The management of complete bone defects in hip and knee periprosthetic infection is still a real surgical challenge.
Material and methodsWe present a technical modification for performing a biarticular total femoral spacer with a femoral nail without the need to approach the proximal tibia.
ResultsThree patients were operated with this technique. There was no intraoperative complication. The infection was resolved in all patients operated at final follow-up. All patients improved their previous functional situation and could walk with different aids.
ConclusionsThis technical modification is an alternative for cases where it is necessary to resect the complete femur, but it is not necessary to approach the tibia.
El manejo de los defectos óseos completos secundarios a una infección periprotésica a nivel de la cadera y/o rodilla continúa siendo un auténtico reto quirúrgico.
Material y métodosPresentamos una modificación técnica para la realización de un espaciador femoral biarticulado con un enclavado femoral, sin necesidad de abordar la tibia proximal, ya sea para obtener la fijación del espaciador o la articulación del mismo.
ResultadosSe han intervenido 3 pacientes mediante esta técnica, no existiendo ninguna complicación intraoperatoria, resolviéndose la infección y mejorando la función previa en todos ellos, volviendo a deambular con diferentes ayudas.
ConclusionesEsta modificación técnica es una alternativa en aquellos casos donde es preciso resecar el fémur completo pero no es necesario abordar la tibia.
Periprosthetic infection is one of the most devastating complications of prosthetic surgery. Two-stage exchange arthroplasty is still the gold standard for treating periprosthetic infection.1–3 Despite the increased popularity of one-stage arthroplasty, principally due to its theoretical lower cost, duration, better functional outcomes, lower morbidity and mortality, and similar cure rates,4 two-stage surgery still achieves better cure rates in specific situations. These situations including multi-operated patients, resistant micro-organisms, polymicrobial infections and in the presence of voluminous bone defects that require tumour implants or massive allografts5 that are increasingly frequent in daily practice.
The implantation of an articulated spacer is particularly complex for cases where the femoral defect is considered massive, once the implant has been removed. This is because the incidence of complications that are routinely observed with the use of spacers (dislocation, peri-implant fracture, and breakage of the spacer itself) considerably increase with this type of bone defect, posing a real surgical challenge for total femoral defects.6
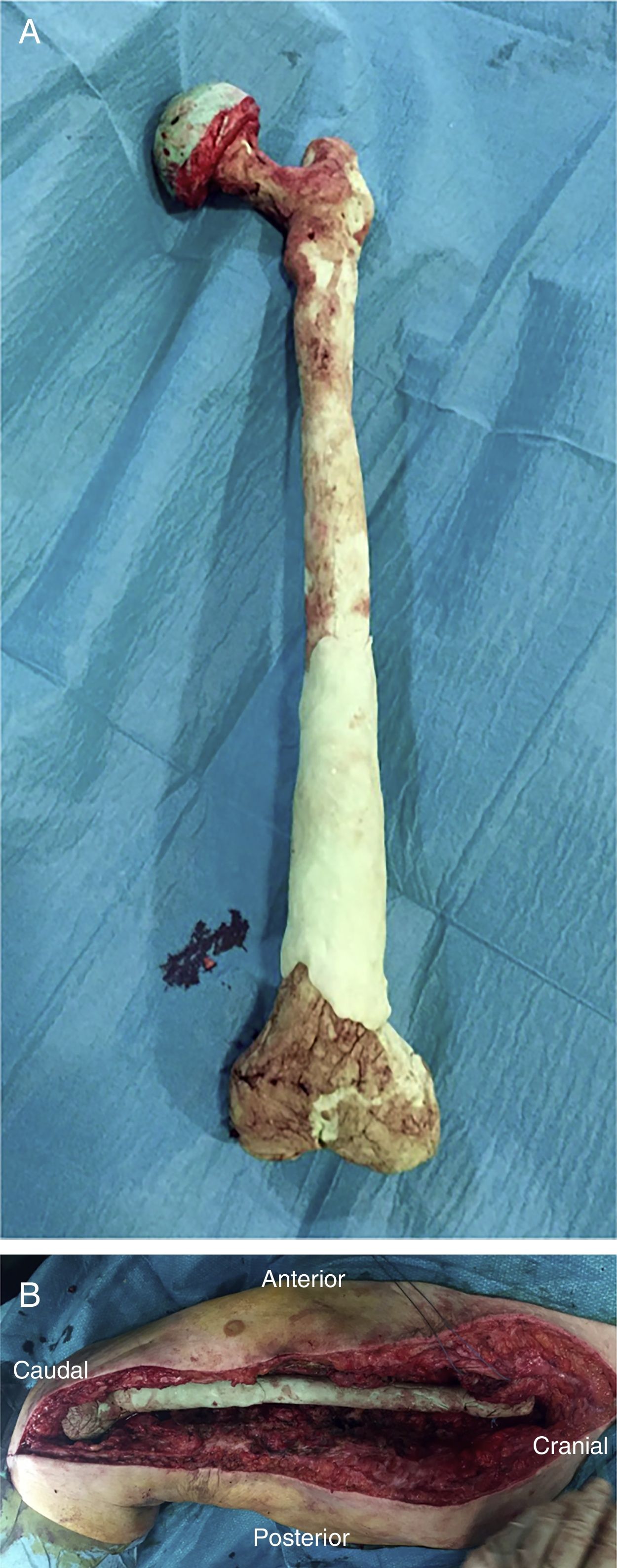
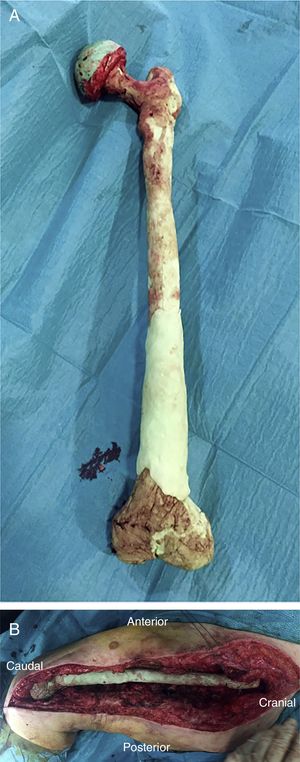
There are currently various different surgical techniques for creating a total femoral spacer, all of which have sacrificing the knee joint in common. In general, these techniques use an intramedullary nail to fix the spacer at the level of the middle third of the tibia, and different “implants” to reconstruct the proximal femur.7–10 We previously described a new technique for a biarticular femur spacer,10 avoiding the need to sacrifice the knee joint. To perform this procedure at the level of the knee, a ball and socket joint is created according to the technique described by MacAvoy and Ries.11 However, this technique involves initial reaming of the proximal surface of the tibia, which can result in a bone defect appearing in the second stage of the surgery. To prevent this bone loss and its associated consequences, we present a modification of the original technique, by manually creating an articulation of the distal femur with the proximal tibia, maintaining both menisci until the second stage (Fig. 1).
Surgical techniquePreparation and approachThe intervention was performed under general anaesthetic with the patient in a lateral decubitus position, enabling access to the hip and knee. It is important when preparing the surgical field to leave the inguinal region free for potential vascular access if necessary during the intervention. At a proximal level, a posterolateral approach is performed, which is the approach the authors use routinely, in addition to a distal extension through the lateral face of the thigh until it becomes an anterolateral approach at the level of the knee. After the superficial dissection, a fascial opening is made between the fascia lata and the gluteus maximus proximally, dissecting the muscle fibres of the latter proximally, and those of the iliotibial band distally reaching the bone plane. Subsequently an L-shaped arthrotomy is performed including the capsule and rotators “en bloc”, mobilising these tissues posteriorly to protect the nerve structures.
After completing the approach, the appearance of the greater trochanter is checked macroscopically, to decide whether part of it is to be preserved, or if it is to be completely sacrificed. If it is preserved, a sliding osteotomy is performed parallel to the longitudinal axis of the femur, leaving a bone plug of the greater trochanter, attempting to maintain the muscular insertions of the gluteus medius and vastus lateralis. If it is not possible to preserve this plug, a subperiosteal dissection is performed releasing the vastus lateralis anteriorly with the help of 2 Hohman-type retractors, taking particular care with haemostasis at the level of the linea aspera.
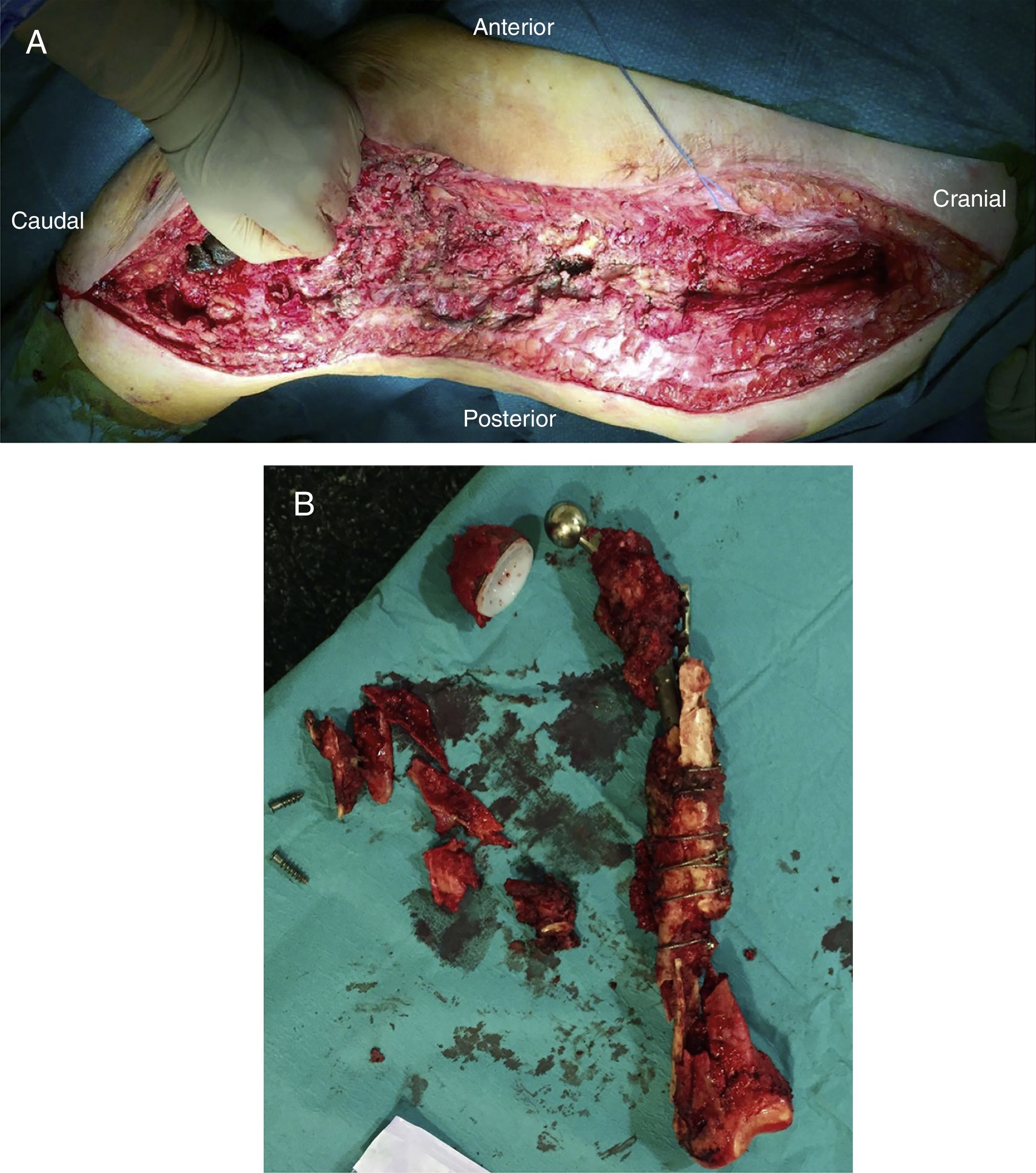
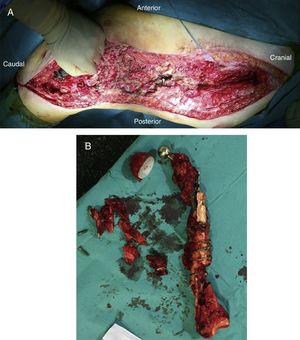
After releasing the entire posterolateral face of the femur, we proceed with the medial dissection, depending on the situation of the femur itself. If the femur is fractured (Fig. 1), the dissection will be performed from its middle area, raising both fragments with a Verbrugge-type clamp, dissecting from the fracture site proximally or distally taking special care to bluntly separate the medial structures in order to be as distant as possible from the vessels. Distally, after releasing the lateral epicondyle, an internal rotation movement is made of the bone fragment, releasing both cruciates in the notch, and finally, the collateral medial ligament at the level of the medial epicondyle, attempting to preserve both menisci.
Once the femur has been extracted, 6 samples are taken for microbiological analysis, along with the explanted implant according to the protocol (Fig. 2A). Then a wide debridement of all the necrotic tissues is performed, until a bleeding bed is created (Fig. 2B), taking special care proximally in the medial area proximally and distally in the posteromedial area to avoid damage to the vascular structures (Fig. 2).
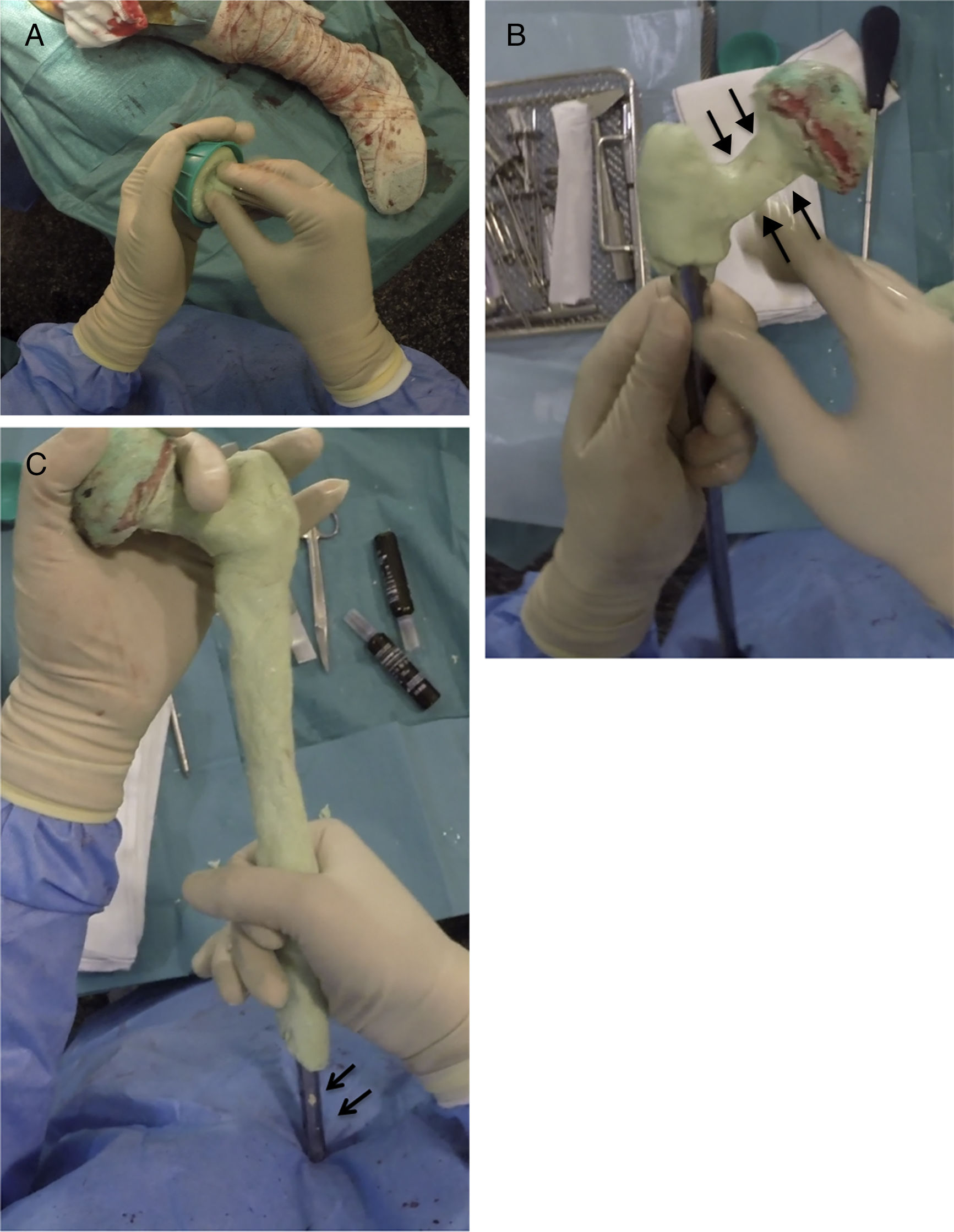
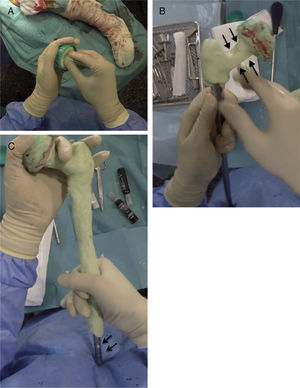
Creating the spacerPrior to the surgery a calibrated radiograph of the complete femur is necessary to decide the length of the implant to be used in the reconstruction. A cephalomedullary nail is used to create the “skeleton” of the spacer, which is at least 6cm shorter than the previously measured femur. The cephalic screw used is usually 75–85mm in length depending on the necessary offset, it is important that the screw does not protrude in the lateral face of the nail to prevent complications when it comes to closure. Once the nail has been mounted, its femoral head is created, using the bulb of a flush syringe as a mould (Fig. 3A). It is important to achieve the best possible head–neck relationship, and completely cover the proximal and middle area of the nail with antibiotic-loaded cement, which will depend on the microorganism causing the infection (Fig. 3B). It is important to leave the distal area of the nail uncovered during this stage to prevent cement entering the interior of the nail channel (Fig. 3C).
Sequence for creating the proximal part and spacer body. (A) Creating the femoral head with flush syringe and cephalomedullary nail with cephalic screw. (B) Creating the neck of the proximal area. Note the favourable head–neck relationship (arrows). (C) Coverage of the spacer body, leaving the distal area free (arrows).
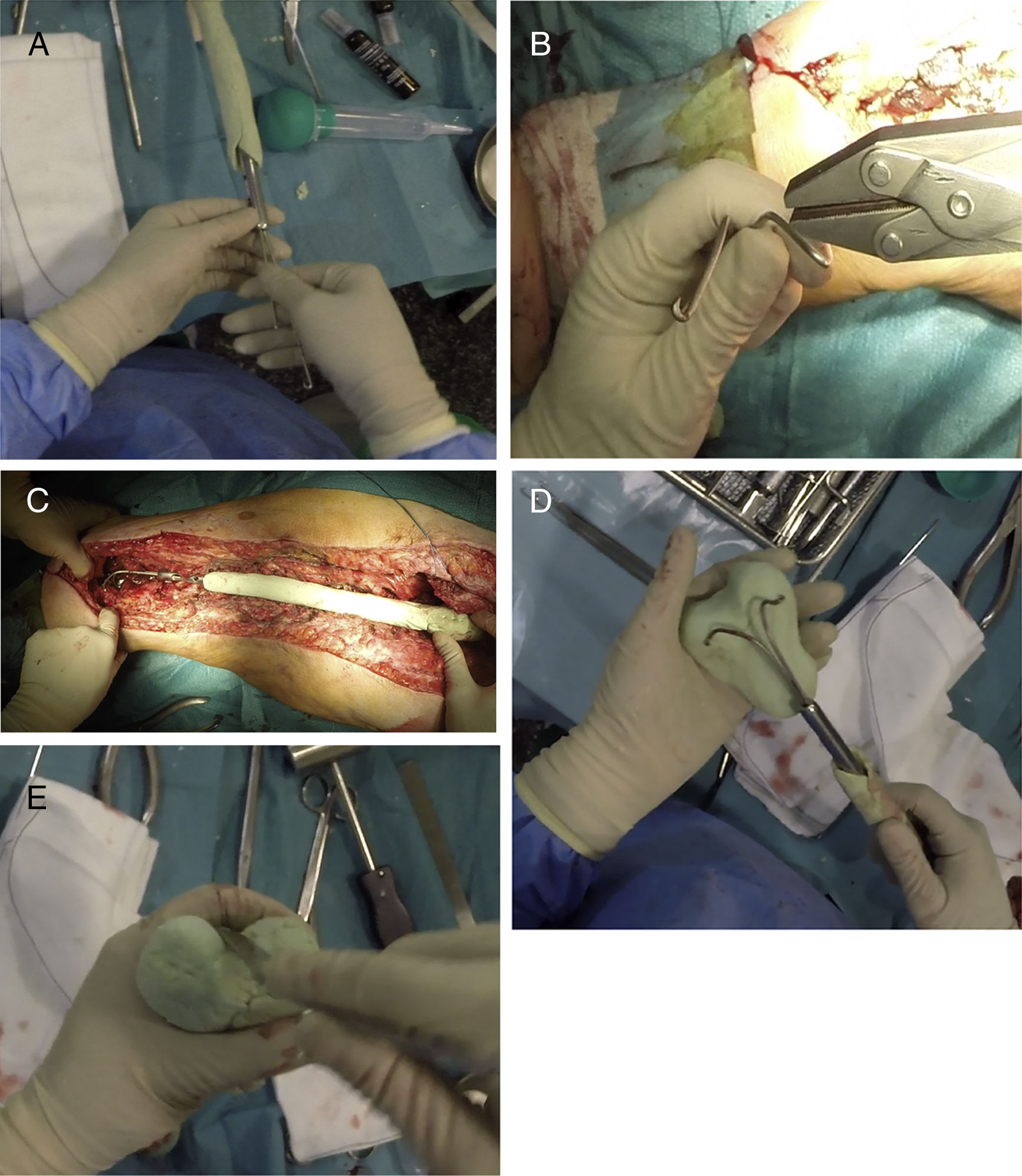
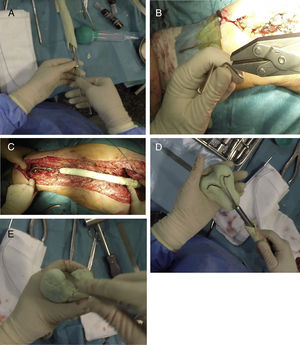
Once the proximal and middle part of the spacer have been completed, 2 Rush-type nails of a small diameter are used, which will be bent as shown in Fig. 4A, inserting them inside the nail, leaving their distal area diverging (Fig. 3B). Before covering these nails with cement, the spacer must be presented in the surgical bed to check that its length is appropriate (Fig. 4C). When we are sure that the spacer is the correct length, these nails are covered with more antibiotic-loaded cement, attempting to imitate the anatomy of the distal femur, with special emphasis on the shape of the posterior condyles (size and offset) and on the femoral trochlea (Fig. 4D and E). It is also important to pay particular attention to the anteversion of these condyles, it is recommended that they are created placing the femoral neck at 20°–30° to the work table. At this time, perforations can be made in the lateral faces of the femur in order to re-anchor the collateral structures, although in the experience of the authors, this is not necessary.
Sequence for creating the distal area of the spacer. (A) Inserting the Rush nails inside the femoral nail. (B) Curving the nails to serve as a “skeleton” for the distal joint. (C) Presentation prior to creating the distal area to “fine-tune” the length of the spacer. (D) Coverage of the Rush nails with antibiotic-laden cement attempting to achieve the shape of a distal femur. (E) Finish of the distal area, placing special emphasis on the trochlea and the femoral condyles.
After creating the spacer (Fig. 5A), it is reduced in the surgical bed, first distally making both condyles coincide with both menisci, and then proximally (Fig. 5B). After checking the stability of the components and that 90° flexion in both joints is possible, a closure in layers is performed, as in any hip/knee approach.
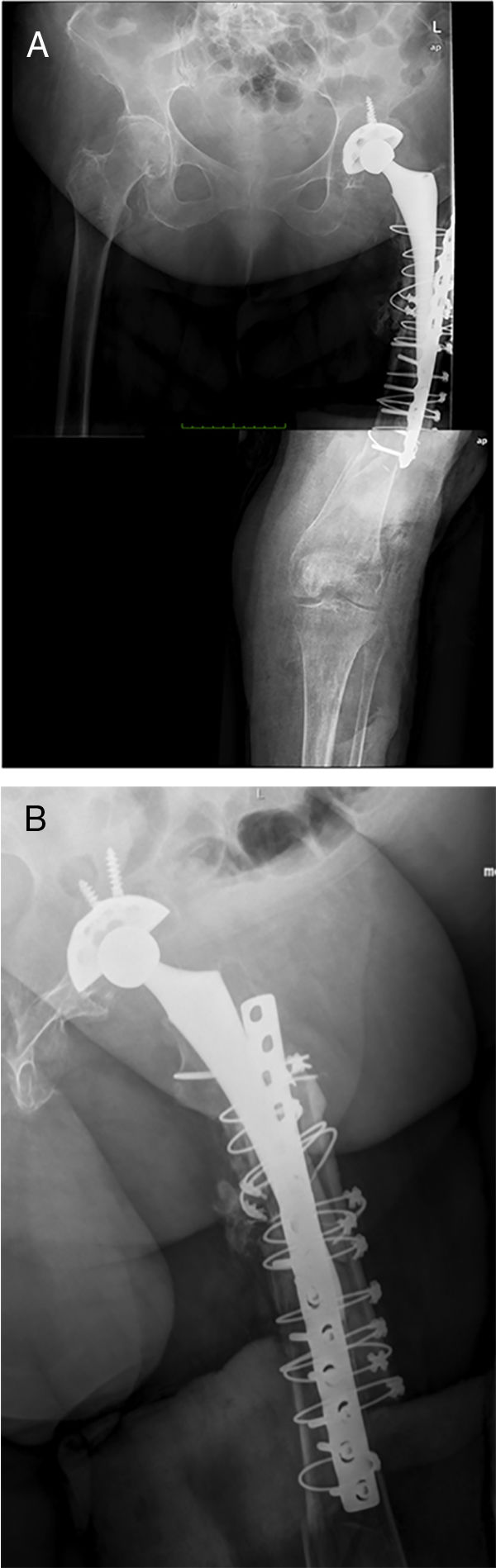
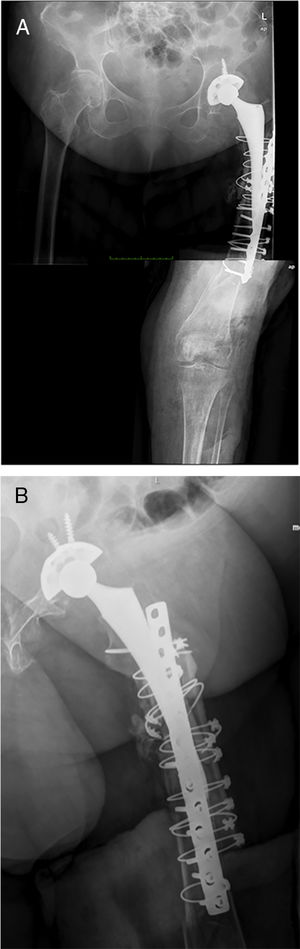
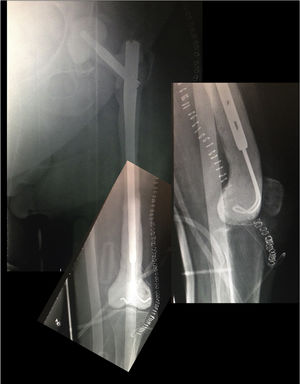
Postoperative periodA radiographic check is carried out after 24h (Fig. 6), to check that the spacer is correctly positioned, and the patient is allowed to sit down, insisting on passive and active flexo-extension exercises of the knee and hip. Partial weight-bearing is allowed, the use of an orthesis at the knee will be necessary to control any residual instability, but is not necessary at the hip.
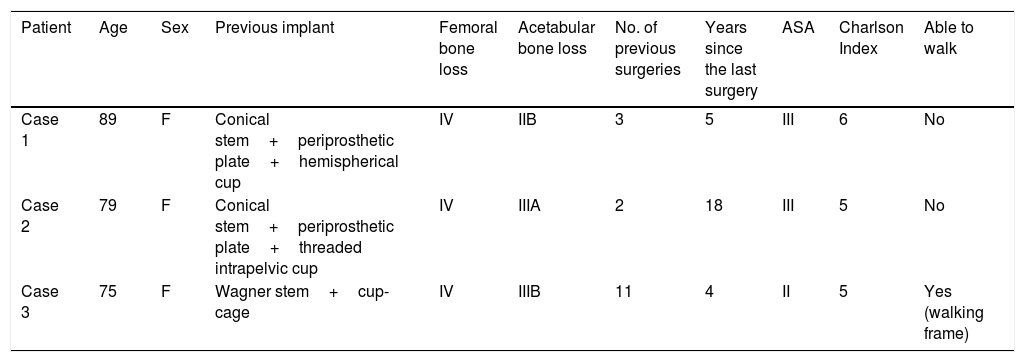
ResultsThree patients underwent this technique with a minimum follow-up of one year after the second stage. The mean age of the patients was 81 (75–89), and they were all female. All the patients had high morbidity, as shown by the high Charlson Index (5 in 2 patients, and 6 in one patient) and preoperative ASA score (2 patients with ASA III, and one ASA II). The mean number of previous surgeries was 5.3 (2–11), the surgical indication was the presence of a periprosthetic infection over a long stem in 2 cases, and a periprosthetic fracture in one patient with chronic suppressive treatment of a previous periprosthetic infection. All the patients had type IV femoral defects that justified using this technique, the associated acetabular defect was type IIB, IIA and IIIB according to Praposky's classification. Only one patient was able to walk with a frame beforehand, the remainder used wheelchairs continually (Table 1).
Demographic data.
| Patient | Age | Sex | Previous implant | Femoral bone loss | Acetabular bone loss | No. of previous surgeries | Years since the last surgery | ASA | Charlson Index | Able to walk |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 89 | F | Conical stem+periprosthetic plate+hemispherical cup | IV | IIB | 3 | 5 | III | 6 | No |
| Case 2 | 79 | F | Conical stem+periprosthetic plate+threaded intrapelvic cup | IV | IIIA | 2 | 18 | III | 5 | No |
| Case 3 | 75 | F | Wagner stem+cup-cage | IV | IIIB | 11 | 4 | II | 5 | Yes (walking frame) |
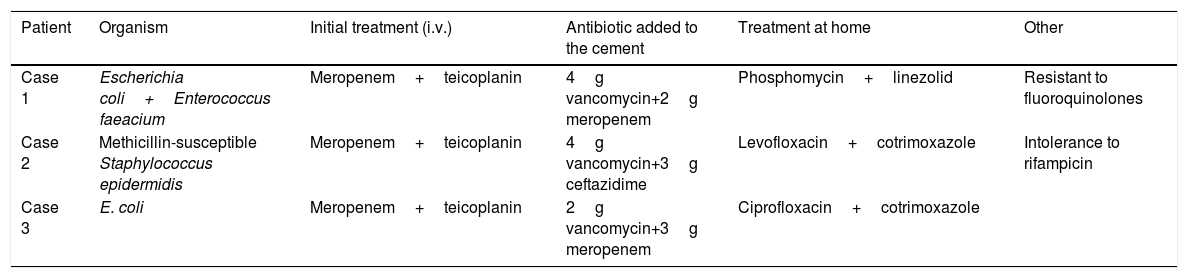
All the patients underwent preoperative aspiration, in 2 cases the cultures were positive for Escherichia coli, and Staphylococcus epidermidis was found in the third case. In 2 cases 4g of vancomycin+2g of meropenem were added to the cement; in the third case, 4g of vancomycin+2g ceftazidime. The oral antibiotic used between the first and second stage is shown in Table 2.
Pathogens and antibiotics used according to the organism identified.
| Patient | Organism | Initial treatment (i.v.) | Antibiotic added to the cement | Treatment at home | Other |
|---|---|---|---|---|---|
| Case 1 | Escherichia coli+Enterococcus faeacium | Meropenem+teicoplanin | 4g vancomycin+2g meropenem | Phosphomycin+linezolid | Resistant to fluoroquinolones |
| Case 2 | Methicillin-susceptible Staphylococcus epidermidis | Meropenem+teicoplanin | 4g vancomycin+3g ceftazidime | Levofloxacin+cotrimoxazole | Intolerance to rifampicin |
| Case 3 | E. coli | Meropenem+teicoplanin | 2g vancomycin+3g meropenem | Ciprofloxacin+cotrimoxazole |
None of the patients had an intrasurgical complication of any type; all of them were able to sit in the first week following the surgeries. There were no fractures or dislocations of the spacer.
The infection resolved in all the patients, defined by negative cultures obtained during the second stage, and the absence of clinical symptoms at the end of follow-up (minimum of one year). All the patients underwent implantation of a total Mega C® femoral prosthesis (Waldemar LINK GmbH, Hamburg, Germany) in the second stage with different acetabular reconstruction systems, and constrained cups were used for all the patients. Spacers at the level of the tibia were not necessary for any of the patients, because there were no bone defects at this level.
At one year following the intervention all the patients were infection-free, and were able to walk with a walking frame (2 patients) and with a crutch (one patient), they are all satisfied with the surgery.
DiscussionAs the number of primary prostheses, revision prostheses and periprosthetic infections continues to grow, the number of two-stage revisions is also increasing.12 Many studies have demonstrated the usefulness of an articular spacer in treating periprosthetic infection, with better rates of infection eradication, facilitating reimplantation of the definitive prosthesis and improving the patients’ functional results.13–16 Commercial spacers are useful for patients with minimal bone loss.17,18 However, bacteria that are resistant to multiple antibiotics or periprosthetic fractures make these spacers unsuitable, particularly for patients with severe bone defects or poor bone quality secondary to multiple surgery; this makes it necessary to create spacers manually according to different published techniques.11,19–21
Despite the theoretical advantages of using a spacer, there are many complications that are inherent to the spacer itself, such as dislocation, peri-spacer fracture, and fracture of the spacer itself.13,19,20,22 There are various factors that encourage these complications. Firstly, a poor head–neck relationship of the commercial spacers, like most of those created manually, is a risk factor for dislocation. A second factor associated with spacer failure is insufficient anchorage in the intramedullary canal, especially in massive bone defects. Leuning et al.22 observed an anchorage of 22±33mm in failed spacers due to fractures (periprosthetic and of the implant itself) compared to anchorage of 57±41mm in the group of spacers with no associated fractures. The use of spacers for massive defects increases the risk of onset of these complications.6,23
Many techniques have been described to create spacers for massive bone defects, using different structures such as the spacer “skeleton”. However, when the defect affects the total femur,7–10 there are only 4 published articles that show how to create a total femoral spacer, and common to all of them is the use of intramedullary fixation at tibial level using a nail as the “skeleton” for the spacer, sacrificing the knee joint between both stages and using a cemented femoral stem, or a PROSTALAC-type spacer proximally to create the proximal part of the spacer. Only Canham et al.7 and Sanz-Ruiz et al.10 have shown an alternative to tibial fixation, by creating a biarticular spacer. Canham et al.7 used 2 commercial spacers, one hip and one knee, that connected using 3 Harrington rods reinforced with cement. Unlike Canham's technique, using a cephalomedullary nail as a “skeleton” for the spacer enables, in addition to choosing the most appropriate femoral head diameter, enables the necessary offset to be determined to maintain appropriate tension of the periarticular structures at the level of the hip, maximising the stability of the spacer itself. Distally creating a ball and socket-type joint increases stability in the absence of ligaments, as demonstrated in different previous series. The solidity of the spacer itself should be added to the abovementioned advantages; it is more resistant to possible failure. This is evident because all the patients who underwent this technique were able to stand with the help of a knee brace. Finally, it is important to stress that the cost of creating a spacer with a cephalomedullary nail and 2 Rush nails is appreciably lower than the technique described by Canham, where the use of 2 commercial spacers (PROSTALAC+commercial knee spacer) considerably increases the cost of this spacer. This is particularly important, bearing in mind that for these patients it will be necessary to implant tumour implants of total femur in a second stage, with costs that are generally very high.
In sum, in this article we present the advantages and possible disadvantages of a technical modification in the distal joint of the previously published biarticular spacer.10 This modification enables the tibial bone to be preserved in patients who, due to the reason for the surgery, do not require it to be resected. In all the patients operated using this modification, the same postoperative protocol was used as for patients undergoing the conventional technique (ball and socket), there were no dislocations of the spacer, although all the patients required a stabilising orthesis in order to stand. In our opinion the possibility of avoiding reaming of the tibia makes the surgery itself less aggressive, reduces postoperative pain to the knee, and completely prevents metaphyseal bone defects at the level of the knee in the second surgical stage. The absence of tibial bone defects in the second stage reduces surgery time in this process, since it avoids the need to reconstruct these defects, and the need for complex reconstruction systems, such as cones, sheaths, etc., with the consequent economic savings.
This article is not free from limitations. The first is the few patients who underwent this technique, which makes drawing clinical conclusions practically impossible. Nonetheless, because there are only 6 published cases of distal femur spacers, we believe that the small size is not a limitation for applying this surgical technique for exceptional situations such as a massive femoral defect in the context of a periprosthetic infection. The second limitation is the short follow-up period of the patients we present (minimum of one year). However the follow-up of these patients is not the aim of this manuscript. We sought to present the safety of a modification of a surgical technique that has been described previously, and its theoretical advantages over the previous technique; there should be no differences in infection control compared to the original technique.
ConclusionModifying the distal joint in a biarticular spacer is useful for treating total femoral defects in the context of a periprosthetic infection when it is not necessary to resect tibial bone, reducing the existing bone defect in a second stage. However these assertions should be viewed with caution given the low number of patients who underwent this technique.
Level of evidenceLevel of evidence V.
FinancingWe had no source of financing.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sanz-Ruiz P, Matas-Diez JA, Calvo-Haro JA, Solans MC, Vaquero-Martín J. Espaciador femoral total biarticulado para el tratamiento de la infección periprotésica de cadera y rodilla con pérdida completa de hueso. Modificación técnica. Rev Esp Cir Ortop Traumatol. 2019;63:192–201.