
SP thymocytes, after positive selection, express the CCR7 receptor, which, guided by the chemokines CCL19 and CCL21, allows migration to the thymic medulla, where it undergoes negative selection9 mediated by the antigen-presenting cells of the thymus. This entire process, from positive selection, migration to the thymic medulla, negative selection and emigration of naïve T lymphocytes, takes a relatively short time, 4 to 5 days.10
Antigen-presenting cellsTECs are non-hematopoietic cells that originate in the endoderm of the third branchial arch and are the most abundant stromal element in the thymus. Fundamentally, these cells support thymopoiesis and lineage differentiation of T lymphocytes.11 Considering their anatomical position and functional qualities, the TECs are classified into two types: cTECs and medullary TECs (mTECs). cTECs are predominantly involved in the process of development and differentiation of thymocytes, where they fulfill some fundamental functions, such as the settlement of lymphoid progenitors, lineage commitment of lymphoid progenitors to T cells, expansion of immature thymocytes, positive selection and MHC restriction of DP thymocytes.6,12 Considering the multiple functions of cTECs, it has been proposed that there are different functional subpopulations, and as an example of this, thymic nurse cells that form multicellular complexes and favor the rearrangement of the α chain of the TCR have been described.6
mTECs fulfill two fundamental functions in the induction of central tolerance: negative selection of autoreactive T cells and generation of regulatory T cells.12 These functions are facilitated by their exceptional capacity to promiscuously express tissue-restricted antigens (TRA) and present them via MHC II molecules to SP thymocytes.12 The expression of TRA is facilitated by the presence of genes such as the autoimmune regulator (AIRE) and the embryonic forebrain zinc finger-like protein 2 (FEZF2) gene.2 Together, these two transcriptional regulators mediate 60% of gene expression in murine mTECs, being these cells able to express around 89% of protein-coding genes; that is, approximately 20,426 genes. This diverse gene expression is supported by post-transcriptional modifications such as the use of alternative splicing and the expression of endogenous retroelements.13
The growth and differentiation of mTECs depend on the activity of the Forkhead Box N1 (FOXN1) transcription factor, which under conditions of deficiency leads to athymia and underdevelopment of mTECs, which in turn cannot recruit hematopoietic precursors.14 The activity of this transcription factor is positively regulated by the classical and non-classical pathways of WNT glycoproteins.15 The development of mTECs is dependent on signals provided by SP thymocytes after positive selection, which importantly mediate the activation of the nuclear factor kappa B (NF-κB) signaling pathway, as is the case of lymphotoxin B, CD40 ligand and RANK-RANKL pathway.6,14,16,17 The LTα1β2/LTβR axis stimulates the development of the mTEC FEZF2+ subpopulation and, in addition, of the CCL21+ mTECs.7 The RANK-RANKL (membrane-bound) signaling pathway has the capacity to activate the canonical and non-canonical NF-κB pathways, which in turn stimulate the proliferation of mTECs and increases the expression of the AIRE gene.6 In line with what has been described, it has been documented a decrease in mTEC count and the presence of manifestations of autoimmunity in mouse models deficient in the RelB component of NF-κB.18 Other determinants of mTEC development are the mTOR signaling pathway, STAT3 and the epithelial-mesenchymal transition factor.14
Antigen presentation in the thymus and regulation of negative selection are not exclusive to mTECs. Dendritic cells are involved in the presentation of ubiquitous antigens and transferred by mTECs, in addition to directing negative selection and clonal deviation.9,12,19 The B cells of the thymus act as antigen-presenting cells; however, through CD40 costimulation from thymocytes, are licensed for the expression of AIRE and to allow the expression of TRA, in a similar way to what is described in mTECs.19,20
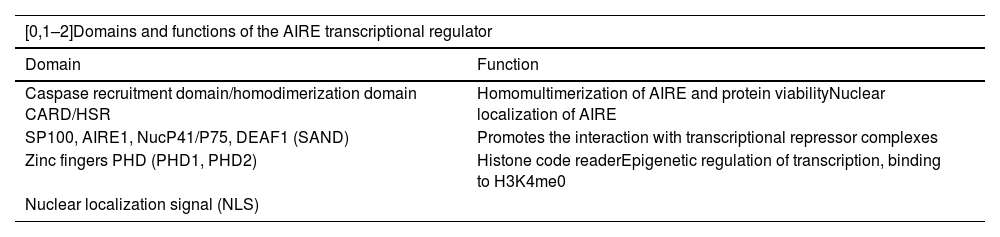
AIREIn the study of the autoimmune polyglandular syndrome type 1, the AIRE gene, located on chromosome 21 at position 21q22.3, was discovered.21,22 This gene is made up of 14 exons and generates the expression of a protein of 545 amino acids, with a molecular weight of 57,727 Da, which in turn has four domains: CARD, SAND, PHD1 and PH2 (Table 1), in addition to two nuclear localization signal regions and four LXLL motifs.23 AIRE is expressed only in mTEChi(2) and is considered a pleiotropic gene, mainly because, from this single gene sequence, it achieves control of different functions, among which the following stand out: functional marker of mTEC differentiation, control of the expression of peripheral tissue antigens, regulation of the differentiation of regulatory T cells, in addition to mechanisms of B cells tolerance, control of mTEC-thymocyte adhesion and mTEC apoptosis.24
Domains of the AIRE gene.
| [0,1–2]Domains and functions of the AIRE transcriptional regulator | |
|---|---|
| Domain | Function |
| Caspase recruitment domain/homodimerization domain CARD/HSR | Homomultimerization of AIRE and protein viabilityNuclear localization of AIRE |
| SP100, AIRE1, NucP41/P75, DEAF1 (SAND) | Promotes the interaction with transcriptional repressor complexes |
| Zinc fingers PHD (PHD1, PHD2) | Histone code readerEpigenetic regulation of transcription, binding to H3K4me0 |
| Nuclear localization signal (NLS) | |
AIRE is a protein of nuclear localization25 and is considered an atypical transcription factor, since it does not have DNA-binding motifs by itself. To promote promiscuous expression of TRA requires acting in association with multiple proteins. The AIRE-PHD1 domain has the ability to recognize the repressive epigenetic marker unmethylated histone H3 lysine 4 (H3K4); however, the integration of a protein complex is needed to achieve the gene expression.26 Among the associated proteins, we can mention the ATF7IP-MBD1 complex, which, in turn, associates with the histone methyltransferase ESET, that allows the recruitment of methylated CpG dinucleotides. In addition, AIRE interacts with the DNA protein kinase that recognizes DNA double-strand breaks, where AIRE is recruited, and has been associated with the initiation of transcription. Finally, AIRE cooperates with the positive transcription elongation factor B (P-TEFb) to achieve the release of stalled RNA polymerase and facilitate RNA elongation.26
The expression of the AIRE gene is regulated in a multifactorial way. Receptors of the tumor necrosis factor family, with the positive regulatory activity of the CD40 and the RANK pathway,2,25 which facilitate the expression of this regulator in mature mTECs stand out. AIRE interacts with nuclear proteins that exert control over its activity, among them the protein deacetylase SIRT1, which deacetylates lysine residues in AIRE and enables AIRE-mediated transcription. In addition, the interaction with the CREB-binding protein, which has acetyl transferase activity, generates a downregulation of AIRE activity. HIPK2 protein decreases the kinase-dependent transactivation activity.26 Human and murine models have documented a differentially higher activity of the AIRE gene in mTEC of male individuals, with no differences in the cell count or in the mTEC/cTEC ratio with respect to female sex. This difference between the sexes appears to depend on the activation of the androgen receptor,27 which is probably an additional factor that explains the higher risk of autoimmunity in the female sex.
Due to its main role in the expression of TRA, AIRE is a determining factor in the negative selection of T cells for the establishment of central tolerance, but it also intervenes in the development and differentiation of mTECs, in addition to mediating the positive selection and differentiation of autoreactive thymocytes into regulatory CD4+ T cells.25 Variations in the AIRE gene prevent the presentation of TRA to SP thymocytes, by allowing autoreactive T cells to exit to the periphery, with the consequent potential development of autoimmunity.23,28
AIRE presents genetic variations in around one per 1,000 people.26 Classically, it has been described that these mutations are biallelic, recessive in nature29 and are represented in the classic autoimmune polyglandular syndrome type 1, which is characterized by the presence of autoimmune lesions in different organs, with a predominance of adrenal insufficiency and hypoparathyroidism, together with susceptibility to fungal infections due to the production of neutralizing antibodies, especially against cytokines such as IL-17, IL-22, the IL-17 receptor and, in addition, neutralizing antibodies against type I interferon, which predisposes to viral infections, including severe presentations of SARS-CoV-2 virus infection.30 This syndrome may be accompanied by other manifestations of autoimmunity, such as type 1 diabetes mellitus, autoimmune thyroid disease, autoimmune hepatitis, pernicious anemia, and non-immune-mediated manifestations, such as ectodermal dystrophy, enamel hypoplasia, and nail dystrophy.31
Dominant-negative monoallelic point mutations of AIRE have been associated with increased susceptibility to late-onset autoimmune diseases with an attenuated phenotype and incomplete penetrance.31 These genetic variations of AIRE are primarily located in the SAND and PHD1 domains, being the major Finnish mutation (p.R257) the most frequent worldwide.32 The clinical manifestations of these mutations are heterogeneous, but they can be represented in the non-classical autoimmune polyglandular syndrome type 1.26,32
As we have mentioned, AIRE expression is not exclusive to thymic epithelial cells. In murine and human models, its expression has been heterogeneously documented in the spleen, lymphatic tissue, embryonic liver, testes, ovaries, dendritic cells and macrophages. In these tissues and cells it probably acts with a transcriptional regulatory role.28 The functions of this gene in peripheral tissues are not sufficiently clear; however, some extrathymic cells that express it are capable of inducing tolerance to autoreactive peripheral T cells.28 AIRE also appears to be involved in the regulation of the expression of pattern recognition receptors and MHC molecules on antigen-presenting cells and in the regulation of the induction and differentiation of peripheral T cells.29
FEZF2There are different TRAs with expression independent of AIRE, which implies the presence of different regulators of TRA expression in the thymus. According to this hypothesis, Takaba et al.33 documented the specific and increased expression in mTEC of a transcriptional regulator called FEZF2, comparable with the regulator AIRE, which is expressed in 30% of mTEClo and in almost all mTEChi of the mouse thymus, and, curiously, in AIRE + mTEC. In addition, in Fezf2 knock-out (KO) mice, it was found a significant decrease in the expression of 16 genes in mTEC, in the presence of normal regulation of AIRE. This finding was accompanied by inflammatory infiltration in the lungs, liver, kidneys, small intestine, salivary glands, brain and testicles. The inflammatory involvement in Fezf2 KO mice is broader and different from that documented in Aire KO deficiency and with apparent greater severity. However, to date there are no systemic or organ-specific autoimmune diseases associated with its variation or deficiency.
The FEZF2 regulator contains an engrailed homology 1 (EH1) domain and 6 zinc finger (ZF) domains, which directly recognize specific DNA motifs. It has not been determined which molecules interact with FEZF2 and how it induces the gene expression of TRA in cooperation with other transcriptional regulators.2 Using embryonic murine models of brain differentiation, it has been documented that Fezf2 interacts with corepressors of the Groucho family, and through its repressive activity restricts the expression of lhx2 and lhx9, which encode two negative regulators of WNT/βcatenin signaling.34 The expression of this gene is regulated by LTβR,33 in contrast to the aforementioned regulation for the AIRE gene.
Antigen presentation in the thymusThe presentation of TRA has three fundamental components: antigen expression in the thymus, antigen processing for the arrangement of peptides and the presence of MHC molecules for the anchoring of TRA-derived peptides.35 The promiscuous expression of genes by mTECs, as a source of TRA, and their ability to present antigens to thymocytes, directly or through the transfer of TRA to dendritic cells, are the most well-known mechanism during the negative selection process.36 However, there are other sources of antigens that are infrequently mentioned. Some peripheral autoantigens (not foreign antigens) can be introduced into the thymic microenvironment by immature (non-activated) migratory dendritic cells, which also have the capacity to make antigen presentation and induce clonal deletion of autoreactive thymocytes.37 In the case of ubiquitous antigens, these are produced spontaneously by different cells resident in the thymus and are predominantly presented in the cortex and in the corticomedullary junction by dendritic cells.19 On the other hand, small peripheral antigens could enter the thymus by direct diffusion38 and are presented by dendritic cells. A fifth form of acquisition of peripheral antigens in the thymus was described by Vollmann et al.,38 who documented that peripheral antigens of macromolecular peptide type are taken up by perivascular dendritic cells known as transendothelial dendritic cells.
Antigen presentation in the thymic medulla leads to the survival of non-autoreactive SP thymocytes. In the opposite direction, autoreactive thymocytes are directed to two destinations that define central tolerance: clonal deletion and clonal deviation (gives rise to regulatory T cells). In this way, the repertoires of non-autoreactive T cells and regulatory T cells are generated.25,39 It has been proposed that the balance between these destinations for SP thymocytes is mediated by the affinity of the TCR binding to the MHC II self-peptide complex. The activation of TCR leads to thymocyte survival when low and prolonged calcium flux with low ERK activity is achieved, as well as to cell death when ERK activity is rapid and robust.11 It is currently known that other factors beyond the intensity of the TCR signal are involved, including the type of antigen-presenting cell, the cytokines in the thymic microenvironment, and costimulatory signals.39 It has been documented recently that B7-CD28 costimulation is necessary for clonal deletion and for the generation of regulatory T cells, for which different cytoplasmic domains of CD28 are needed; for example, for the generation of regulatory T cells, the PYAP domain is essential but not the YMNM domain, and in the case of clonal deletion, other domains that are not currently defined are necessary.39
Clonal deviation mediates the production of regulatory T cells, and to do so this subtype of cells must undergo two fundamental steps. First, induced by TCR activity, there is an upregulation of the interleukin 2 receptor, and also of GITR, OX40, TNFR2, which generates the CD4+ FOXP3-CD25+ regulatory T cell precursor. Secondly, this progenitor undergoes a cytokine-mediated conversion, due to the expression of the transcription factor FOXP3, which generates the CD4+ FOXP3+ CD25+ regulatory T cell, which finally emigrates from the thymus.5 Thymic regulatory T cells are characterized by their ability to suppress the activation of CD4+ or CD8+ T lymphocytes, to decrease the expression of costimulatory ligands by antigen-presenting cells, and by the secretion of anti-inflammatory cytokines.4
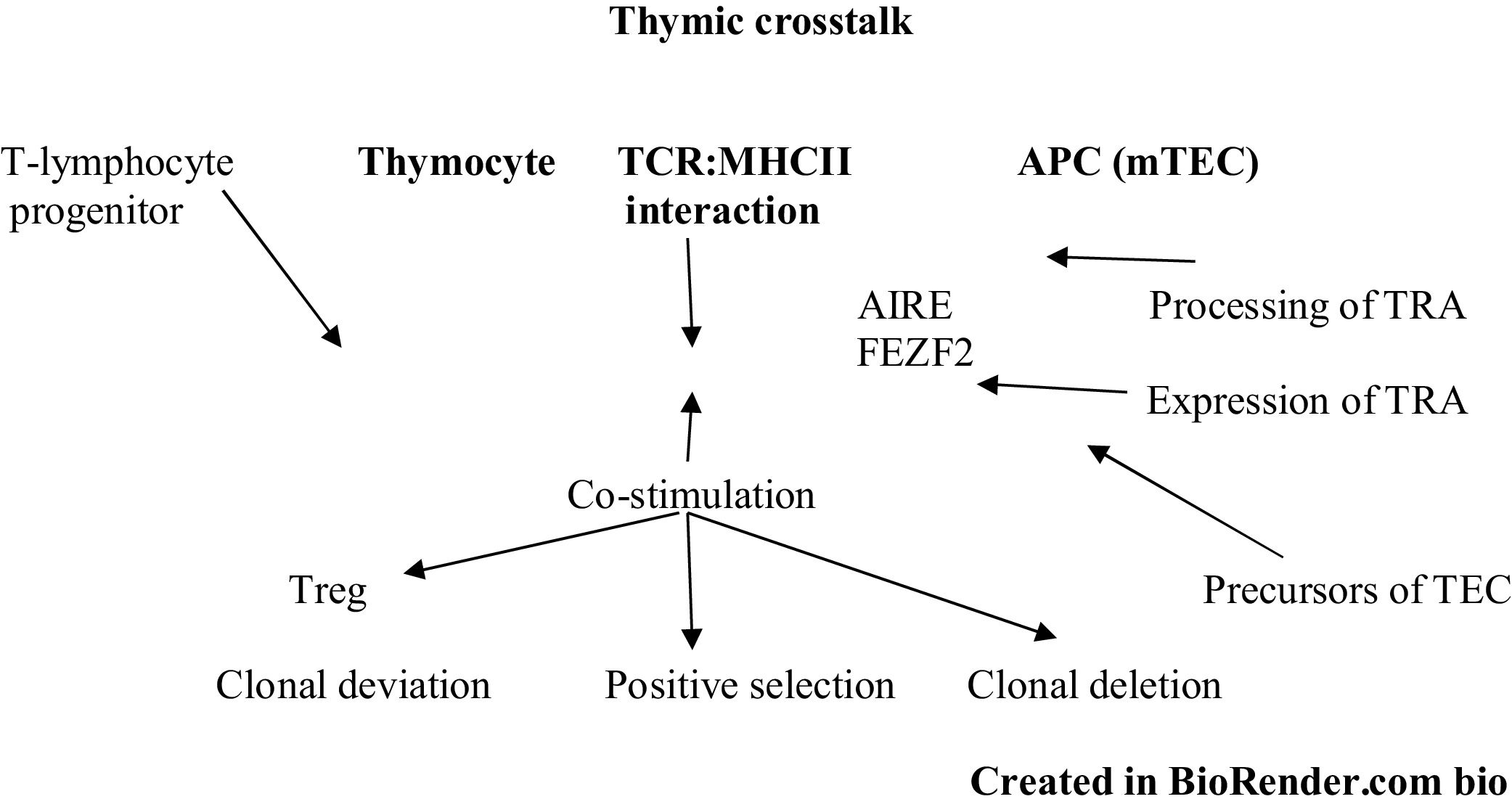
The interaction between mTEC and thymocytes goes beyond clonal deletion and formation of regulatory T cells. In the same way that mTECs have the ability to affect the development of thymocytes, existing evidence demonstrates that SP thymocytes have the ability to induce transcriptional regulators in mTEClo, which lead to their differentiation into mature stages and increase the expression of TRA, chemokines. and adhesion molecules related to the development of thymocytes, a phenomenon known as thymic crosstalk.40 In line with this, independently of the expression of TRA, alterations in the TCR-MHC II interaction facilitate the formation of mature autoreactive thymocytes that can cause autoimmunity,40 as has been documented both in thymic samples from mouse models and in humans MHC II-deficient, in which a decrease in the volume of the thymus medulla, a decrease in the expression of molecules associated with the maturation of mTECs, an attenuation of the expression of AIRE-dependent TRAs and a lower expression of components of the NF-κB signaling pathway related to the development of mTECs are relevant.41
Factors that alter central toleranceThe alterations in central tolerance go beyond the variations in the AIRE and FEZF2 genes that affect the production of TRAs. Summarizing all the concepts illustrated throughout the article, we can say that multiple factors affect thymic crosstalk (Fig. 2), which alters its components, such as: thymocytes (development, differentiation, and response to TCR signaling); antigen-presenting cells and thymic microenvironment (development, maturation of thymocytes, capacity of expression of TRA, antigen processing and expression of MHC molecules), and the interaction of the self-peptide-MHC II complex (MHC deficiencies or absences), which leads in different proportions to immunological deficiencies associated with T-lymphocytes, as well as to manifestations of autoimmunity.
The thymus begins its development and function in fetal life and reaches its greatest volume and production before the first year of life. After that, thymic function decreases rapidly between the first and eighth years of life, followed by a progressive deterioration until old age.42 Probably, associated with this progressive deterioration of thymic structure and function, aging is associated with detriment of the immune function, which represents, in a progressive state of immunodeficiency, the presence of chronic inflammation and the increased risk of autoimmunity evident in the elderly.43 Thymic involution, which is associated with a decrease in the expression of TRA and a less efficient negative selection process, is mediated by factors such as changes in the thymic microenvironment and impaired development of T cells.43 In murine models, aging remodels the thymic epithelium, decreases the differentiation of precursor cells into mTEC, attenuates promiscuous gene expression,44 and is also associated with defective negative selection of polyclonal T cells, due to loss of the efficiency of clonal deletion of autoreactive SP thymocytes.45 The regenerative capacity of the thymus has been proposed, but functional alterations in negative selection did not improve when thymic atrophy was delayed in transgenic mice.45 The decrease in negative selection mediated by aging is compensated with an increased production of regulatory T cells.42
Early changes in thymic structure and function may be related to an increased risk of autoimmunity and serious infections, as is the case of individuals with thymic aplasia and DiGeorge syndrome, in whom there is an altered preconceptional thymic structure and function. Conversely, when adequate thymic development is achieved at the end of pregnancy, there is no evidence of an increase in said risk, at least in the medium term, as is the case of neonatal or early thymectomy associated with cardiovascular surgery.42 Likewise, viral infections in early life stages (gestational and postnatal) with thymotropic viruses have been linked to defects in T cell-mediated immunity. In murine models, an association has been found between infection with murine thymotropic roseolavirus in the early postnatal stage, with the appearance of autoimmune gastritis in late stages of life, without chronic infection being documented.46In vitro mouse models have linked infection with the Coxsackievirus B4 enterovirus (both at embryonic and postnatal stages) with alterations that decrease the expression of insulin-like growth factor 2, and secondary to this, a nexus with the appearance of type-1 diabetes mellitus in mice.47
Alterations in antigenic processing in the antigen-presenting cells of the thymus can be reflected in defects in central tolerance.47 Starvation-independent autophagy in mTECs contributes to the processing of endogenous antigens for their anchoring in MHC class II molecules, and their alteration can lead to inflammatory infiltration of peripheral tissues.48. An example of these abnormalities is the variation of the CLEC16A gene, which can modify the negative selection and lead to the appearance of type 1 diabetes mellitus.35,48
MHC molecules and the TCR are the central elements in antigen presentation, both in the thymus and in the periphery. For this reason, variations in any of their components decrease the MHC-TCR binding affinity and alter central tolerance. For example, an MHC molecule less affine to an endogenous peptide may not present it to the SP thymocyte, leading to subsequent peripheral autoreactivity.35 TCR signaling can vary when there are alterations in its regulation, as is the case of variations in the PTPN2 and PTPN22 genes, which, associated with loss of their function, decrease phosphatase activity and increase TCR stimulation, which has been related to changes in the effectiveness of central tolerance.35
Importantly, the processes of thymocyte differentiation and the processes of positive and negative selection depend both on the intensity of the activation of TCR and the bonding time. The capicua transcriptional repressor (CIC), initially documented in Drosophila melanogaster and widely conserved in mammals, regulates the RTK-MAPK signaling pathway, downstream of the TCR receptor.49 It has been documented previously that CIC variations generate autoimmunity phenotypes in murine models, probably by hyperactivation of peripheral T cells.50 However, it has been suggested that this gene mediates the differentiation and selection of T lymphocytes. In a study of murine models, Kim et al.51 demonstrated that CIC regulates the development of double-negative thymocytes and the process of positive and negative regulation, especially at the double-positive stage, associated with a significant attenuation of TCR signaling.
ConclusionsCentral tolerance is a fundamental mechanism for the prevention of autoreactivity and autoimmune diseases. However, it is not an infallible mechanism, since the random recombination of TCRs that gives it a variety of responses against thousands of foreign agents, also makes it susceptible to autoimmunity. Its function is based on the presentation of antigens to thymocytes in the thymus medulla. Thymic stromal cells generate an ideal microenvironment for the development of T cells, and TECs are the most abundant stromal component in this organ. The exceptional ability of mTECs to generate TRAs and present them to thymocytes allows the process of negative selection, which is also possible due to the presence of the transcriptional regulators AIRE and FEZF2. However, these do not explain all the promiscuous gene expression in the thymus, so there are probably transcriptional factors to be investigated and described. Beyond this, the existence of three fundamental components for central tolerance: thymocytes, the thymic microenvironment (antigen-presenting cells and other stromal components) and the TCR:MHC interaction must be recognized. Multiple congenital or acquired mechanisms of metabolic, infectious, or even natural characteristics that compromise the functioning of any of these axes alter central tolerance and favor autoreactivity. Aging as a natural process entails both a structural and functional deterioration of the components of the thymus, among which there is a deterioration of negative selection that is not susceptible to reversal or slowing down with the delay of thymic atrophy. Alterations in the process of central tolerance can contribute, predominantly, to the appearance of organ-specific diseases, but they are not sufficient for their appearance, since there are several control points within peripheral immune tolerance.
FundingNone.
Authors’ contributionAll authors were involved in the conception of the work, the preparation of the initial draft, the editing and the final approved version.
Conflict of interestNone.