To evaluate the results of bloc resection and vascular reconstruction of leiomyosarcomas with involvement of main vessels in the lower extremities.
Material and methodsFrom January 1983 to December 2016, 42 patients with leiomyosarcomas were diagnosed. Six of these leiomyosarcomas affected main vessels of the lower extremities (called vascular). Epidemiological data, imaging studies, surgery performed, adjuvant treatments, complications, as well as recurrences and mortality were retrospectively recorded.
ResultsAll the patients were affected by high-grade leiomyosarcomas (II–III FNCLCC classification), with a larger tumour average diameter of 9.1cm (6–15) and a mean follow-up of 23 months (7–36). The average age was 64 years (29–84). The first symptom was a palpable tumour in 4 of them. The other 2 cases debuted with thromboembolic phenomena. In 5 cases the origin was the femoral vessels, while one case was at the popliteal level. Although all cases preserved the limb, in 3 cases (50%) they presented pulmonary dissemination, 2 cases (33%) hepatic dissemination and one case had local recurrence. Two cases died at the end of the study and there was one case of loss to follow-up.
Discussion and conclusionsVascular leiomyosarcomas are highly aggressive tumours with a low survival rate at 5 years. In our study, 50% of the patients remain in complete remission with a mean follow-up of 23 months. Their onset frequently associates the presence of tumour mass with thrombotic phenomena (33% of our cases). Tumour resection surgery usually compromises the main vascular structures, which implies resection and vascular reconstructive techniques to salvage the limb.
Evaluar los resultados de la resección en bloque y la reconstrucción vascular de leiomiosarcomas con afectación de vasos principales en las extremidades inferiores.
Material y métodosDesde enero de 1983 a diciembre 2016 se diagnosticaron 42 pacientes con leiomiosarcomas, de los que 6 afectaban a vasos principales de las extremidades inferiores (denominados vasculares). Se registraron retrospectivamente datos epidemiológicos, estudios de imagen, cirugía realizada, tratamientos adyuvantes, complicaciones, así como las recidivas y mortalidad.
ResultadosTodos los pacientes fueron afectos de leiomiosarcomas de alto grado(ii-iii FNCLCC), con un diámetro mayor tumoral de 9,1cm de media (6-15) y un seguimiento medio de 23 meses (7-36). La edad media fue de 64 años (29-84). El primer síntoma fue una tumoración palpable en 4 de ellos. Los otros 2 casos comenzaron con fenómenos tromboembólicos. En 5 casos el origen fueron los vasos femorales, mientras que un caso fue a nivel poplíteo. A pesar de que todos los casos preservaban la extremidad, 3 (50%) presentaron diseminación pulmonar, 2 (33%) hepática y un caso presentó recidiva local. Dos casos fueron exitus al finalizar el estudio y hubo un caso de pérdida de seguimiento.
Discusión y conclusionesLos leiomiosarcomas vasculares son tumores altamente agresivos con baja tasa de supervivencia a los 5 años. En nuestro estudio, el 50% de los pacientes se mantienen en remisión completa con un seguimiento medio de 23 meses. Es frecuente que su inicio asocie la presencia de una masa tumoral con fenómenos trombóticos (33% de nuestros casos). La cirugía de resección tumoral habitualmente compromete las estructuras vasculares principales, hecho que implica una resección y técnicas reconstructivas vasculares para el salvamento de la extremidad.
Leiomyosarcomas (LMS) are mesenchymal tumours composed of spindle-shaped cells, which is derived from smooth muscle. They constitute 5–10% of all soft tissue sarcomas.1 Regardless of their body location, they are histologically similar, but their prognosis and treatment are markedly different. For that reason, LMS have been categorised into four large groups according to their origin:
Vascular LMS, or LMS of vascular origin (in some articles, of intravascular origin), is rare, corresponding to 6% of all LMS cases and approximately 0.7% of soft tissue sarcomas.6 They tend to arise in the low pressure vascular systems, primarily in the middle media (muscular) lining the walls of the major blood vessels (although there are cases originating in arterial structures).7 Most publications on vascular LMS refer to a series of isolated cases or of few patients, which is why there is no uniform consensus on its treatment.
The objective of this study was to present the clinical and histological results and the prognoses obtained in a series of 6 vascular LMS in the lower extremities, which were treated using en block resection and vascular reconstruction.
Material and methodsFrom January 1983 to December 2016, a total of 42 patients with soft tissue LMS in the extremity (we excluded bone LMS cases) were diagnosed. Of the 42 patients, 6 were of vascular origin, affecting the major blood vessels in the extremities, all inferior. Epidemiological data, such age and sex, were recorded, as well as symptom onset, location and size, imaging studies, histological grade, operation performed, adjuvant therapy and evolution followed for each patient. Early and late complications, reoperations, recurrences and mortality were also registered.
The Oncological Orthopaedic Surgery Unit followed the patients up postoperatively 1 month after surgery and every 3 months during the first 2 years. If the patient remained in remission, follow-up was performed every 6 months until 10 years postoperatively. If there was local recurrence, follow-up was adapted to the therapeutic decision of the Sarcoma Committee, which depended on whether new operation and/or adjuvant therapy.
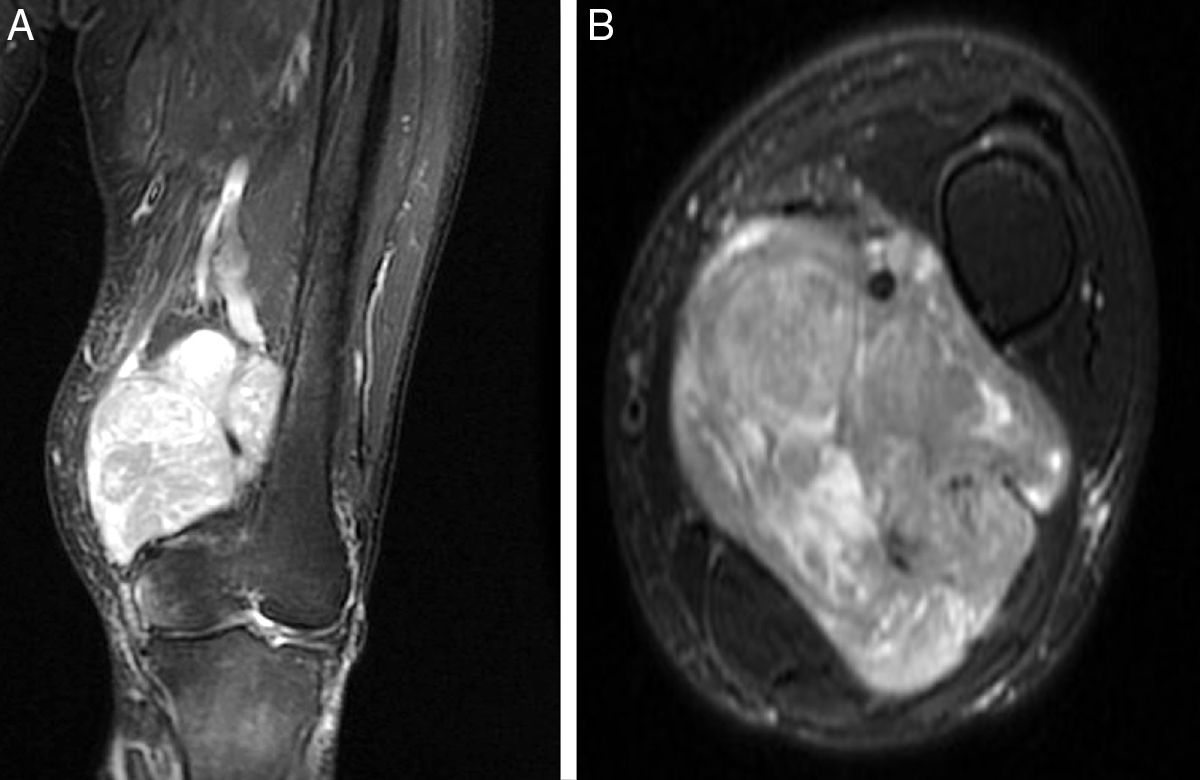
All patients received complementary examinations to establish the grade of vascular involvement: simple X-rays and magnetic resonance imaging (MRI) of the affected extremity (both pre- and post-adjuvant therapy in the cases that received preoperative chemotherapy and/or radiation therapy), thoracoabdominal computerised tomography (CT) scan as an extension study and angiographic studies (whether with angiography or angio-CT).
For the preoperative pathology diagnosis, a core needle biopsy was performed in all cases. Histological grade was also reviewed in all cases based on the French staging classification of the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC),8 both the biopsies and the final tumour resection piece from which we established the definitive histological grade. The FNCLCC classification evaluates 3 parameters: (1) tumour differentiation, (2) mitotic count and (3) tumour necrosis. Each item is assigned a score and, from their sum, 3 grades are set. Grade I has a total score of 2–3, grade II, of 4–5, and grade iii, of between 6 and 8. The oldest cases were reviewed again histologically based on the FNCLCC classification to standardise the study.
As for treatment, all the patients were evaluated by the hospital's Multidisciplinary Committee on Sarcomas, in order to match the best medical-surgical treatment for each specific case.
ResultsThis was a retrospective study on 6 patients with vascular LMS in the lower limbs, with a mean follow-up of 23 months (range, 7–36). At the end of the control, 2 patients had died, 1 was lost to follow-up and 3 were alive and in clinical remission. Mean age at diagnosis was 64 years (29–84), with the same number of male and female cases. With respect to the first symptom, 4 cases reported the appearance of a clinically-palpable tumour mass (painful in 2 of these patients). The other 2 cases began with thrombo-embolic phenomena, 1 of them with a pulmonary thromboembolism and the other with a deep thrombosis of the lower limb.
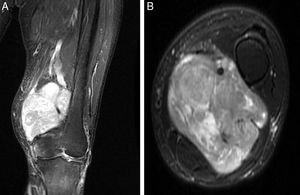
The LMS arose from the femoral vein in 5 cases, in the limb root; in the remaining case, it originated at the level of the popliteal fossa, affecting the popliteal vein. Injury morphology and degree of involvement of the adjacent vascular bundle were revealed in the MRI (Fig. 1). Mean diameter of the tumour mass was 9.1cm (6–15). Histopathological study of the preoperative biopsies showed that the disease was high-grade LMS (II–III in the FNCLCC classification) in 100% of the cases. The extension study at time of diagnosis revealed the presence of pulmonary dissemination in 2 of the cases (33%).
All the patients received surgery for the resection of the tumour in our centre. To get oncologically proper in a soft tissue sarcoma we have to bear in mind the tumour involvement in both the adjacent tissues and the arteries and muscles, as well as the tissue where the tumour originated (in our cases, the superficial femoral vein and popliteal vein). In curative preoperative planning, if more than 50% of the perimeter of the artery adjacent to the LMS is surrounded by tumour mass, it is a candidate for resection and reconstruction.9
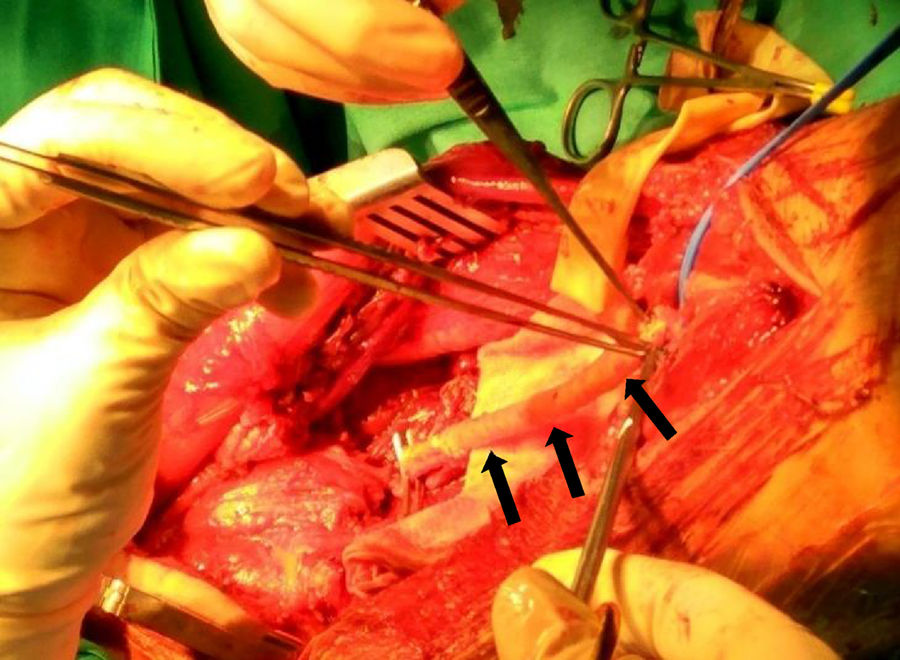
All of our patients presented extraluminal tumour growth that surrounded more than 50% of the main artery (detected in the preoperative imaging studies), a fact that required us to carry out an en block arterial resection with the tumour mass.10 The margins obtained in 3 cases were R0, and, in the other 3, R1. As for the arterial reconstruction, it is preferable to use an autograft (such as the contralateral saphenous vein) because it has a lower risk of infection and resistance if radiation therapy is needed in the surgical bed. When there is no proper autograft available, the choice is an artificial vascular graft. Consequently, we used the contralateral internal saphenous vein for the arterial reconstruction in 3 cases, and an artificial vascular graft in the other 3 (GORE® PROPATEN®, Omniflow® and polytetrafluoroethylene, respectively) (Fig. 2).
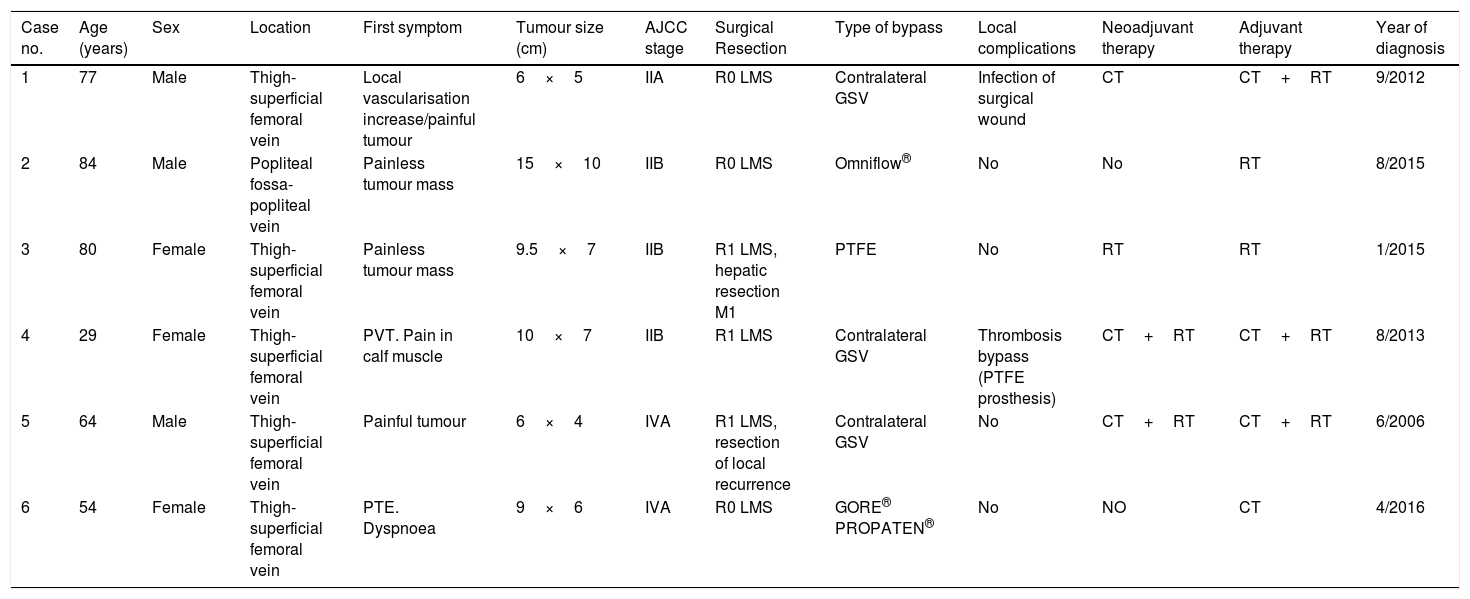
In all cases, the final resection piece revealed that histologically there was a high-grade LMS (2–3 FNCLCC), all of them coinciding with the grade obtained in the biopsy. As for preoperative adjuvant oncological treatments, 1 patient received chemotherapy; another patient, just radiation therapy; and 2 patients, combined therapy with both chemo- and radiation therapy. With respect to postoperative adjuvant therapy, chemotherapy was administered to 1 patient; isolated radiation therapy in the surgical bed, to 2 patients; and combined chemo- and radiation therapy to the 3 remaining patients. The indications of our centre's Committee on Sarcomas were always followed according to the protocol in force at the time (Table 1).
Clinical and epidemiological leiomyosarcoma data in our centre.
| Case no. | Age (years) | Sex | Location | First symptom | Tumour size (cm) | AJCC stage | Surgical Resection | Type of bypass | Local complications | Neoadjuvant therapy | Adjuvant therapy | Year of diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | Male | Thigh-superficial femoral vein | Local vascularisation increase/painful tumour | 6×5 | IIA | R0 LMS | Contralateral GSV | Infection of surgical wound | CT | CT+RT | 9/2012 |
| 2 | 84 | Male | Popliteal fossa-popliteal vein | Painless tumour mass | 15×10 | IIB | R0 LMS | Omniflow® | No | No | RT | 8/2015 |
| 3 | 80 | Female | Thigh-superficial femoral vein | Painless tumour mass | 9.5×7 | IIB | R1 LMS, hepatic resection M1 | PTFE | No | RT | RT | 1/2015 |
| 4 | 29 | Female | Thigh-superficial femoral vein | PVT. Pain in calf muscle | 10×7 | IIB | R1 LMS | Contralateral GSV | Thrombosis bypass (PTFE prosthesis) | CT+RT | CT+RT | 8/2013 |
| 5 | 64 | Male | Thigh-superficial femoral vein | Painful tumour | 6×4 | IVA | R1 LMS, resection of local recurrence | Contralateral GSV | No | CT+RT | CT+RT | 6/2006 |
| 6 | 54 | Female | Thigh-superficial femoral vein | PTE. Dyspnoea | 9×6 | IVA | R0 LMS | GORE® PROPATEN® | No | NO | CT | 4/2016 |
| Follow-up period (months) | Survival | Metastasis at diagnosis | Dissemination during developmental period | Demise |
|---|---|---|---|---|
| 24 | Death at 24m | No | Mesenteric, hepatic and pulmonary dissemination | Yes |
| 20 | In clinical remission | No | No | No |
| 24 | In clinical remission | No | Hepatic dissemination | No |
| 36 | Lost to follow-up | No | No | No |
| 26 | Death at 26months | Pulmonary metastasis | No | Yes |
| 7 | In clinical remission | Pulmonary metastasis | No | No |
AJCC: American Joint Committee for Cancer; CT: chemotherapy; GSV: great saphenous vein; PTE: pulmonary thromboembolism; PVT: portal vein thrombosis; RT: radiation therapy.
One early complication was 1 case of superficial infection of surgical wound, which was solved with antibiotic therapy. Another early complication was another case that developed acute limb ischaemia from acute bypass blockage, which required a new bypass (this time without any complications).
Oncologically speaking, although all the cases had a functional limb at the end of the study, 3 cases (50%) presented pulmonary dissemination (2 of them when the primary tumour was diagnosed and 1 in the developmental follow-up). Likewise, 2 patients (33%) developed hepatic metastasis during the evolution (pulmonary metastasis also affected 1 of them). With respect to tumour progression, the patient with initial pulmonary metastasis (Case 6 in Table 1) remains in clinical remission after receiving coadjuvant chemotherapy, as well as the patient with resected hepatic metastasis (Case 3 in Table 1). The other 2 patients with systemic dissemination died.
One patient presented a local recurrence. For that reason, it was resected surgically.
As for mortality, 2 patients had died at the end of the study, with a mean of 2 years from diagnosis (24–26 months). Three patients remained in complete remission from the disease, with a mean of 17 months from diagnosis (7–24 months). Follow-up data for Case 4 in Table 1 is only available up to 36 months post-diagnosis.
DiscussionVascular LMS is rare, which is why there are few published cases of it. Consequently, experience is scant as to its clinical presentation and the therapy to follow. In this study, there are important limitations such as the small sample size and the long selection period, which we consider relevant given the rarity of the cases analysed.
LMS arises from the smooth muscle cells of vascular walls, fundamentally venous. Although the vena cava is the main source involved in these tumours,12 they have also been described in the major veins of the lower limbs,13 less frequently in the upper limbs14 and, rarely, in vascular arterial structures.15,16 In our study, we have just 6 cases corresponding to LMS affecting major vessels, 14% of all the soft tissue LMS cases in limbs treated in our hospital's Sarcomas Unit.
Diagnosis of suspected LMS is difficult if it is based only on the physical examination, because there is no parameter that distinguishes vascular LMS from other soft tissue sarcomas in lower limbs. The primary finding that usually raises suspicion is the presence of a palpable tumour mass, normally painless and slow growing in the path of a main vessel (generally in the inguinal or popliteal area). We should sometimes suspect the diagnosis of LMS when there is a history of thromboembolic phenomena associated with an inguinal mass. In our study, 2 patients (33% of the cases), began with thromboembolic clinical picture without presenting any relationship to a greater systemic dissemination of the disease during its progression.
At age of onset, the patient is generally between 50 and 70 years old, with a similar male–female incidence for all types of LMS.17 However, sex dominance in vascular LMS varies from one study to another, sometimes appearing more frequently in men than in women and sometimes, vice versa.4,17,18 Both age and sex in our review coincide with the literature published.
Once the diagnosis of suspected soft tissue sarcoma has been made, imaging studies must be started. These should include simple X-rays of the area affected, as well as an MRI, which is ideal for assessing the true anatomical extent of the tumour.19 The angiographic study is particularly important in diagnosing LMS, to evaluate secondary vascular involvement. Vascular LMS cases can either be intraluminal, spreading only within the vessel, or extraluminal, surrounding the affected vessel completely. This face is fundamental in surgical therapy, because if tumour growth is intravascular, the vein involved can probably be resected from the adjacent artery without sacrificing it. In contrast, if the growth is extravascular, arterial resection and posterior reconstruction are normally needed; this can occur in up to 33% of the cases.17,20 In our series of vascular LMS, it was 100%.
Once the presumptive diagnosis of vascular LMS has been obtained, it must be confirmed histologically though biopsy. We always prefer using a core needle biopsy for this, to affect as little tissue as possible and avoid tumour ulcerations and infections. In our centre, we have been using computed tomography (CT)-guided core needle biopsies because that is the way to avoid injuring healthy neurovascular structures. In this study we have not needed to perform an incisional biopsy, which we reserve for cases in which the core needle biopsy is not diagnostic. Histologically, samples can vary significantly. However, it is common to see a highly cellular field with abundant eosinophilic cytoplasm. The cells are arranged in bundles that (in well differentiated tumours) often form right angles; this makes it possible to identify both the longitudinal and transversal areas within a field. The nuclei are usually found in the centre and with a cigarette shape. One of the most important tumour characteristics is the presence of longitudinal myofibrils that span the entire cell longitude. However, as the cells stop being differentiated, they become disorganised and begin to lose their distinctive characteristics,21 consequently increasing the level of tumour malignancy.
Therapy has to include complete resection of the tumour and of the vascular segment involved with negative margins, together with the healthy adjacent tissues that cover the tumour (whether muscle, fascia, fat or lymph) (Fig. 3).17,22 The vascular axis is considered infiltrated when there is a stenosis and/or a thrombosis, or when the tumour mass surrounds more than 50% of the vessel perimeter. Given that the vascular LMSs arise from major blood vessels, extraluminal tumour expansion changes them by definition into extra-compartmental tumours. In the absence of sufficient collateral arterial circulation, vascular reconstruction with an interposition graft is needed, whether the graft is from the patient's own saphenous or using a vascular prosthesis. Vein reconstruction will depend on the presence of an incompetent superficial system and being able to prevent venous hypertension and its consequences.22 However, it is not free from complications, such as occlusions and chronic oedema.
As for adjuvant therapies, radiation therapy is an essential tool, especially in patients with high-grade LMS. Chemotherapy can be of great help in cases of high-grade LMS, recurring tumours and/or disseminated disease.
According to the bibliography consulted, local recurrence and metastasis generally appear in the first 3 years after diagnosis; the lung is the organ most often affected by distant metastasis, as occurred in our patients. We generally agree with the literature in that vascular LMS cases constitute aggressive tumours with a poor prognosis; among all the soft tissue sarcomas, survival rates for vascular LMS are the lowest, less than 50% at 5 years.23 Ascertaining the value of chemotherapy in improving survival will depend on future studies with a greater number of cases. Even so, assessment and planning should be multidisciplinary to offer the most appropriate therapy in each case, both systemic and local, given the continuous advancements in oncology.
Level of evidenceLevel of evidence IV.
Conflict of interestThe authors declare no conflict of interests.
We wish to thank Dr Ignasi Proubasta Renart for his teaching skills and dedication, as well as for how he constantly motivates us to advance scientifically in our speciality.
Please cite this article as: Rojas Sayol R, Trullols Tarragó L, Grau Blanes A, Martinez Zaragoza J, Britez Altamirano E, Peiró Ibañez A, et al. Leiomiosarcomas de grandes vasos en las extremidades inferiores. Rev Esp Cir Ortop Traumatol. 2018;62:401–407.





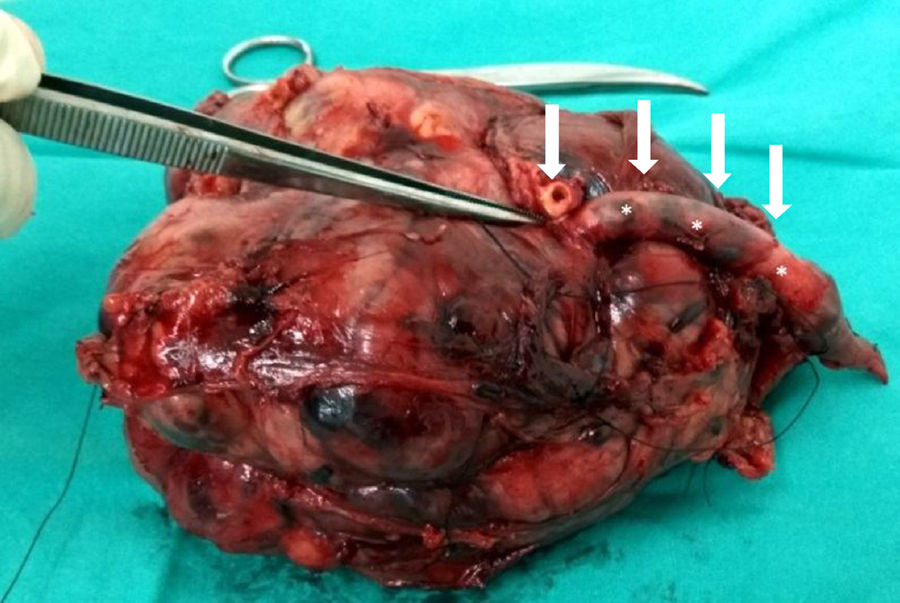
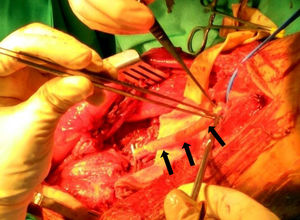
![Macroscopic view of a vascular LMS after its resection at the thigh (superficial vascular femoral bundle [arrows]). Macroscopic view of a vascular LMS after its resection at the thigh (superficial vascular femoral bundle [arrows]).](https://static.elsevier.es/multimedia/19888856/0000006200000006/v1_201811240652/S1988885618300762/v1_201811240652/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
