There is an increase in the levels of metals in the serum and urine after the implantation of some models of metal–metal hip prosthesis. It has recently been demonstrated that there is an association between these levels and the levels found in hair. The aim of this study is to determine the presence of metals in hair, and to find out whether these change over time or with the removal of the implant.
Materials and methodsThe levels of chromium, cobalt and molybdenum were determined in the hair of 45 patients at 3, 4, 5, and 6 years after a hip surface replacement. The mean age was 57.5 years, and two were female. Further surgery was required to remove the replacement and implant a new model with metal–polyethylene friction in 11 patients, 5 of them due to metallosis and a periarticular cyst.
ResultsThe mean levels of metals in hair were chromium 163.27ppm, cobalt 61.98ppm, and molybdenum 31.36ppm, much higher than the levels found in the general population. A decrease in the levels of chromium (43.8%), molybdenum (51.1%), and cobalt (91.1%) was observed at one year in the patients who had further surgery to remove the prosthesis.
ConclusionsHigh concentrations of metals in the hair are observed in hip replacements with metal–metal friction, which decrease when that implant is removed. The determination of metal ions in hair could be a good marker of the metal poisoning that occurs in these arthroplasty models.
Tras el implante de algunos modelos de prótesis de cadera metal-metal se produce una elevación de los niveles de metales en suero y orina. Recientemente se ha demostrado que hay concordancia entre estas cifras y los niveles encontrados en el cabello. Nuestro objetivo ha sido estudiar la presencia de metales en cabello y conocer si ello se modifica con el paso del tiempo o con la extracción del implante.
Material y métodoEn 45 pacientes con una artroplastia de superficie se ha realizado una determinación de los niveles de cromo, cobalto y molibdeno en cabello a los 3, 4, 5 y 6 años desde el implante. La edad media fue de 57,5 años, 2 eran mujeres. En 11 pacientes, en 5 de ellos por metalosis y quiste periarticular, fue necesaria una reintervención para extracción de la artroplastia e implante de un nuevo modelo con fricción metal-polietileno.
ResultadosLas cifras medias de metales en cabello fueron cromo 163,27ppm, cobalto 61,98ppm y molibdeno 31,36ppm, muy por encima de los niveles referidos en la población general. En los pacientes reintervenidos para extracción de la artroplastia se observó al año de la intervención una disminución del 43,8% en los niveles de cromo, del 51,1% en molibdeno y del 90,3% en cobalto.
ConclusionesEn las artroplastias de cadera con fricción metal-metal se aprecia una alta concentración de metales en el cabello, que disminuye cuando dicho implante es extraído. La determinación de iones en cabello puede ser un buen marcador de la intoxicación por metales que sucede en estos modelos artroplásticos.
The main long-term complication in hip arthroplasties is wear of components, followed by onset of osteolysis and, ultimately, failure caused by mobilization of components. In recent years, the search for solutions to this problem, especially worrying among young patients, has led to the introduction of new polyethylenes, some new designs and a re-emergence of metal–metal (M-M) friction couplings. It is known that some M-M arthroplasty models may produce various clinical conditions resulting from the presence of metal ions in blood and urine, particularly chromium (Cr) and cobalt (Co). Although no cases of cancer or fetal complications have been reported to date, high levels of these metals can cause kidney and liver disorders, as well as local lesions, such as cysts and pseudotumors known as ALVAL (aseptic lymphocyte-dominant vasculitis-associated lesion).1,2 In addition to Cr and Co, other metals, like molybdenum (Mo), are also employed in the manufacture of these models. Nevertheless, it is striking how little impact the elevation of the levels of this metal has had in the abundant literature on these models, considering that elevated levels of Mo can cause negative effects on fertility. This is important when we take into account that the population group eligible for implantation of a hip prosthesis of this type includes young patients, and therefore, has implications at a reproductive level.3
Different follow-up protocols have been published including guidelines for normal levels of metal ions in blood and urine, maximum admissible levels4 and the importance of monitoring. These analytical determinations require special care in the collection and transfer of samples and are not routinely performed by the laboratories of general hospitals. Moreover, they can occasionally be altered by the intake of certain foods, the contribution of certain drugs and environmental conditions.
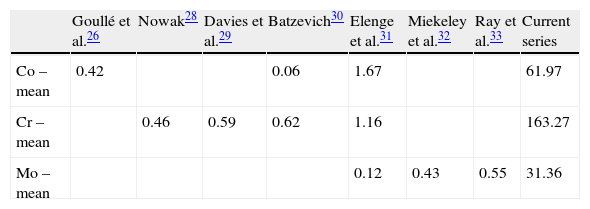
Hair has a remarkable potential as a biomarker, as it reflects historical exposure to various substances, including heavy metals.5 Its use in the determination of metal levels avoids invasive measurements and discomfort for patients, is independent of occasional situations and shows the true level of intoxication that these ions can cause. The levels among the general population are known to range between 0.11 and 0.52ppm for Cr, 0.004 and 0.14ppm for Co, and 0.01 and 0.028ppm for Mo,6 so their determination can be used as an indicator of metal poisoning caused by metal–metal arthroplasties, with a high level of agreement.7 The objectives of our study were:
- 1.
To know whether the hair of patients with M-M hip prostheses presented an elevated presence of metals.
- 2.
To study the variation in these figures depending on the period elapsed since implantation.
- 3.
To assess whether the figures for metals in hair decreased by removing M-M arthroplasties, through revision surgery.
This work was part of a comparative study of diagnostic and consistency tests in the monitoring of Co and Cr levels in serum and urine and Cr, Co and Mo in hair obtained from 45 patients with De Puy™ ASR hip resurfacing metal–metal prostheses (De Puy Orthopedics Inc., Warsaw, IN, USA). The arthroplasties were implanted during the period between 2006 and 2009. The study was approved by the Regional Ethics Committee (reference number 037/2011) and all patients signed an informed consent form for the use of their blood, urine and hair, as well as their clinical and imaging data. The initial series consisted of 49 arthroplasties, but 1 case was a bilateral implant so it was removed on suspicion that its determinations could introduce a confounding bias. A further 2 arthroplasties were lost during follow-up.
The mean age was 57.5±9.05 years (range: 35–76 years) and the mean BMI was 29.42±5.37 (range: 19.48–43.58). Two patients were female and the remaining 43 were males. At the time the study was conducted, a mean period of 51.8 months (range: 29–85 months) had elapsed since the arthroplasty was implanted. During the follow-up period, revision surgery was performed to remove the surface arthroplasty in 11 cases; 2 cases due to fractures of the femoral neck, 1 due to recurrent dislocation, 5 due to high metal levels and presence of a periarticular cyst confirmed by magnetic resonance and 3 due to local pain with an unexplained cause. A conventional model with metal–polyethylene friction was implanted in these patients and they abandoned the study. However, a determination of metal ions in hair was carried out at 12 months of this second intervention.
Hair samples were taken less than 4cm away from the skin in the right side of the head of each patient, with samples weighing at least 0.5g, following the recommendations established by several validated studies. The samples were sent to the laboratory in polyethylene bags identified by random numbers.
Hair analysis consisted of several phases. In the pretreatment stage, the samples were conditioned by a process involving washing in an ultrasonic bath to remove traces adhered to the surface that could alter the results. After this process, samples were introduced in an oven at 50°C for 12h. Dry samples were stored at room temperature until analysis. The samples were stored at −20°C until their analysis, which was performed through the inductively coupled plasma mass spectrometry (ICP-MS) technique.
Quantification of metals was carried out using an Element high-resolution mass spectrometer (Thermo Fisher, Inc., Waltham, MA, USA). This device was equipped with a Meinhard type concentric nebulizer, an uncooled Scott type double-pass nebulizing chamber, a Fassel type torch with a 1mm internal diameter injector tube and 2 nickel interface cones, a sampler and a skimmer. It offered 3 different resolution levels, which were predefined and selectable at the time of executing a study.
The data analysis was carried out using the statistical package SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA). Comparisons of metals according to years of follow-up were carried out through the nonparametric Kruskal–Wallis test. The significance level for statistical differences was defined at P<.05.
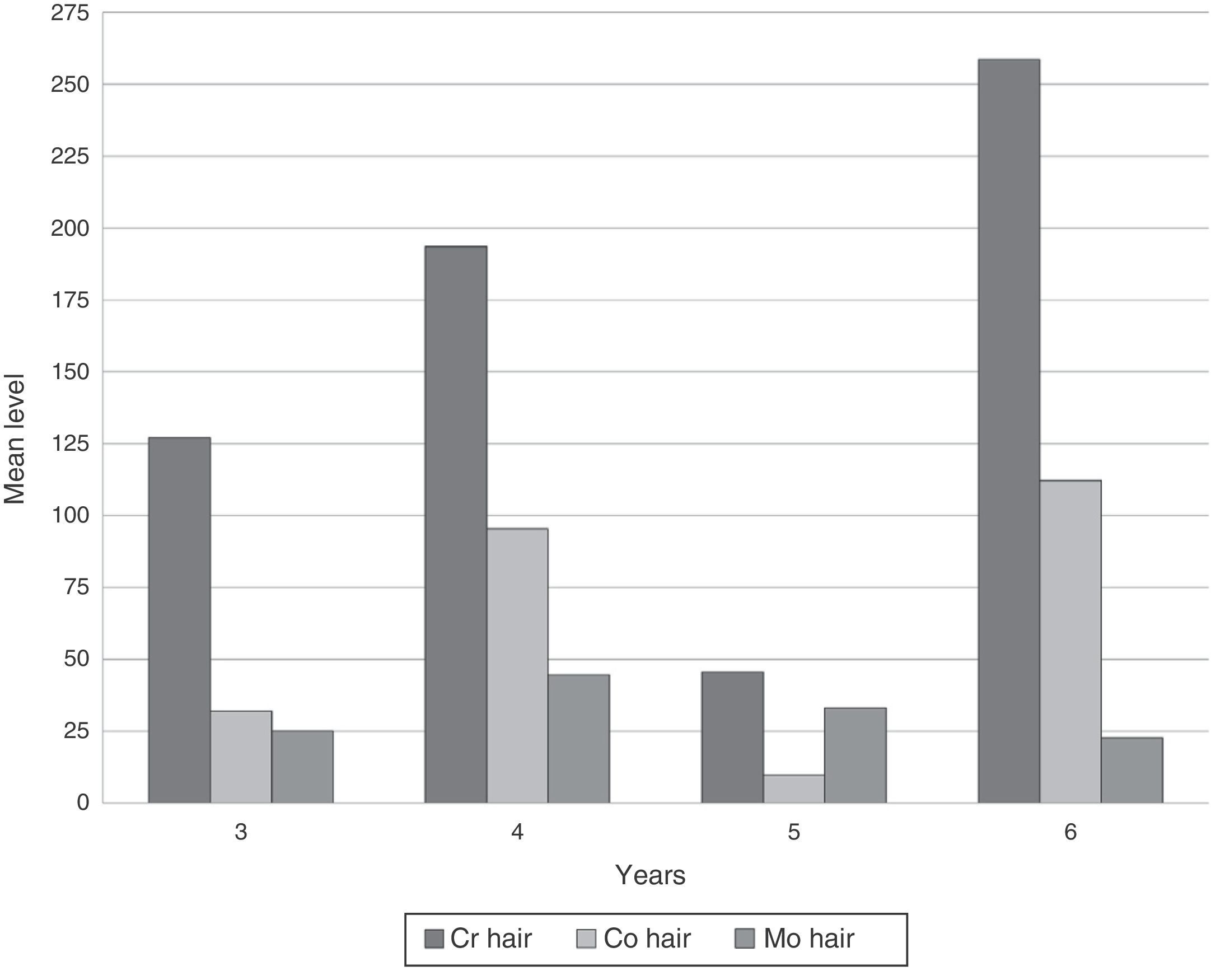
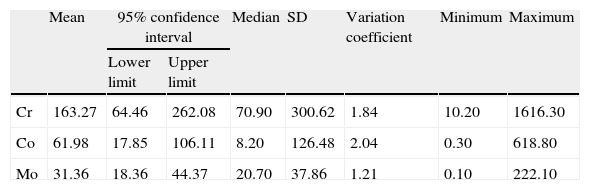
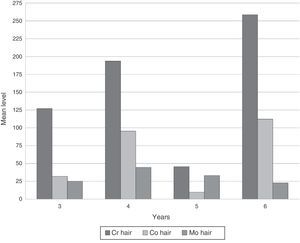
ResultsDeterminations of Cr, Co and Mo in hair in our series reached very high levels (Table 1). No significant differences in metal concentrations were detected according to the follow-up time in years (Kruskal–Wallis). Both Cr and Co increased at 4 years, decreased at 5 and rose again at 6. However, Mo decreased progressively after 4 years (Fig. 1).
Levels of metals in hair (in ppm).
| Mean | 95% confidence interval | Median | SD | Variation coefficient | Minimum | Maximum | ||
| Lower limit | Upper limit | |||||||
| Cr | 163.27 | 64.46 | 262.08 | 70.90 | 300.62 | 1.84 | 10.20 | 1616.30 |
| Co | 61.98 | 17.85 | 106.11 | 8.20 | 126.48 | 2.04 | 0.30 | 618.80 |
| Mo | 31.36 | 18.36 | 44.37 | 20.70 | 37.86 | 1.21 | 0.10 | 222.10 |
SD: standard deviation.
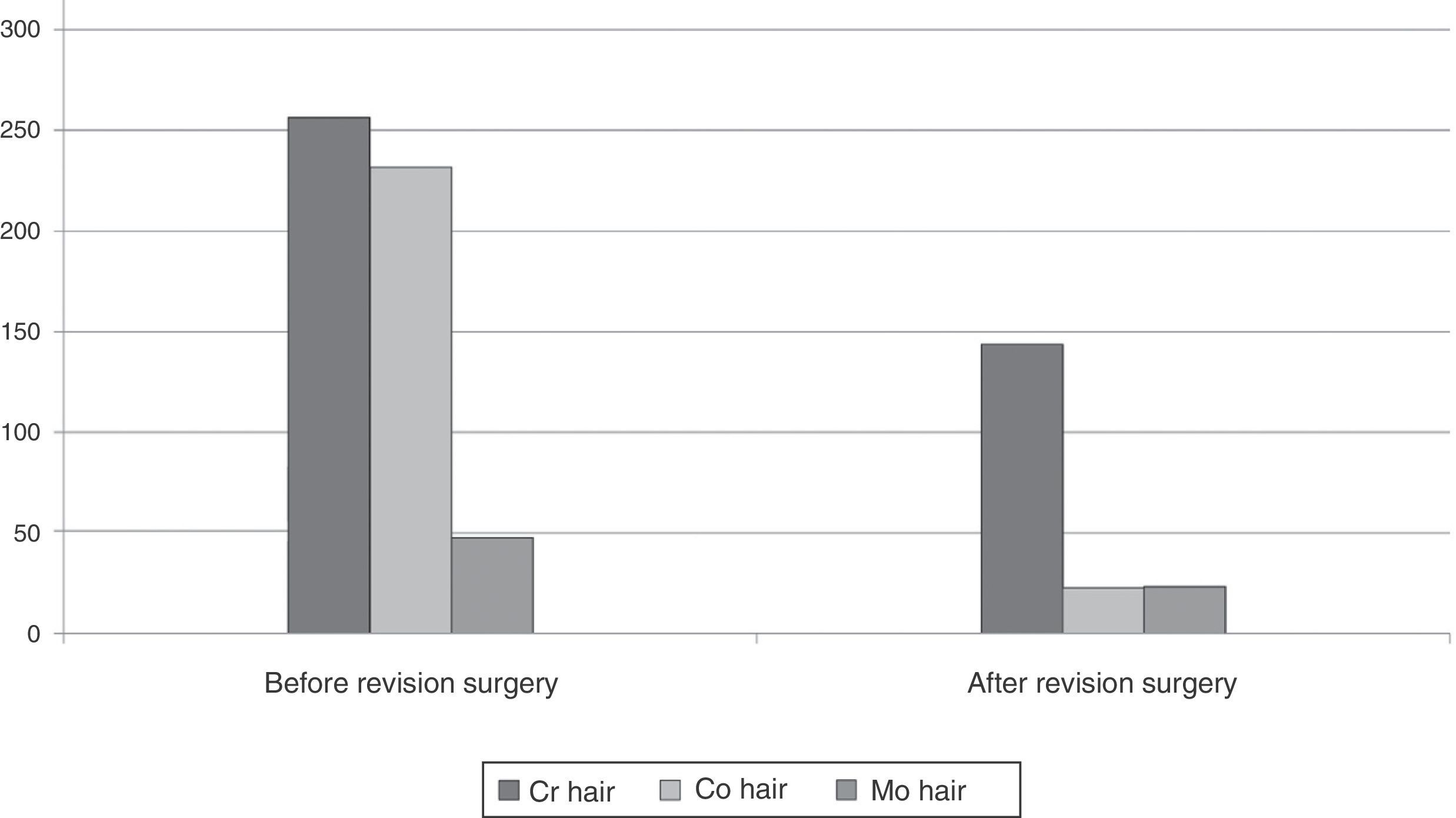
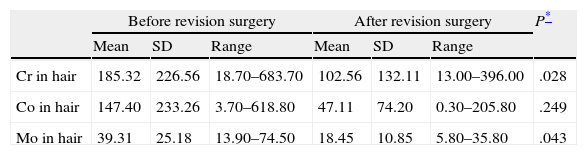
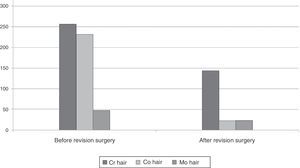
In order to determine whether the levels of these ions in hair were modified by removing the implant, we specifically studied the group of 11 patients who were reoperated, comparing the levels of metals before and after the revision surgery (Table 2). We observed significant differences in the decrease of Cr and Mo levels, and a trend toward significance in the case of Co. By exclusively studying the 5 patients who were reoperated due to metallosis we were able to observe a considerable difference between the measurements carried out before and after revision surgery, which represented a 43.8% decrease in the levels of Cr, 51.1% in Mo and 90.3% in Co (Fig. 2).
Comparative results following implant extraction (in ppm).
| Before revision surgery | After revision surgery | P* | |||||
| Mean | SD | Range | Mean | SD | Range | ||
| Cr in hair | 185.32 | 226.56 | 18.70–683.70 | 102.56 | 132.11 | 13.00–396.00 | .028 |
| Co in hair | 147.40 | 233.26 | 3.70–618.80 | 47.11 | 74.20 | 0.30–205.80 | .249 |
| Mo in hair | 39.31 | 25.18 | 13.90–74.50 | 18.45 | 10.85 | 5.80–35.80 | .043 |
SD: standard deviation.
The release of metal ions from M-M hip prostheses, especially in surface models, is one of the best studied types of exposure to metals, especially since local tissue reactions were detected.2 The importance of studying the consequences of this release is increased by the fact that one third of hip surgeries performed in the US during 2009 employed M-M prostheses and that there are over 500,000 patients carrying these types of prostheses in the country, most of them implanted between 2003 and 2010. Over a million M-M hip joints have been implanted worldwide since 1996.
Although metal contamination with an internal origin has shown no teratogenic or carcinogenic effects so far, poisoning from external sources has been associated with different effects on reproduction, renal and cardiac function. There have also been reports of bone marrow, spleen and liver alterations among patients with M-M friction arthroplasties. The most commonly affected systems and organs in the medium-term (10–20 years) are the hematopoietic and urogenital systems and the skin. In the long-term (20–40 years), solid organs can be affected,8 but in comparison with other types of implants there is no evidence that M-M surface implants are associated with an increased risk of cancer diagnosis in the 7 years following the intervention.9
Due to these effects, and especially to the possibility of unknown medium- and long-term complications, regular monitoring has been recommended in patients with an M-M arthroplasty10–12 and especially in those with hip surface models. The ASR model has shown a high rate of medium-term failures,13 which has led international agencies14 and the manufacturing company itself to recommend its withdrawal from clinical practice since 2010.
The main objective of our study was to know whether these models of M-M hip prostheses were associated with higher levels of metal detection in hair compared to the normal population and also to investigate whether removing the implant decreased those levels.
The study of metals in hair was carried out to assess their relationship with the development of certain diseases, such as Parkinson's, fibromyalgia, autism, schizophrenia, multiple sclerosis, etc.15–20
There are various techniques available for the analysis of trace metals in hair,21,22 including anodic dissolution voltammetry, X-ray fluorescence, spectroscopic elemental analysis by energy dispersion through an electron detector coupled to a scanning microscope, neutron activation analysis, attenuated total reflectance Fourier transform infrared, synchrotron radiation and ICP-MS. In our study we used ICP-MS because it is a new generation qualitative and quantitative technique which offers high resolution, is extensively validated and is cost-effective compared to the more novel synchrotron radiation technique.
Several studies23–25 have shown that levels of metals accumulated at concentrations above 10–50% in hair and their determination enable the analysis of the historical exposure of a patient, and not just the release of ions, which fluctuates over time. Hair is a slow-growing tissue, so it does not reflect rapid fluctuations in the levels of metals, but in order to assess the state of health it is more desirable to know the tissue load of metals than the amount being transported at a given time. The normal amounts of metals in the hair of unexposed populations are well known, including some small variability due to the use of different equipment for their determination.26–33 The figures for Cr in hair range between 0.46 and 1.16ppm, those for Co between 0.06 and 1.67ppm and those for Mo between 0.12 and 0.55ppm. As can be seen (Table 3), the figures in our series were much higher, and this can only be due to internal contamination produced by the prosthetic models.
In animals, continued exposure to Mo has originated fatty degeneration in the liver and kidneys, as well as altered levels of group B vitamins.34 Mo is a cofactor for several enzymes (xanthine oxidase, aldehyde oxidase, sulfite oxidase) and its presence is necessary for protein metabolism, sulfur metabolism, hydrolysis of phosphate esters and the transport and utilization of iron. Mammalian studies have observed different toxic effects derived from a high presence of this metal, particularly weight loss, diarrhea, alopecia and anemia. Its reproductive toxicity is known since the mid-twentieth century and several studies have proven gonadal involvement at a morphological and functional level (decreased sperm concentration, motility and morphological alterations), as well as embryotoxicity of male origin.
The ultimate objective of our study was to determine whether the levels of metals in hair decreased by removing M-M arthroplasties. We observed that these levels decreased after removing the prostheses among cases suffering metallosis. The decrease was over 40% in the levels of Cr, 50% in Mo and 90% in Co.
However, although we observed that the figures dropped considerably after revision surgery, they did not return to normal levels 1 year after the second intervention. This may be due to the effect of metal deposition and will presumably decrease over time. Another explanation may be that the new, metal–polyethylene friction implants also increase circulating metal levels.
The determination of metals in hair has several advantages. Its analysis does not require specific technical personnel to obtain samples, which can even be provided by patients themselves, thus avoiding the costs of specific healthcare staff and equipment for collection. There is no need to maintain the cold chain and the samples can be stored at room temperature for a long time without changes in composition. The determination of metals in hair is a low cost technique which also offers indirect savings, as patients do not need to visit healthcare centers during business hours as is unavoidably the case for blood collection and delivery of urine samples. Moreover, it also prevents the exposure of healthcare staff to biological fluids which could represent a medium for transmission of infections. Furthermore, an incorrect extraction and transport of blood samples can alter the results, whilst urine tests are tedious for patients, since they require the urine of 24h, which implies discomfort and increases uncertainty about the correct collection of samples, as there is no technical staff present. Both fluids require specific material for transport and storage of samples, and the cold chain must be maintained at all times.
The limitations of our study are, firstly, the absence of a control group; we have accepted the levels of metals in the hair of a normal population described in the literature and we have noted the considerable difference between the published figures and those obtained in our work. We do not know if metal levels in hair also increase with models of arthroplasties using other types of friction, like metal–polyethylene, but this does not invalidate the results obtained. Our series included few patients and a limited follow-up period. Due to the small number of patients who underwent revision surgery, the decrease in the levels of metals in hair could offer no statistical differences. However, the extraordinarily large differences between the measurements before and after implant removal confirmed that the levels of these metals in hair were modified through the placement and subsequent removal of M-M arthroplasties. A final limitation of our study is the absence of a link between metal levels in hair and general or local alterations in the medium- and long-term, which can only be established with the passage of time.
The results of our study show that the levels of Cr, Co and Mo in the hair of patients with this type of arthroplasty are higher than among the normal population, and that they decrease following implant removal. This indicates that this determination is a good biological indicator for the monitoring and study of the toxicokinetic behavior of metals released by M-M hip prostheses. This simple determination can be useful to screen for metal poisoning from joint prostheses among large populations, which can subsequently be monitored by standardized determinations in serum and urine.
Level of evidenceLevel of evidence iii.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work obtained a research grant from the Spanish Society of Orthopedic Surgery and Traumatology (SECOT) in 2011.
Please cite this article as: Hernandez-Vaquero D, Rodríguez de la Flor M, Fernandez-Carreira JM, Sariego-Muñiz C. Detección de iones metálicos en cabello tras artroplastia de cadera metal-metal. Rev Esp Cir Ortop Traumatol. 2014;58:267–273.