Many substances (growth factors and hormones) have osteoinduction properties and when added to some osteoconduction biomaterial they accelerate bone neoformation properties.
MaterialsThe materials included 15 New Zealand rabbits, calcium phosphate cement (Calcibon®), human growth hormone (GH), and plasma rich in platelets (PRP).
MethodsEach animal was operated on in both proximal tibias and a critical size bone defect of 6mm of diameter was made.
The animals were separated into the following study groups:
- -
Control (regeneration only by Calcibon®).
- -
PRP (regeneration by Calcibon® and PRP).
- -
GH (regeneration by Calcibon® and GH).
All the animals were sacrificed at 28 days. An evaluation was made of the appearance of the proximal extreme of rabbit tibiae in all the animals, and to check the filling of the critical size defect. A histological assessment was made of the tissue response, the presence of new bone formation, and the appearance of the biomaterial. Morphometry was performed using the MIP 45 image analyser. ANOVA statistical analysis was performed using the Statgraphics software application.
ResultsThe macroscopic appearance of the critical defect was better in the PRP and the GH group than in the control group. Histologically greater new bone formation was found in the PRP and GH groups.
No statistically significant differences were detected in the morphometric study between bone formation observed in the PRP group and the control group. Significant differences in increased bone formation were found in the GH group (p=0.03) compared to the other two groups.
ConclusionGH facilitates bone regeneration in critical defects filled with calcium phosphate cement in the time period studied in New Zealand rabbits.
Determinadas sustancias (factores de crecimiento y hormonas) tienen propiedades osteoinductivas y añadidas a un biomaterial osteoconductivo aumentan sus propiedades de neoformación ósea.
MaterialesQuince conejos de la raza Nueva Zelanda.
Los biomateriales y factores utilizados fueron: cemento de fosfato tricálcico (Calcibon®), hormona de crecimiento humana y plasma rico en plaquetas PRP.
MétodoCada conejo fue intervenido en ambas tibias donde se le realizó un defecto de 6mm.
Los animales de experimentación se repartieron en los siguientes grupos:
- -
Grupo control (regeneración solo con TCP).
- -
Grupo PRP (regeneración con TCP+PRP).
- -
Grupo GH (regeneración con TCP+GH).
Todos los animales fueron sacrificados a los 28 días. Se valoró el aspecto del defecto crítico comprobando su relleno. Histológicamente valoramos la respuesta tisular, la presencia de tejido óseo neoformado, y el aspecto del biomaterial. Se realizó la morfometría con analizador de imágenes MIP 45. Usamos el test ANOVA para el estudio estadístico mediante el programa Statgraphics.
ResultadosEl aspecto macroscópico del defecto crítico, fue mejor en el grupo PRP y en el grupo GH que en el grupo control. Histológicamente se observó mayor neoformación ósea en los grupos PRP y GH.
El estudio morfométrico no detectó diferencias significativas en la neoformación ósea entre el grupo PRP y control. Se detectó mayor neoformación ósea en el grupo GH (p = 0,03) frente a los otros dos grupos.
ConclusiónLa GH facilita la regeneración ósea en defectos críticos, rellenos con cemento de fosfato cálcico, en el período de tiempo estudiado en conejos de Nueva Zelanda.
The need to achieve bone regeneration in situations where a patient's spontaneous regenerative capacity is insufficient is a common situation in daily clinical practice.
Most bone lesions which present cavities are self-regenerating as they are not of a sufficient size to be termed “critical defects”. A cavity of a size which does not close or regenerate spontaneously is defined as a “critical defect”.
The formulation of calcium phosphate ceramics as bone cement has introduced new materials for bone regeneration since, in most cases, its reaction process produces a hydroxyapatite which is very similar to biological hydroxyapatite.
In addition to bone substitutes, the development and use of diverse growth factors and hormones are another line of research towards promoting bone regeneration.
Growth factors from platelet-rich plasma (PRP),1 are the simplest to apply because of the way they can be gathered.
Platelets transport platelet growth factors2: platelet derived growth factor and vascular endothelial growth factor, as well as transforming growth factor-beta and bone sialoprotein.
Furthermore, in terms of the role played by hormones in bone regeneration, numerous studies have shown the relationship between growth hormone (GH) and bone metabolism. In fact, growth hormones have a significant action at bone level on the bone forming cells.3 The increased proliferation of osteoblasts brought about by GH, would translate as a greater number of these cells appearing at the level of the critical defect, and early cellular differentiation would significantly accelerate the synthesis and mineralization of the osteiod matrix. All of which would favor the process of bone regeneration in the critical defect.
Despite the vast arsenal of bone grafts and substitutes, the problem of how to achieve entirely satisfactory bone regeneration with their use has as yet not been fully solved.
This work is a further step towards finding new methods of achieving total bone regeneration in bone cavities with critical defects.
Materials and methodologyMaterials15 New Zealand rabbits were used as experimental animals for this work. They were healthy, adult males of between 8 and 12 months old from the Animal Experimentation Centre of the Faculty of Medicine of the University of Alcalá which complies with national and international animal experimentation regulations.
The animals were alive and in good health up until 28 days after surgery; they had direct access to water and food ad libitum.
Surgical instruments: the usual instruments for bone tissue surgery were used. A contra-angle coupled to a surgical motor with an irrigation system and a trephine drill 6mm in diameter adapted to the contra-angle.
The following products were used:
- A.
Tricalcium phosphate cement (Calcibon®): a calcium phosphate-based cement. It consists of two separate components: powder and liquid. When the mixture is made up, at the time of surgery, calcium carbonate phosphate apatite forms which comprises small crystals similar to the inorganic phase of bone tissue.
- B.
Growth hormone. Norditropin Simplex® 15mg/1.5ml. It contains a biosynthetic human growth hormone called somatropin which is identical to human growth hormone.
- C.
PRP. This comes from the animal's own blood and is obtained using the specific method which is described later.
Histological and morphometric material:
- A.
Histological cutting equipment: EXAKT® Cutting Grinding System (Exakt Apparatebau, Nordersted, Germany) for hard tissues, comprising a diamond saw and polisher with discs of different grain diameters.
- B.
Histological stain solutions.
- C.
Two-dimensional imaging software for quantitative study of the samples (MIP-45).
- D.
Optical microscope and conventional optics camera for histological study.
- E.
Morphometric material: optical microscope, computer system and application for MIP-45.
- F.
Statgraphics plus v. 5.1 software for statistical analysis.
The animals were divided into three groups:
- •
Control Group, comprising 5 animals in which only tricalcium phosphate was used as the filler material for the defect created in the tibial metaphysis.
- •
PRP Group, comprising 5 animals using tricalcium phosphate bathed in PRP as the filler material.
- •
GH Group, comprising 5 animals using tricalcium phosphate bathed in growth hormone as the filler material.
A. Surgical technique:
- –
All the experimental animals were anaesthetized using ketamine chlorydrate at a dose of 5mg/kg body weight.
- –
Preparation of PRP: The PRP group, comprising 5 animals, required platelet rich plasma to be gathered prior to surgery. The technique described by Anitua et al.4 was used to gather the PRP from each animal.
- –
Preparation of the tricalcium phosphate (Calcibon®):
The solid and liquid components of the tricalcium phosphate were prepared by mixing. 3.3g of Calcibon® powder was dissolved in 1cc of Calcibon® liquid, mixed for 1min and left for 4min before being placed in the bone defect.
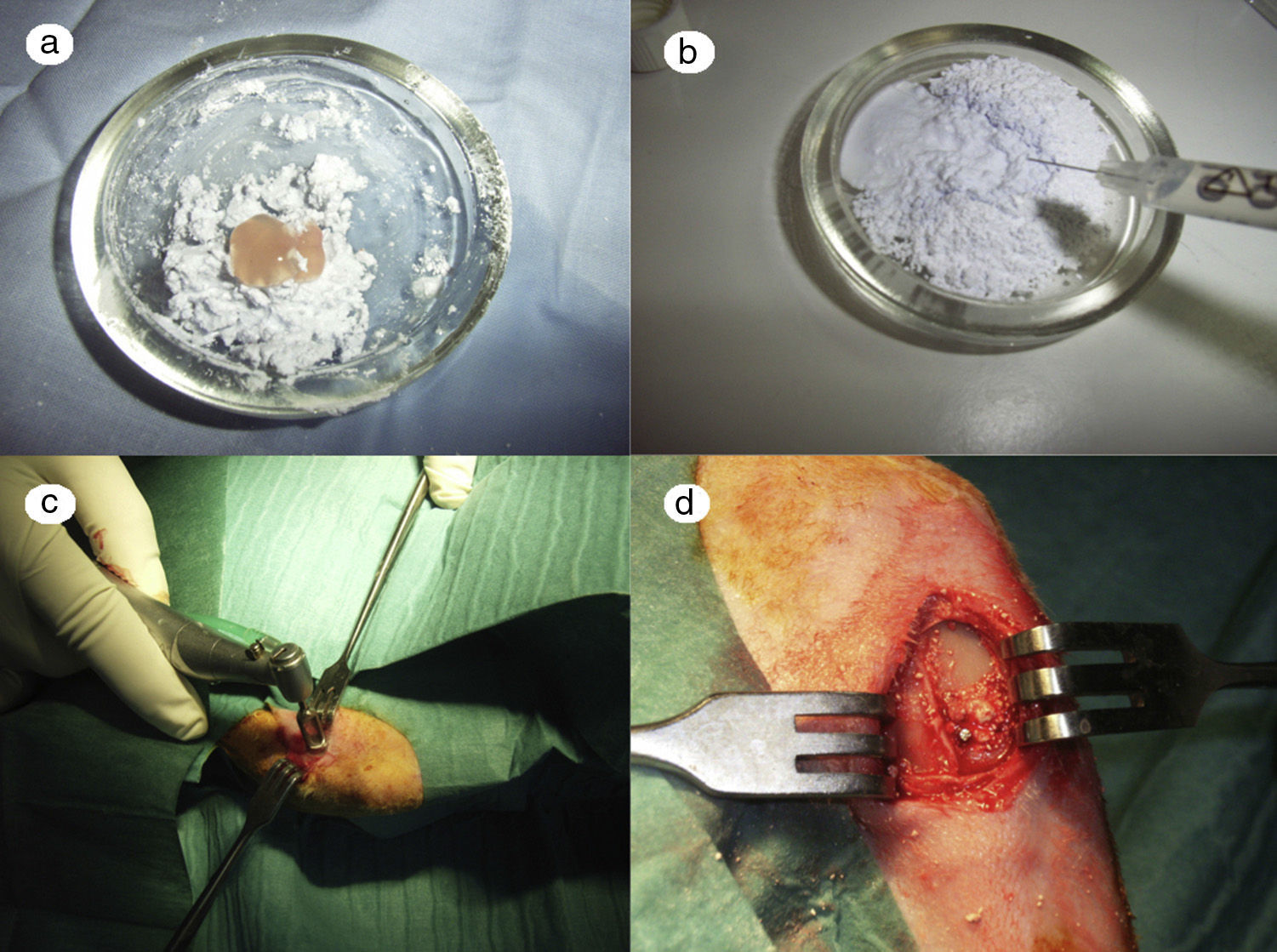
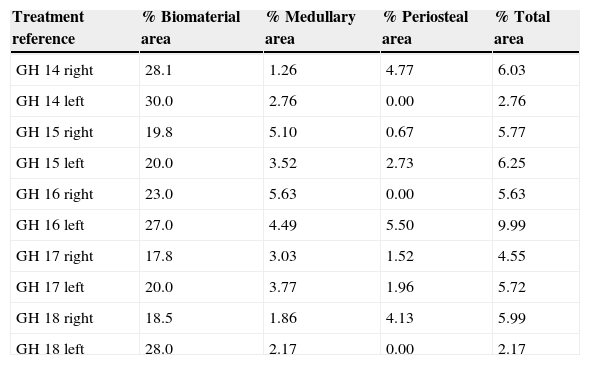
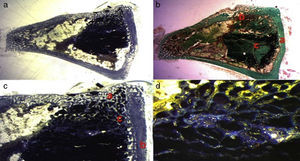
In the PRP group, the filler material was made up mixing tricalcium phosphate cement with 1cc. PRP (Fig. 1a), mixing for 1min.
In the GH group, tricalcium phosphate bathed in growth hormone (Fig. 1b) was used as the filler material, adding 4 UI of growth hormone.
– Preparation of the bone defect: both tibias of each rabbit were operated successively making bone defects in each. Both defects were treated with the same type of filler in the two tibias according to the animal's experimental group (control, PRP or GH).
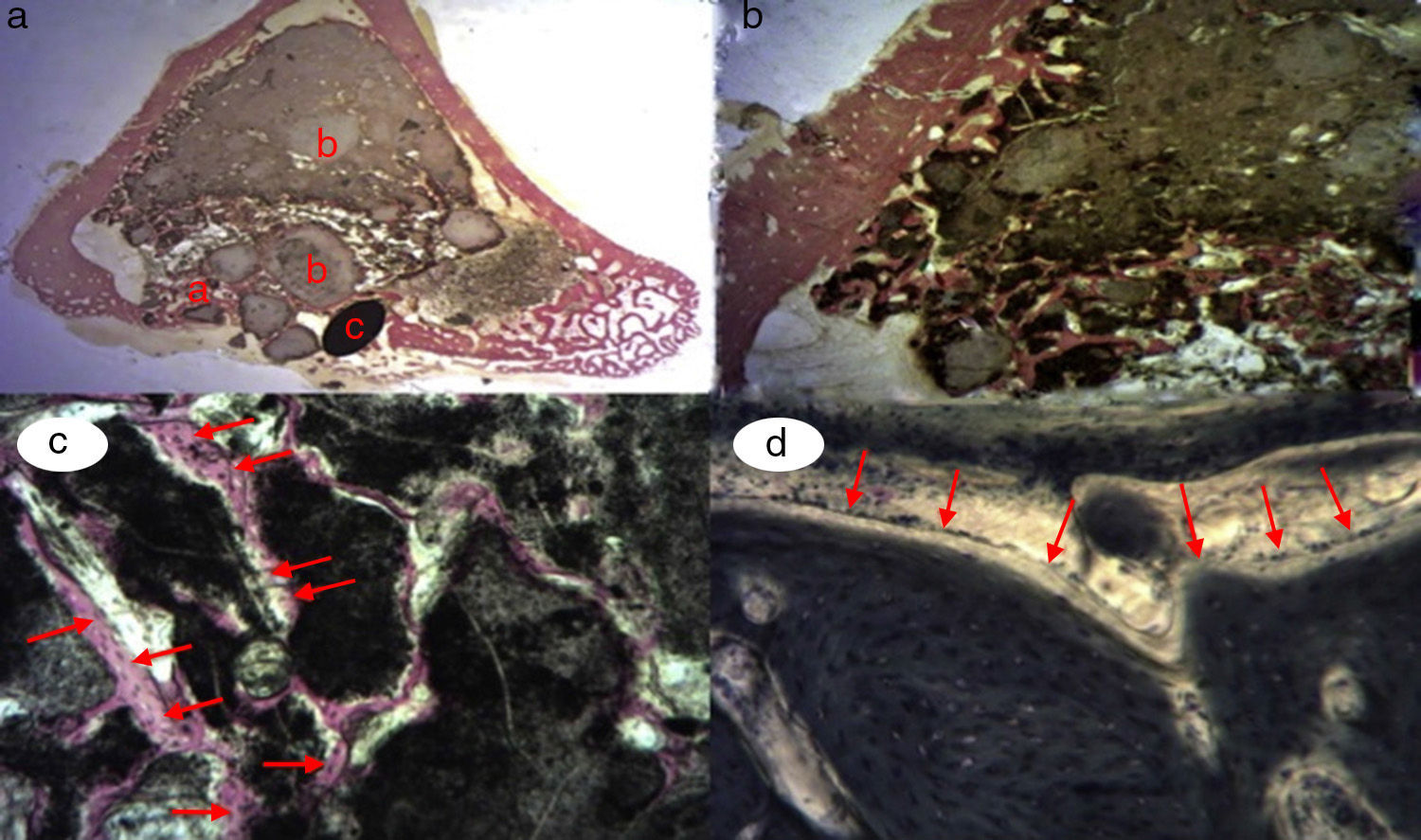
After shaving the operation site above the tibia, the skin surface was disinfected using povidone-iodine. Then a sagittal incision was made of about 4cm, at the level of the internal face in the proximal end of the tibia, carefully separating the periosteum and exposing the bone. An experimental circular defect was made using a trephine drill of 6mm in diameter mounted on a contra-angle (Fig. 1c). We chose this site based on previous references, as the metaphysiary region responds more intensely to stimuli than the tibial diaphysis.5
The bone defects were subsequently filled with tricalcium phosphate alone, or with tricalcium phosphate and PRP or GH, depending on the experimental group (Fig. 1d). The operation was performed on both limbs of the animal.
The site of the filled defect was marked with a 1.2mm×4mm Kirschner wire in order to identify it later in the histological study (Fig. 1d).
Finally, the periosteum was carefully sutured with absorbable suture and the skin was sutured with silk.
Taking the study samples: all the animals were sacrificed 28 days after surgery by lethal injection of barbiturates (sodium pentothal).
B. Macroscopic study: macroscopic study assessed the appearance of the proximal region of the tibia of the animals once the soft parts had been dissected. The appearance of the proximal part of the tibia was assessed in the 3 experimental groups, checking the filling of the critical defect in all of the animals.
C. Optical microscopy study
Once the tibias had been taken, they were fixed in 5% formalin buffered at pH 7 to prevent decalcification. The samples were then processed in the Human Anatomy and Embryology Department of the Faculty of Medicine of the University of Alcalá, where their fixation in formalin continued to be monitored.
The bone regeneration was then examined histologically and histomorphometrically in undecalcified bone tissue samples. The Exakt® cutting and polishing system was used for this purpose.
The next step was to put the sample into a polymer. Therefore a block of Methacrylate was made containing the tibias. Then the samples were cut with the diamond cutting band of the Exakt® system, obtaining 5 cuts per sample for subsequent histological study. The cuts were made perpendicular to the longitudinal axis of the tibial diaphysis, following a transverse plane, in order to be able to assess the entire cavity, from the inside to the surface.
The usual stains were used for the bone tissue study in the laboratory:
- –
Masson's Trichrome.
- –
Hematoxylin–eosin.
- –
Toluidine blue.
The following histological variables were assessed:
- •
Mature bone tissue: characterized by the fact that its bone matrix (mineralized interstitial substance) organizes itself forming bone lamellae 3–7μm thick. Cells of mature bone are osteocytes and occupy spaces called lacunae, arranged concentrically, like lamellae.
- •
The lamellae are parallel and concentric.
- •
Newly formed bone tissue: contains a relatively higher proportion of osteocytes, with larger lacunae than those which appear in mature bone and collagen fibers which follow various directions.
- •
Quantity and appearance of the biomaterial (calcium phosphate cement): the appearance of the biomaterial placed inside the cavity which had been made experimentally in the tibia was also studied histologically.
- •
Response of the bone tissue to the biomaterial: signs of inflammatory reaction were looked for, such as the appearance of lymphocytes or foreign body cells. Finally, signs of allergic reaction were investigated, such as the presence of eosinophyls.
D. Morphometric study
After the qualitative histological study, quantitative analysis was undertaken using two-dimensional morphometry.
This enabled us to objectively quantify the amount of newly formed bone tissue which had regenerated in the cavities of the different groups. To do this, we used image-analyzing software, called MIP-45.
D.1. Study technique:
We used a point template as a measurement estimator designed so that each point had an associated area of 1mm.2 The count of impact points enabled us to obtain the values of our variables.
D.2. Variables of the histomorphometric study. In order to obtain the study variables, we had to calculate the following areas beforehand:
- 1.
Total area of the sample: this includes the area occupied by the entire bone, and the area occupied by the medullary cavity.
- 2.
Area of cortical bone.
- 3.
Area of newly formed bone which we subdivided into:
- a.
Area of newly formed bone inside the medullary cavity.
- b.
Area of newly formed bone outside the medullary cavity.
- a.
- 4.
Area occupied by the remaining biomaterial.
Once the above areas had been calculated, the study variables were determined in the five sections or cuts taken from each animal's tibia. These variables were as follows:
- 1.
Density of the area of the remaining biomaterial (% biomaterial area):
- 2.
Density of the area of new bone formation in the medullary cavity (% medullary area):
- 3.
Density of the area of new bone formation outside the medullary cavity (% periosteal area):
- 4.
Density of the total area of new bone formation (% total area):
D.4. Study of the reliability of the method. A prior pilot study for dependent samples was undertaken to check the degree of reliability in order to establish the morphometry, with the objective of evaluating the accuracy of the estimator or probe (measurement unit) used, by calculating the error coefficient for this probe. This study enabled a measurement probe to be designed for the samples, comprising a point template, each point with an associated area of 1mm.2
The reliability study, its calculation and methodology were as follows:
Calculation of sampling reliability for dependent samples: cuts parallel to each other, perpendicular to the longitudinal axis of the sample (Cavalieri cuts): the error coefficient is a statistical concept which allows us to know the degree of precision of the estimator used. Its value will depend on the number of sampling units and the size of the estimator (in other words, the distance between the points of the measuring template). This information enables us to identify the minimum value we require of cuts from one single sample, and the minimum distance between the points used to measure them, so that the data obtained using these two constants enable us to make a reliable statistical calculation.
E. Statistical study: the significant differences between the study groups were assessed by analysis of variance (ANOVA) using the Stagraphics program version 5.1.
Results- I.
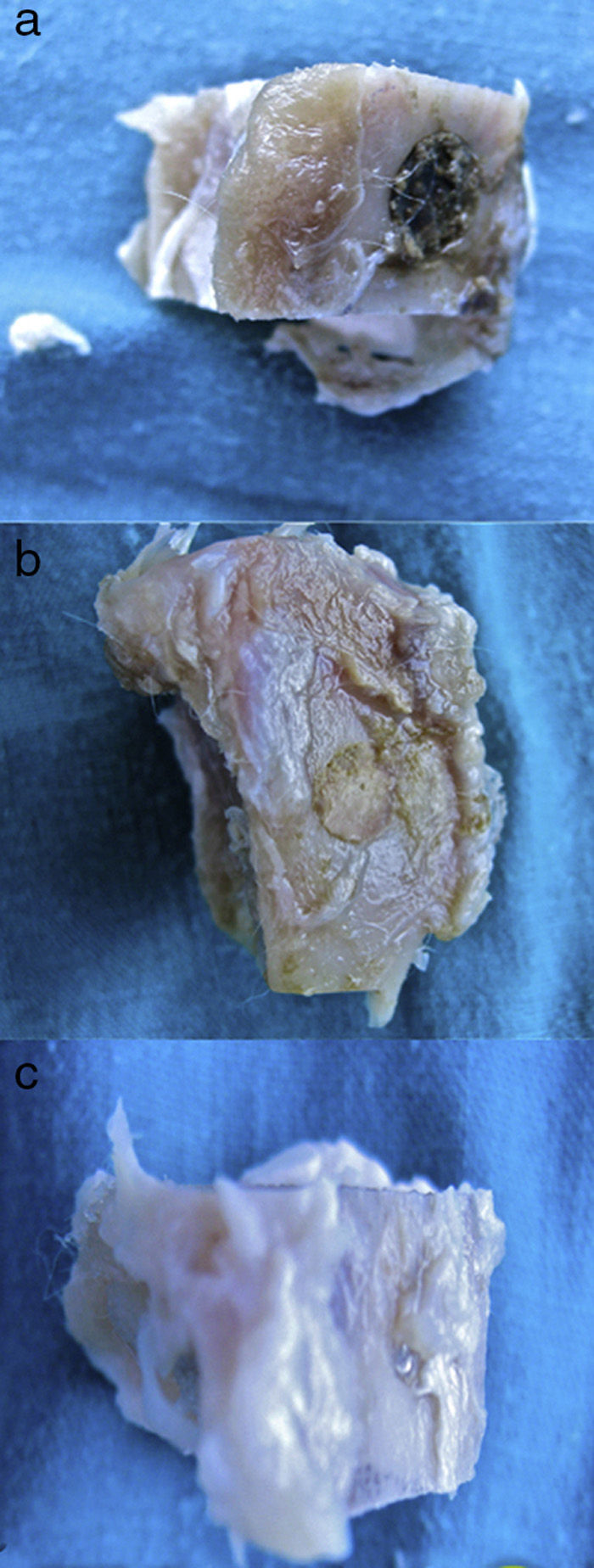
Macroscopic results: the site in the tibias which had been operated 28 days earlier was screened.
- 1.
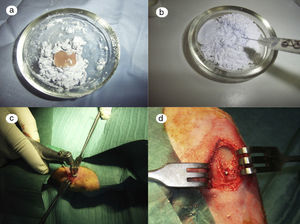
In the control group (Fig. 2a) the samples did not show that the calcium phosphate cement which had been placed during surgery had integrated. The site of the critical defect was clearly differentiated; the cement appeared as an amorphous mass which filled the defect with no integration of any type.
- 2.
In the PRP group (Fig. 2b), on the one hand, it was observed that the critical defect was well filled, and on the other, the cement appeared to have produced better bone integration than in the control group.
- 3.
On observing the critical defect made in the tibias of the rabbits in the GH group (Fig. 2c), macroscopically the filling appeared to be better osseointegrated and it was difficult to distinguish the bone tissue from the filled defect. The site of the critical defect can be seen in Fig. 2c, marked with a Kirschner wire, which is difficult to distinguish from the rest of the cortex.
- 1.
- II.
Histological results: the histological study enabled us to histologically evaluate and compare bone tissue formation and the presence of biomaterial inside the critical defect between the 3 groups studied. Mature bone, immature bone, residual biomaterial, the Kirschner's wire which served to mark the critical bone defect, and the presence of connective tissue and bone marrow could be observed in the transverse cuts.
- 1.
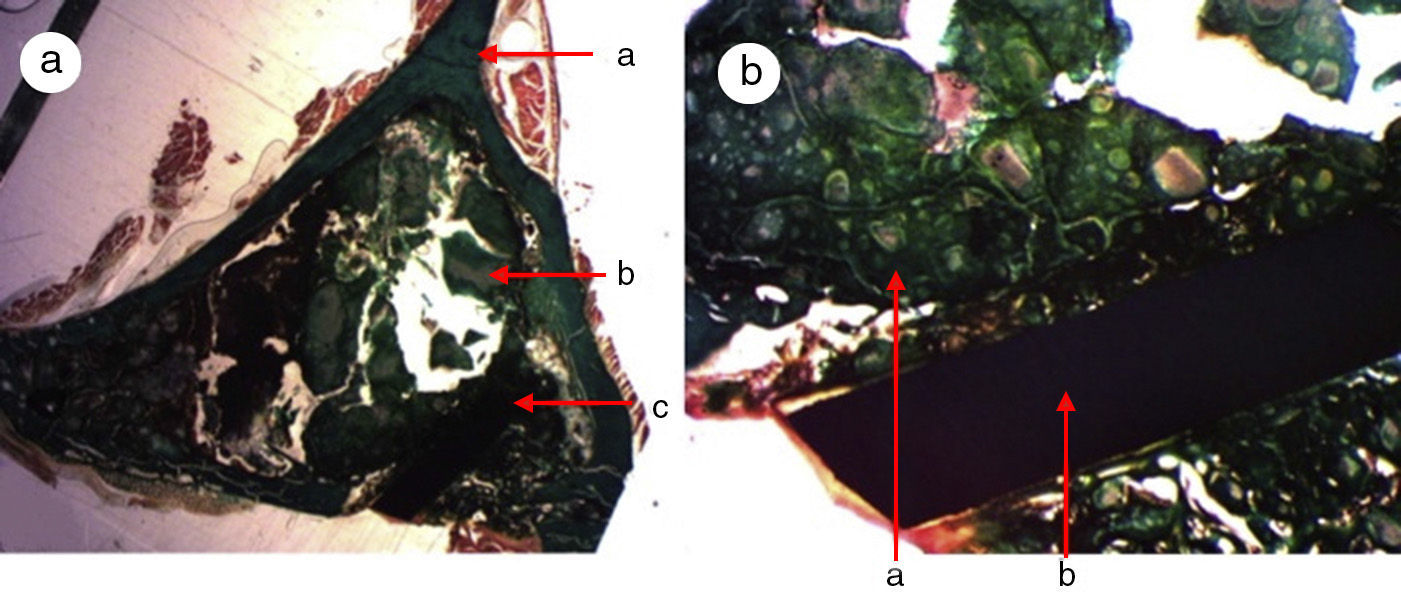
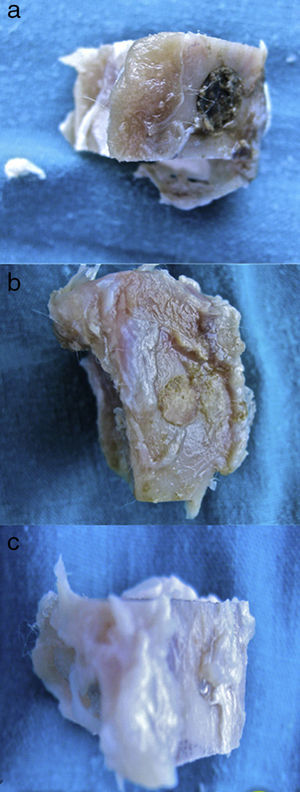
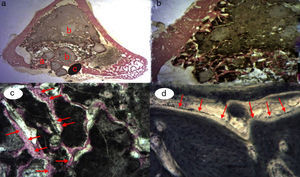
A great many tricalcium phosphate (Calcibon®) particles were observed in the control group (Fig. 3a and b) distributed throughout the entire area of the sample, lightly stained by hematoxylin, with a greenish shade in the Masson's Trichrome preparations and a grayish appearance in the Toluidine blue preparations.
Figure 3.Control group histology. (a) Rabbit control group 15 increases. Transverse cut Masson's trichrome stain. General appearance. The bone tissue is distinguished from the cortex (a), the calcium phosphate cement which fills the medullary channel (b) and the cortical critical defect marked with Kirschner wire (c). (b) Rabbit control group 30 increases. Masson's trichrome stain. Kirschner wire used as a marker (a), surrounded by biomaterial (b) in the site of the critical defect. No bone tissue formation is observed.
(0.16MB).Little new formation was noticed, with some young, short and isolated trabeculae, close to the external and internal surface of the cortex and between the particles of calcium cement. No osteoblasts or osteoclasts were found.
- 2.
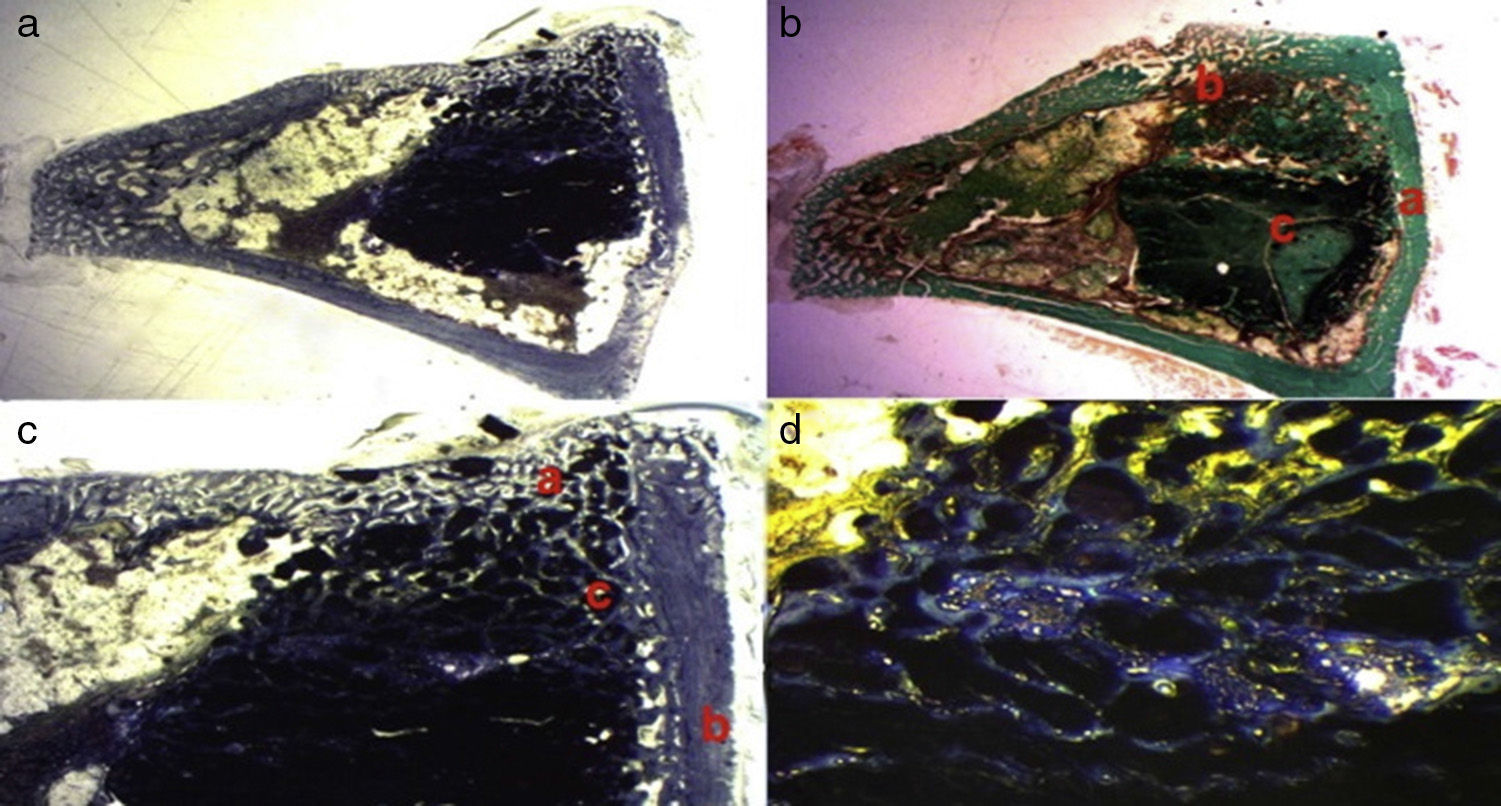
Greater formation of immature bone tissue was observed in the PRP Group (calcium phosphate cement+PRP filler) (Fig. 4a–d) filling both the critical defect and the spaces between the particles of biomaterial compared with the control group. No inflammatory tissue was found and only a little connective tissue. The tricalcium phosphate presented as an amorphous mass and we observed no osteoclastic activity.
Figure 4.(a) Transverse cut. Masson's trichrome stain. Overall appearance of calcium phosphate+PRP: greater bone formation is observed in the site of the critical defect and between the particles of calcium cement. (b) Rabbit PRP group, 30 increases. Two-fold increase from the previous image. (a) Newly formed bone (immature) filling the defect. (b) Mature bone of the cortex. (c) Bone between the particles of the biomaterial. (c) Rabbit PRP group, 15 increases. Transverse cut. Masson's stain. General appearance: (a) cortex, (b) newly formed bone y (c) biomaterial. (d) Rabbit PRP group, 30 increases. Immature bone between the particles of calcium phosphate. The color of this figure can only be appreciated in the electronic version of the article.
(0.24MB). - 3.
We observed the greatest degree of new bone formation in the form of immature bone tissue in the GH group (calcium phosphate cement+GH filler) (Fig. 5a–d) compared with the histological cuts of the control group and the PRP group. Numerous bone trabeculae were seen between the particles of biomaterial. It is worth noting that the formation of the bone tissue was greater in the defect and inside the medullary channel compared with the cuts from the control group and the PRP group. These observations suggest greater osteoblastic activity due to the effect of the GH in this group. No osteoclastic activity or inflammatory tissue was found.
Figure 5.GH group histology. (a) Rabbit GH group, 15 increases. General image hematoxylin–eosin stain. (a) Newly formed bone tissue between the particles of the biomaterial (b) in the site of the cortical defect, marked with Kirschner wire (c). (b) Rabbit GH group, 30 increases. Hematoxylin–eosin stain. Newly formed bone tissue in contact with biomaterial. (c) Rabbit GH group, 60 increases. Newly formed bone tissue (arrows) in contact with the biomaterial (tricalcium phosphate+GH). (d) Rabbit GH group, 100 increases. Toluidine blue stain. Front of osteoblasts (arrows). The color of this figure can only be appreciated in the electronic version of the article.
(0.25MB).
- 1.
- III.
Morphometric results: once the reliability of the study had been confirmed the results were obtained of the proposed variables (Tables 1–3).
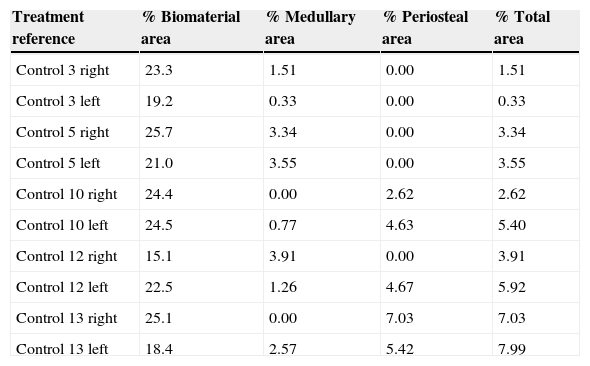
Table 1.Results of the control group morphometry.
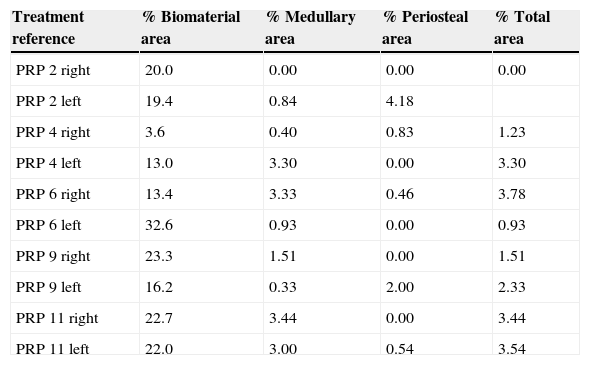
Treatment reference % Biomaterial area % Medullary area % Periosteal area % Total area Control 3 right 23.3 1.51 0.00 1.51 Control 3 left 19.2 0.33 0.00 0.33 Control 5 right 25.7 3.34 0.00 3.34 Control 5 left 21.0 3.55 0.00 3.55 Control 10 right 24.4 0.00 2.62 2.62 Control 10 left 24.5 0.77 4.63 5.40 Control 12 right 15.1 3.91 0.00 3.91 Control 12 left 22.5 1.26 4.67 5.92 Control 13 right 25.1 0.00 7.03 7.03 Control 13 left 18.4 2.57 5.42 7.99 Table 2.Results of the PRP group morphometry.
Treatment reference % Biomaterial area % Medullary area % Periosteal area % Total area PRP 2 right 20.0 0.00 0.00 0.00 PRP 2 left 19.4 0.84 4.18 PRP 4 right 3.6 0.40 0.83 1.23 PRP 4 left 13.0 3.30 0.00 3.30 PRP 6 right 13.4 3.33 0.46 3.78 PRP 6 left 32.6 0.93 0.00 0.93 PRP 9 right 23.3 1.51 0.00 1.51 PRP 9 left 16.2 0.33 2.00 2.33 PRP 11 right 22.7 3.44 0.00 3.44 PRP 11 left 22.0 3.00 0.54 3.54 Table 3.Results of the GH group morphometry.
Treatment reference % Biomaterial area % Medullary area % Periosteal area % Total area GH 14 right 28.1 1.26 4.77 6.03 GH 14 left 30.0 2.76 0.00 2.76 GH 15 right 19.8 5.10 0.67 5.77 GH 15 left 20.0 3.52 2.73 6.25 GH 16 right 23.0 5.63 0.00 5.63 GH 16 left 27.0 4.49 5.50 9.99 GH 17 right 17.8 3.03 1.52 4.55 GH 17 left 20.0 3.77 1.96 5.72 GH 18 right 18.5 1.86 4.13 5.99 GH 18 left 28.0 2.17 0.00 2.17 - IV.
Statistical results of the morphometric data: using Statgraphics 5.1. software, we applied the ANOVA test to look for statistically significant differences between the three study groups:
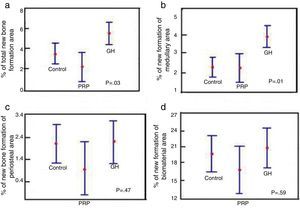
- 1.
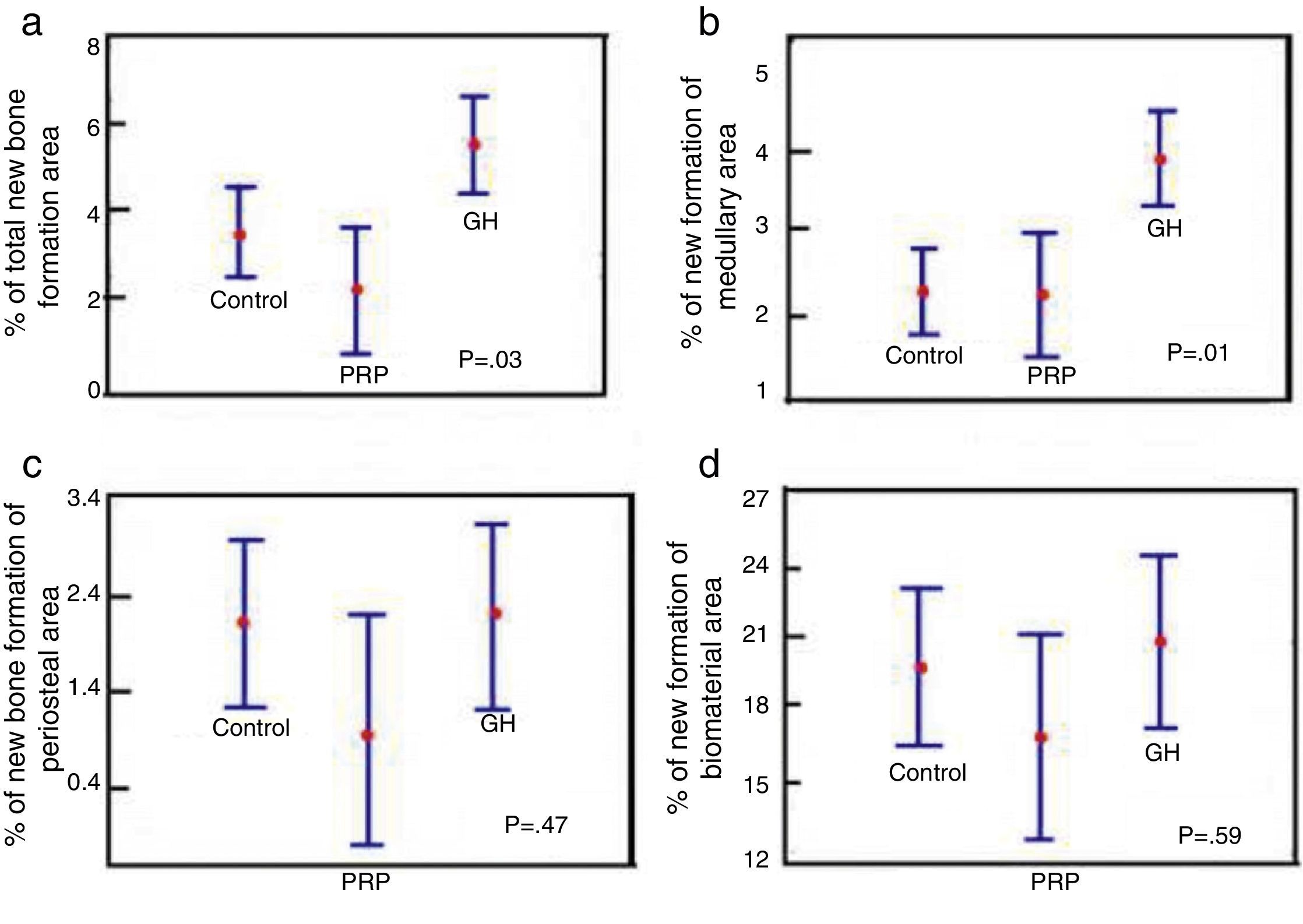
Comparison of % total area between the control groups, PRP and GH: in terms of the total newly-formed bone in the control, PRP and GH groups, we found percentage values which were clearly greater in the GH group (P<.05), (Fig. 6a).
Figure 6.Statistical results of the morphometric study. (a) ANOVA of the density of the total new bone formation area. (b) ANOVA of the density of the new bone formation area in the medullary cavity. (c) ANOVA of the density of the new bone formation area outside the medullary channel. (d) ANOVA of the density of the area of the remaining biomaterial.
(0.19MB). - 2.
Comparison of % medullary area between the control groups, PRP and GH: we found more new bone formation inside the medullary channel or canal in the GH group compared with the control group and the PRP group. The difference was statistically significant (P<.05). This result demonstrates that GH has a positive effect on new bone formation compared with PRP (Fig. 6b).
- 3.
Comparison of % periosteal area between the control groups, PRP and GH: on comparing the control, PRP and GH control groups, we found no major differences in the percentage value of newly formed bone outside the filled defect. This could be explained by the surgical technique used, where the periosteal closure was completed through the suture in all the animals, therefore no growth factor or hormone would have left the periosteum. This would explain the new bone formation being similar in the three groups (Fig. 6c).
- 4.
Comparison of % biomaterial area between the control, PRP and GH groups: no significant differences were found in the percentage of biomaterial between the control, PRP and GH groups. The reabsorption process of the calcium phosphate cement appears not to have started, in the time frame studied of 28 days (Fig. 6d).
A/. Animal species:
Rabbits were chosen as the experimental models because they reach “skeletal maturity” at around 8–10 months and have true cortex remodeling with faster bone turnover than that of other species.6 This makes them appropriate models for the study of drugs which regulate this remodeling. The most suitable models are certain species of primates, but research using these models is expensive, laborious and is not free from risk.6
The animal's tibia is the most frequently chosen site as it is easy to access.7,8
B/. Influence of gender: males were used in our study because they are not subject to cyclical hormonal changes. We believe that using females might have increased the variability of the results.9
C/. Influence of age: a series of morphological differences have been described in rabbits which might explain a lower intensity of regeneration in old animals.10
Animals of between 8 and 12 months of age were used in our study, which had completed their skeletal growth. At this age rabbits no longer display the excellent osteo-regenerative capacity of those younger than 2 months.11
Site and size of the bone defectThe studies of bone regeneration in rabbits were undertaken on different anatomic sites: the mandible,12 the femur,13 and the calvaria,14 in order to assess bone repair after induced damage.
The tibia has been used by some authors15,16 to induce bone defects. The anatomo-surgical site of the medial face of the proximal tibia, in particular, is susceptible to induced bone defects, as it has a wide, slightly convex surface which has no muscular insertions. The repair of bone defects will depend on their size rather than the anatomic site.17
In literature, the tibial bone defects most used in rabbits have been full cortical thickness circular in shape and 3, 6 and 8mm in diameter.14
We chose to use defects of 6mm, in accordance with authors such as Katthagen et al.18 who consider that these defects do not regenerate spontaneously.
Biomaterials usedHistorically, bone autografts and allografts have been used in addition to a great variety of biomaterials to repair bone defects.
The ideal bone substitute would be biocompatible, bioabsorbable, osteoconductive, of a similar structure to bone, easy to use clinically and economically priced.
Ceramic biomaterials are amongst the groups of greatest interest in the reconstruction of bone defects due to their acknowledged osteoconductive properties, proven biocompatibility, biodegradation capacity, unlimited availability, suitability as drug vehicles and because they have the appropriate conditions to be used as casts for tissue engineering. Alpha-tricalcium phosphate cement belongs to this group.19,20
However, after examining the literature, we have found no studies where an evaluation and comparison has been made of the effect produced by the mixture of calcium phosphate cement with platelet factors or growth hormone on spontaneous bone regeneration, using a quantitative methodology and a bone defect model in animals.
Growth factors usedA/. PRP: PRP is considered to be a regenerative biomaterial which favors the circulation of stem cells, the proliferation of fibroblasts and synthesis of type I collagen.21
Our morphometric results did not demonstrate a positive effect of PRP on bone regeneration. We agree with the work of other authors who have not demonstrated a positive effect of PRP on bone generation either.22
Considering the proven positive effect of platelet growth hormones on the processes of bone regeneration, it appears that the reason for the differences between studies is due to the different procedures used by the authors for collecting PRP. It has been found that the concentration of growth hormones varies greatly depending on the methodology used for collecting them.23
For the future, it appears that this methodology needs to be protocolized in order to obtain the exact concentration of platelet growth factors to achieve an optimal effect on bone regeneration.
B/. Growth hormone: GH stimulates the production of collagen and non-collagen proteins by osteoblasts; and, moreover, by stimulating the synthesis by the liver and the osteoblasts themselves of insulin-like growth factors (IGF), favors the differentiation of preosteoblasts and the proliferation of osteoblasts.24,25
These actions on bone tissue at the level of critical defects are interesting because of the possibility of applying GH locally at clinical level.
The data obtained in our research work, with the local application of GH, demonstrated significant histomorphometric differences between the control group and the GH group. 28 days after surgery, GH at a local dose of 4 UI (1.2mg), significantly increased the parameters of bone regeneration: the density of the area of new bone formation in the medullary cavity.
In this regard, we agree with the data obtained by Blom and Tresguerres.26,27
In the references we found mentions of the use of GH28 as a therapeutic agent, through systemic application, in the treatment of fractures. All the authors agree on its positive effect in curing fractures, however, the majority reported adverse systemic effects.28,29 Nonetheless, there are few studies on their local use, and those which we managed to find, did not report systemic effects.30 This could justify the local application of the hormone being of clinical interest, which would achieve the benefits of this hormone in curing bone lesions, and avoid its possible side effects when applied systemically.
ConclusionsThe following conclusions can be drawn from the results obtained from this study:
- 1.
The macroscopic appearance of the critical defect, after treatment, is better in the PRP group and in the GH group than in the control group.
- 2.
New bone formation was observed histologically, both inside and outside the defect, in the three groups.
- 3.
However, the quantitative morphometric study did not detect statistically significant differences between the new bone formation achieved in the PRP group and the control group.
- 4.
Statistically significant differences were found which indicated more new bone formation in the GH group compared to the other two groups.
Final conclusion: GH facilitates bone regeneration in critical defects, filled with calcium phosphate, in New Zealand rabbits in the period of time studied.
Evidence levelEvidence level I.
Ethical responsibilitiesProtection of human beings and animalsThe authors declare that the procedures followed conform to the ethical standards of the committee for responsible human experimentation and in accordance with the World Medical Association and the Helsinki Declaration.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Emilov-Velev K, Clemente-de-Arriba C, Alobera-García MA, Moreno-Sansalvador EM, Campo-Loarte J. Regeneración ósea en animales de experimentación, mediante cemento de fosfato cálcico en combinación con factores de crecimiento plaquetarios y hormona de crecimiento humana. Rev Esp Cir Ortop Traumatol. 2015;59:200–210.