To evaluate the safety and efficacy of a single intra-articular injection of 2% hyaluronic acid (HA)+mannitol in symptomatic knee osteoarthritis (KOA).
Material and methodsPilot, multicentre, open, non-comparative study performed in eighty patients with painful KOA, of whom 79 completed the study. They received one injection of 2ml of 2% HA+0.5% mannitol (Day 0) and were followed-up for 6 months. On Days 0, 15, 30, 60, 90, 120, 150 and 180, pain and joint function were assessed using a visual analogue scale (VAS) and WOMAC index. Efficacy and safety by investigator and patient, and rescue medication, as an indirect measure of pain, were also recorded.
ResultsA significant reduction in joint pain, stiffness and functional disability compared with baseline was observed at every follow-up visit (P<0.001). Joint function improved by 38.7% on Day 30, reaching 47.5% on Day 180. Rescue medication use decreased from 58.2% at baseline to 2.5% on Day 90, increasing in the last visits. Efficacy and safety were positively evaluated by investigators and patients. No serious adverse events were observed. Mild side effects were reported in 4 patients (local pain and swelling in the infiltration area).
DiscussionThere is evidence that repeated intra-articular injections of HA improve symptoms in KOA. However, studies with a single injection of HA have shown mixed results. This study demonstrates that one single intra-articular injection of non-cross-linked HA reduces joint pain and increases function in patients with KOA over a period of at least 6 months.
Evaluar la eficacia y seguridad de una única inyección intraarticular de ácido hialurónico (AH)+manitol en artrosis de rodilla (AR).
Material y métodoEstudio prospectivo, abierto, no-comparativo con 80 pacientes diagnosticados de AR, de los cuales 79 finalizaron el estudio. Recibieron una inyección intraarticular de 2ml de AH al 2%+manitol al 0,5% (día 0) y fueron monitorizados durante 6 meses. Los días 0, 15, 30, 60, 90, 120, 150 y 180 se evaluaron dolor y funcionalidad articular mediante una escala analógica visual (EAV) y el índice WOMAC, eficacia y seguridad según médico y paciente, y medicación de rescate como medida indirecta del dolor.
ResultadosDisminución significativa del dolor articular, rigidez e incapacidad funcional en comparación con el valor inicial en todas las visitas (p<0,001). La funcionalidad articular mejoró un 38,7% 30 días tras la inyección, alcanzando un 47,5% el día 180. El consumo de medicación de rescate descendió desde un 58,2%, inicial, hasta un 2,5% el día 90, aumentando en las últimas visitas. Investigadores y pacientes valoraron positivamente eficacia y seguridad. Solamente se reportaron efectos adversos leves en 4 pacientes (dolor leve e inflamación en la zona de infiltración).
DiscusiónEstá demostrado que inyecciones intraarticulares repetidas de AH mejoran los síntomas en AR. Sin embargo, estudios con una única inyección de AH han proporcionado resultados mixtos. Este estudio demuestra que una inyección intraarticular de AH no crosslinked mejora el dolor y la funcionalidad articular en pacientes con AR durante un periodo mínimo de 6 meses.
Osteoarthritis is a disease that affects the synovial joints and is characterised by degradation and loss of articular cartilage with subchondral bone remodelling, osteophyte formation and inflammation of the synovium. Clinical signs include fluctuating joint pain, swelling, stiffness and loss of mobility, increasing in severity as the disease progresses.1–3 It is one of the most common causes of long-term disability among adults.4–7
Given the absence of a cure for the disease, to date the main treatment goals for osteoarthritis have been the reduction of symptoms, minimisation of functional disability and limitation of the progression of structural alterations.1–4,8 Current treatment options include non-pharmacological measures such as weight loss, the use of orthopaedic aids, exercising and physical therapy. Among the pharmacological measures we highlight the use of analgesics or NSAIDs, SYSADOA (symptomatic slow-acting drugs in osteoarthritis, mainly glucosamine, chondroitin sulphate and diacerein), opioids, intraarticular (ia) corticosteroid and hyaluronic acid (HA) injections and, in more advanced stages, surgical treatment.3–5,8–10
Intraarticular hyaluronic acid (ia HA) is widely used as a treatment to improve pain and joint function.4,9,11 It is an endogenous glycosaminoglycan of high molecular weight which is distributed throughout the organism, mainly in the hyaline cartilage, synovial fluid of joints, skin, vitreous humour and the connective segment of soft tissues.8,9 HA lubricates synovial joints, provides shock absorption and structure stabilisation and has direct effects on the function of synovial cells.8,9
Synovial fluid contains a lower concentration of HA in arthritic joints than in healthy joints,3,8–10 causing a substantial reduction in viscoelasticity and decreasing their lubricating and shock absorbing properties.7,9 This increases the mechanical load of joints and causes changes in the cartilage,7 subchondral bone and synovial membrane. This set of changes ultimately produce pain and limited movement of the affected joint. Since the elasticity and viscosity of synovial fluid are directly proportional to the content and integrity of HA, an i.a. injection of HA represents a rational approach for the treatment of osteoarthritis.8,9,11 It has been used successfully as a direct i.a. injection in degenerative processes of the articular cartilage, seeking to enhance the action of synovial fluid and resulting joint function.12–14 In addition, several clinical trials have shown that repeated i.a. injections of HA at different doses improve symptoms and especially pain in osteoarthritis.7,11,14–18
Nevertheless, HA infiltration can cause adverse side effects, some of which are related to the origin of the product (derivatives of animal proteins such as cockscomb) and are attributable to biological impurities.8 Other adverse side effects associated with HA infiltration, such as pain and swelling, are associated with the high molecular weight and concentration of some pharmaceutical HA specialties available, which are derived through cross-linked semisynthesis11 (HA chains stabilised synthetically by cross-linking). Since the majority of HA products available on the market require multiple injections (3–5) to achieve the desired efficacy (due to their rapid degradation within the joint8,16), their stabilisation and consequent increase in time within the joint may reduce the number of injections required to achieve long-term efficacy in the treatment of osteoarthritis.6–8 A single i.a. injection of HA may represent an alternative option to the current treatment regimes in terms of tolerability, logistics and cost, as a result of fewer injections and visits to the physician. This would provide greater comfort and safety for patients by reducing the risks associated with repeated injections, as well as an economic and logistic advantage for the hospital or medical centre.
This study was conducted with the primary objective of assessing the long-term effectiveness of treatment with Ostenil plus® (Masterfarm Laboratories SL, Barcelona, Spain) in terms of pain relief and joint functionality improvement. This agent is a natural, highly purified, clear solution of 2ml sodium hyaluronate at 2%, obtained by fermentation and devoid of animal protein, which also contains 0.5% of mannitol, a free radical scavenger which helps to stabilise sodium hyaluronate chains, thus increasing their residence time within the joint without increasing their molecular weight. We mainly studied the effect of a single i.a. injection of HA on the symptoms of knee osteoarthritis. The secondary objective was to study and define the safety of this treatment, by assessing tolerance and monitoring any adverse effects.
Material and methodWe conducted an exploratory, prospective, open, non-comparative and multicentre pilot study in phase IV. It was carried out by the Orthopaedic Surgery and Traumatology (OST) Services of the following centres: Hospital Universitario Virgen de la Macarena in Seville, Hospital Universitario Principe de Asturias in Madrid and Hospital Universitario Virgen de la Arrixaca in Murcia.
A total of 80 patients (aged 40 years or older) diagnosed with class III knee osteoarthritis according to the American College of Rheumatology (ACR) criteria were included in the study. Inclusion criteria for subjects were: at least class III joint functionality in the knee to be treated, diagnosed according to the requirements of the ACR (radiographs, symptoms and signs) and suffering pain and discomfort in the affected knee most days during the last 3 months. We excluded from the study those patients who suffered from other diseases which could confuse or interfere with the assessment of effectiveness, those who had received i.a. steroid and/or HA injections within the past 180 days, patients who had undergone arthroscopic lavage in the last year and those who were taking oral chondroprotective agents such as glucosamine or chondroitin sulphate, or enzymatically hydrolysed collagen supplements within 2 months before the start of the study. Additionally, we also excluded those patients who had participated in other clinical trials during the last 30 days and pregnant women. All subjects gave their written informed consent prior to participating in the study, which was approved by the Clinical Research Ethics Committees of each of the aforementioned centres.
The study design did not include a control group because it aimed to assess the long-term efficacy of a single injection of non-cross-linked HA+mannitol, given that the effectiveness of HA infiltration has already been established previously.
The 80 patients received an i.a. injection of sodium hyaluronate at 2%+mannitol at 0.5% (Ostenil Plus®) during their first visit and were monitored for 6 months, with evaluations in 8 visits on days 0, 15, 30, 60, 90, 120, 150 and 180. The primary efficacy parameters evaluated were clinical evolution of pain and joint function measured using a visual analogue scale (VAS) of 10cm for pain and the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) scale to measure pain and joint function (stiffness and difficulty). Both parameters affect the physical function and quality of life of affected patients. We also recorded the opinion of the physician and patient regarding treatment efficacy and tolerance, and monitored the possible occurrence of unwanted effects, both locally and in general. Moreover, during the study patients were allowed to consume 1g of paracetamol and/or 400mg of ibuprofen up to a maximum of 3g or 1200mg, respectively, provided that pain in the knee was equal to or greater than 7 on the VAS, as measured subjectively by patients. This intake of rescue medication was also recorded and analysed as an indirect measurement of pain, taking into account whether the intake was habitual, sporadic or did not take place.
Statistical analyses were based on the principle of intention-to-treat (ITT). ITT analysis was performed using the latest data recorded for each patient.
Data were analysed using IBM SPSS 19.0 for Windows. We conducted a descriptive analysis of all variables analysed in the study (n, mean, standard deviation and graphs with mean and 95% confidence interval of the mean for each variable). Regarding inferential statistics, we carried out an analysis of variance for repeated measures (linear mixed model) in order to analyse the evolution of different variables across visits. We made 2 to 2 comparisons with respect to controls using Bonferroni correction. The first visit was considered as the control for the VAS and WOMAC variables, whereas the second visit was considered as the control for the opinion of the physician and patient on efficacy and tolerance. The confidence level (1−α) was set at 95%, with a significance level of 0.05 and a statistical power of 90%.
ResultsWe recruited a total of 80 patients for our study. One patient did not turn up to follow-up visits after treatment, so he was excluded from the study. Thus, the analysis included 79 patients who continued monitoring. Of the 79 patients, 6 dropped out during the follow-up period for reasons not related to the study: 2 patients discontinued monitoring due to accidents, in the third and sixth visit, respectively, and 4 patients dropped out because they did not attend the last assessment visits without a reason. The latest data obtained from these patients were carried until the end of the study in order to conduct statistical analysis, as stipulated in the protocol.
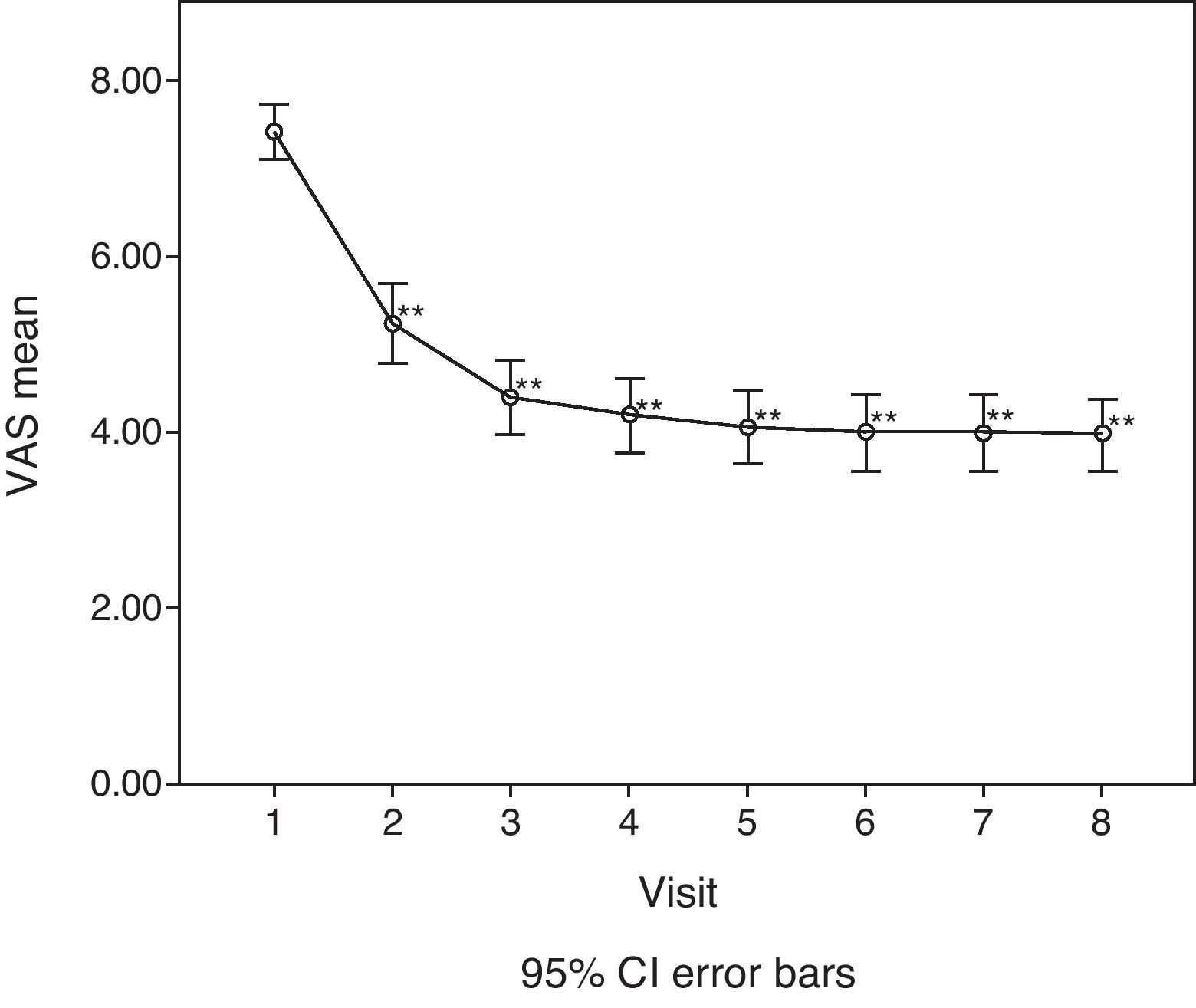
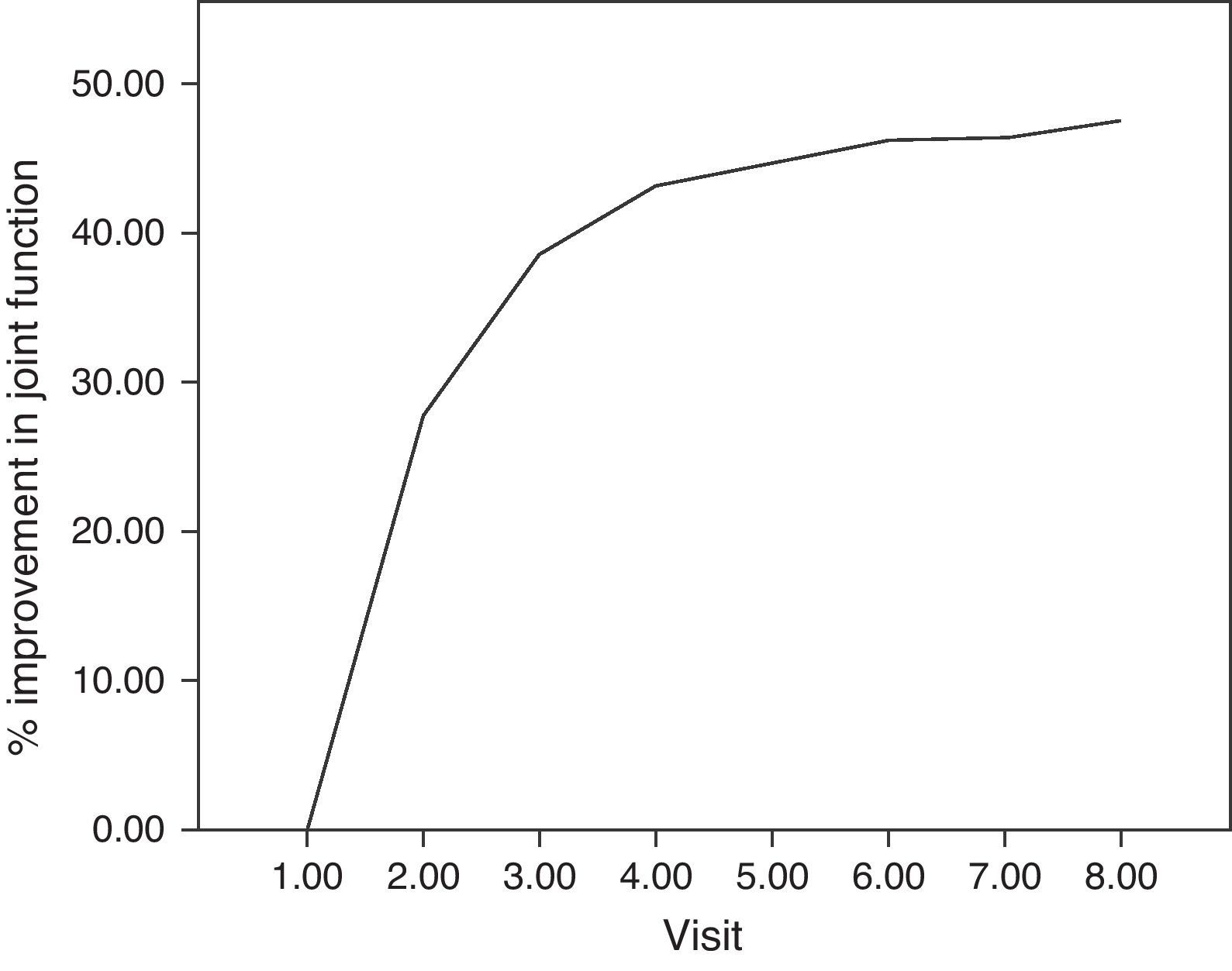
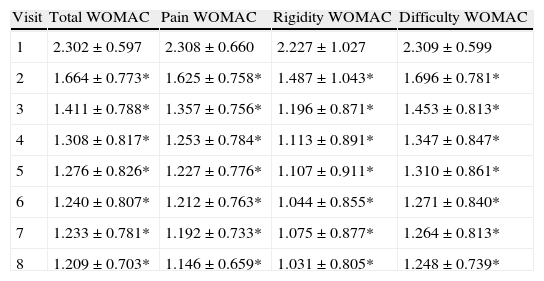
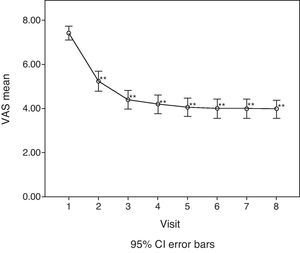
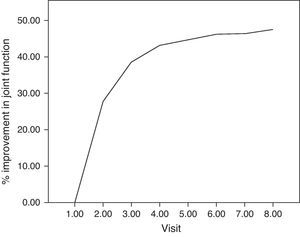
For the main efficacy parameters studied, the extent of joint pain assessed by VAS showed a statistically significant decrease (P<0.001) after the first follow-up visit (15 days) compared to the initial value (before HA infiltration). This decrease was maintained until the last visit (6 months). Fig. 1 shows the differences in knee joint pain from the start of the study, where the mean value was 7.41 (out of 10), until its end, where it reached a mean value of 3.97. In addition, the assessment of quality of life, specifically joint pain and function as measured by the WOMAC index (score from 0=none to 4=extreme) showed a statistically significant decrease (P<0.001) compared to the baseline after the second visit, whether studied in its overall assessment or divided into the pain, stiffness and functional disability components. This decrease was maintained in subsequent visits, up to 6 months after treatment. Table 1 summarises the changes in the WOMAC scale throughout the different visits. Moreover, we also calculated the percentage of patients who presented improved joint function reflected by the WOMAC index values in its overall assessment (Fig. 2). We observed that, after 30 days of treatment, joint functionality had improved by 38.7% compared with the initial assessment, reaching an improvement of 47.5% at 180 days.
Evolution of the mean assessment of the WOMAC index.
| Visit | Total WOMAC | Pain WOMAC | Rigidity WOMAC | Difficulty WOMAC |
| 1 | 2.302±0.597 | 2.308±0.660 | 2.227±1.027 | 2.309±0.599 |
| 2 | 1.664±0.773* | 1.625±0.758* | 1.487±1.043* | 1.696±0.781* |
| 3 | 1.411±0.788* | 1.357±0.756* | 1.196±0.871* | 1.453±0.813* |
| 4 | 1.308±0.817* | 1.253±0.784* | 1.113±0.891* | 1.347±0.847* |
| 5 | 1.276±0.826* | 1.227±0.776* | 1.107±0.911* | 1.310±0.861* |
| 6 | 1.240±0.807* | 1.212±0.763* | 1.044±0.855* | 1.271±0.840* |
| 7 | 1.233±0.781* | 1.192±0.733* | 1.075±0.877* | 1.264±0.813* |
| 8 | 1.209±0.703* | 1.146±0.659* | 1.031±0.805* | 1.248±0.739* |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Mean±standard deviation for WOMAC scale (Total, Pain, Rigidity and Difficulty).
Statistically significant differences (*P<0.001) for 2 to 2 comparisons with respect to the first visit (Bonferroni method).
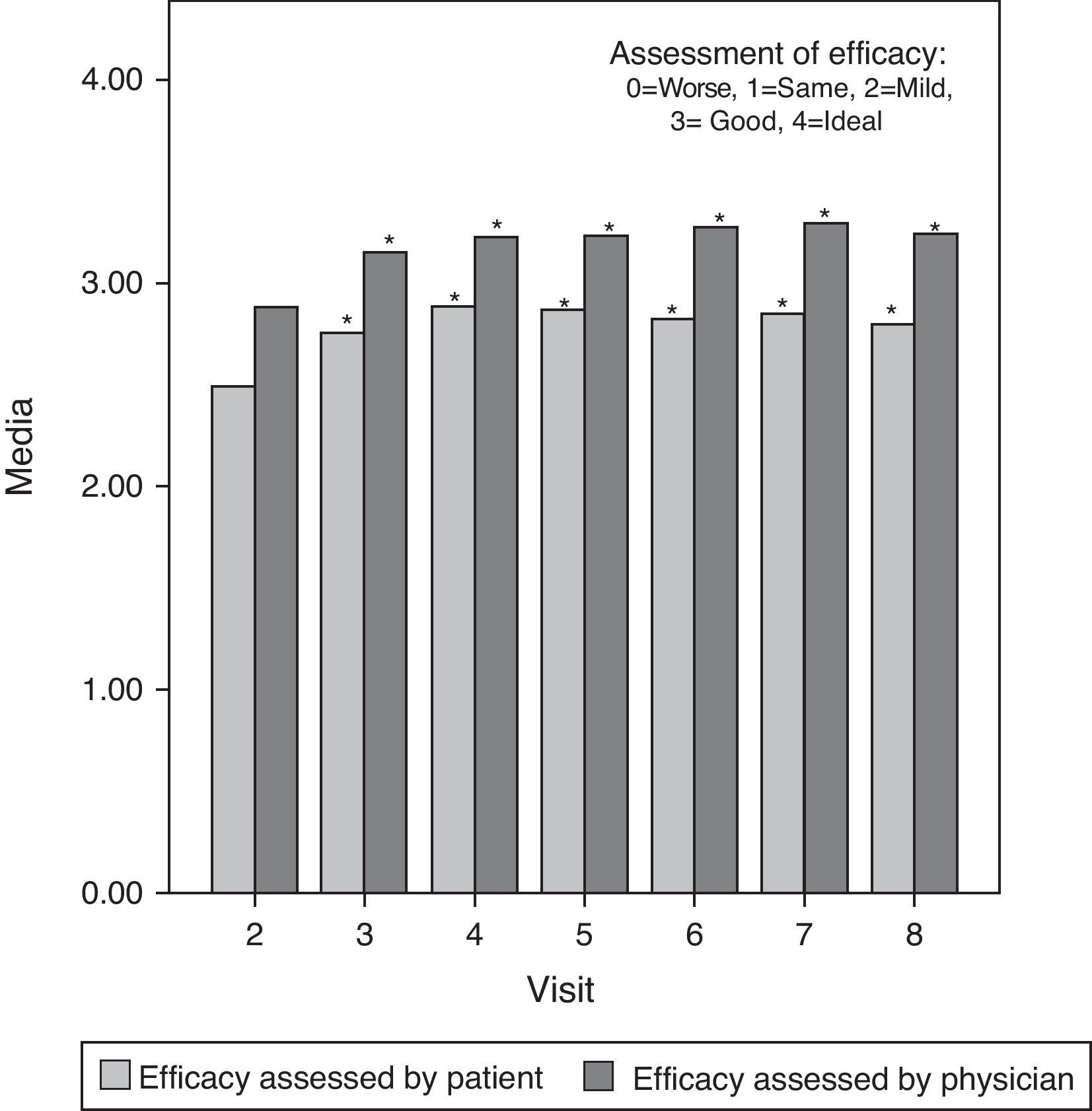
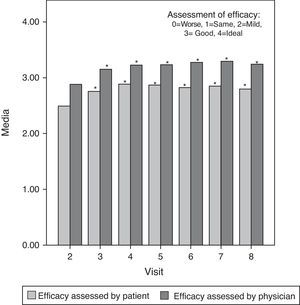
Moreover, the mean assessment of effectiveness, scored from 0 (worst) to 4 (ideal) both by the investigator and the patient, was good or very good throughout the study. There were statistically significant differences (P<0.05) between the first assessment of efficacy (second visit) and subsequent evaluations in all cases except for patient assessment at the last visit, which showed no significant differences compared with the baseline. This last assessment by patients suggested that some of the initial symptoms started to manifest once again 6 months after treatment. Fig. 3 shows these results graphically.
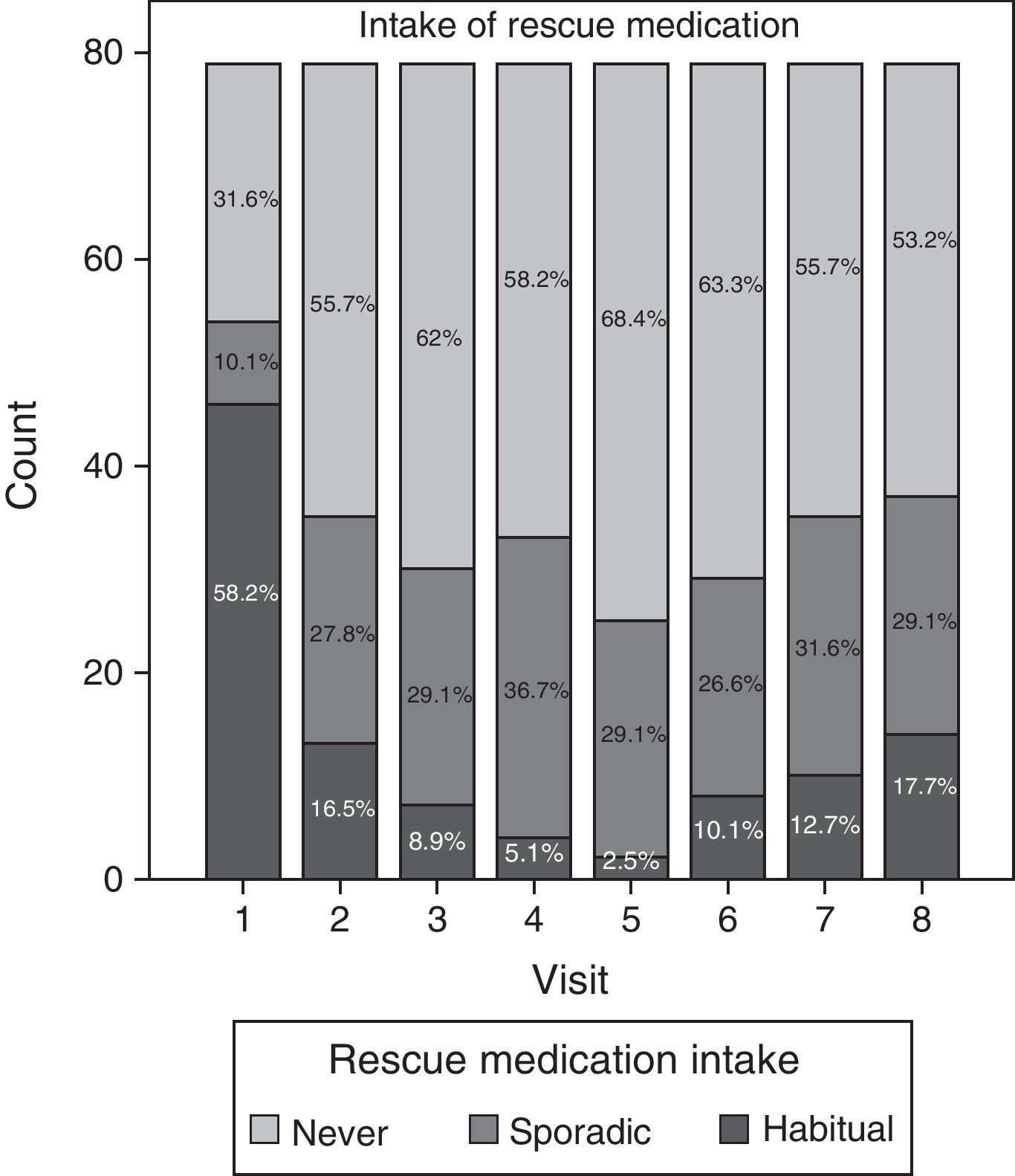
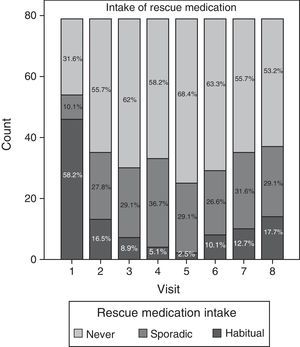
Data relating to consumption of rescue medication during the study are summarised in Fig. 4. Although most patients (58.2%) regularly took analgesic and anti-inflammatory agents during the initial visit, the consumption of such medication decreased considerably as the study progressed. Regular use of medication was lowest (2.5%) at visit 5 (Day 90). Nevertheless, consumption tended to increase once again after visits 6 and 7, both sporadically and habitually, reaching a regular consumption of paracetamol and/or ibuprofen of 17.7% during the last visit.
In order to assess tolerance, the investigator and the patient scored this parameter at each visit. No significant differences were observed over time, since this outcome was excellent from the start of the study (infiltration visit) until its the end.
Regarding safety, no severe adverse effects were observed during the study. Mild adverse effects were reported in the second follow-up visit by 5.06% of patients (n=4). These adverse effects consisted of mild pain and swelling in the area of infiltration in all cases, and disappeared during subsequent visits.
DiscussionAt present, many of the pharmaceutical HA products available on the market are administered in repeated doses (3–5 injections). Several clinical studies have shown that they improve symptoms and, especially, pain in osteoarthritis.4,10,11,14 More recently, clinical studies have been carried out in order to demonstrate the efficacy and safety of a single injection of HA in the treatment of knee and hip osteoarthritis with mixed results.7,8,19,20 In most cases, these studies employed HA formulations with a high molecular weight (cross-linked or semisynthetic laboratory products) and obtained results which demonstrated efficacy, although with unequal safety and duration of response and with more mild local adverse effects than with untreated HA (non-cross-linked).7,20 Only one study, conducted by Richette et al.,6 employed a single injection of untreated HA, obtained by fermentation and with a medium molecular weight. This study included patients with hip osteoarthritis and the results obtained after 3 months monitoring were not satisfactory, since there were no differences in pain reduction between the placebo and treatment groups. Different hypotheses, such as a high placebo effect, the study design or lack of efficacy of the treatment itself related to the concentration and/or dosage, could explain the lack of efficacy of a potentially active treatment in a clinical trial.
Taking all the above into account, our study was designed with the purpose of administering a single i.a. injection of HA for different reasons. First, repeated injections could lead to an increased risk of local side effects. Second, reducing the number of injections and visits to the physician was convenient for patients and represented an economic and logistic advantage for the hospitals and medical centres. Lastly, there are no recent studies on knee osteoarthritis employing a single injection of non-cross-linked HA with a medium molecular weight which assess safety and efficacy results in the long term. Therefore, a single i.a. injection of HA may represent a therapeutic alternative to current treatment regimes in terms of efficacy, safety and comfort for patients, and logistics for medical centres.
The results obtained in this study demonstrate that a single i.a. injection of HA at 2%+0.5% mannitol (Ostenil plus®) is effective in reducing long-term pain in patients with knee osteoarthritis. Its special composition including mannitol increases the stability of HA and its salts when injected directly into the joint, thus prolonging the mean residence time of HA within the joint cavity by protecting it from degradation.21,22 The mean value of the efficacy assessments (VAS and WOMAC index) indicated a clear improvement after treatment which was statistically significant for all parameters measured, both those related directly to pain and those related to joint functionality and quality of life. Moreover, this improvement was maintained in a statistically significant manner (P<0.001) throughout the study (6 months).
Similar studies have employed the same evaluation parameters, using questionnaires other than the WOMAC index, such as the Lequesne index.10 In this study we decided to use the WOMAC index because it is an instrument which has been specifically validated for osteoarthritis and which is clinically useful to assess pain, joint stiffness and functional capacity in affected patients. The Lequesne index was developed to evaluate the severity of hip osteoarthritis, although there is a specific version for the knee. Its assessments include pain, maximum walking distance and daily activities.
Despite the limitations of the study (open, non-comparative), these efficacy results enabled us to establish that the therapeutic effects of our treatment lasted for the entire follow-up period of 6 months. This finding was reinforced by data collected from the records of rescue medication consumed by patients, which showed that, after 6 months, some patients started to consume analgesics and anti-inflammatory agents in a sporadic manner, without returning to their initial habitual consumption. At this point, subsequent clinical monitoring of patients became necessary in order to decide when the treatment should be repeated.
Another objective of the study was to evaluate the safety profile of the treatment. We observed that treatment was well-tolerated and this observation was confirmed both by investigators and patients, and by the low incidence (5.06%) of adverse events during the study. These results are in contrast with those obtained by previous studies with HA formulations of high molecular weight (cross-linked or obtained by semisynthesis), in which a high incidence of pain and swelling in the area of infiltration took place during the days after injection.7,20 The excellent safety profile of the treatment resulted in a positive benefit/risk ratio for patients.
In conclusion, this study was the first to show that a single i.a. injection of non-cross-linked HA at 2%+0.5% mannitol is an effective treatment for knee osteoarthritis, as it decreases pain and improves joint functionality for a minimum period of 6 months. In addition, it has a low incidence of associated mild adverse effects.
In daily practice, a favourable benefit/risk ratio of a single i.a. injection of 2ml HA at 2%+mannitol represents a good option for decreasing the number of HA injections from between 3 and 5 to just one single injection per treatment cycle. More extensive studies are needed in order to determine the duration of the treatment cycle beyond 6 months follow-up.
Level of evidenceLevel of evidence III.
Ethical responsibilitiesProtection of people and animals. The authors declare that this investigation did not require experiments on humans or animals.Confidentiality of data. The authors declare that they followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in it.Right to privacy and informed consent. The authors obtained informed consent from all patients and/or subjects mentioned in the article. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflicts of interest to declare.
The authors wish to thank Anna Delgado García and Luisa Varela Sende for their cooperation, allowing us to obtain bibliographic and biostatistics data to bring this study its conclusion.
Please cite this article as: Borrás-Verdera A, et al. Eficacia y seguridad de una única inyección intraarticular de ácido hialurónico al 2% más manitol en artrosis de rodilla durante un periodo de 6 meses. Rev Esp Cir Ortop Traumatol. 2012;56:274–80.