To investigate the molecular mechanisms of tissue response after treatment with the Intratissue Percutaneous Electrolysis (EPI®) technique in collagenase-induced tendinopathy in Sprague-Dawley rats.
MethodsTendinopathy was induced by injecting 50μg of type i collagenase into the patellar tendon of 24 Sprague Dawley rats of 7 months of age and weighting 300g. The sample was divided into 4 groups: the control group, collagenase group, and two EPI® technique treatment groups of 3 and 6mA, respectively. An EPI® treatment session was applied, and after 3 days, the tendons were analyzed using immunoblotting and electrophoresis techniques. An analysis was also made of cytochrome C protein, Smac/Diablo, vascular endothelial growth factor and its receptor 2, as well as the nuclear transcription factor peroxisome proliferator-activated receptor gamma.
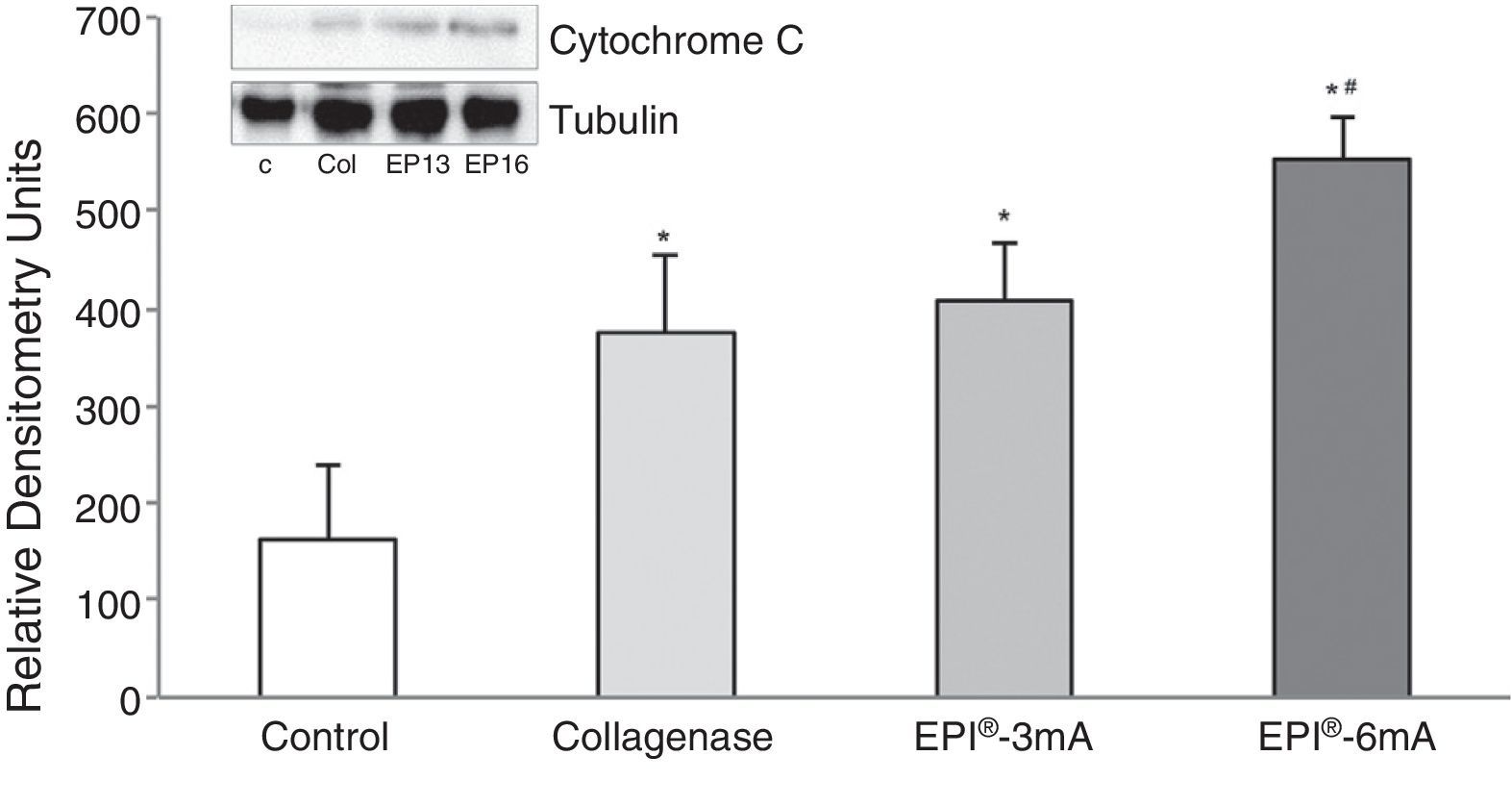
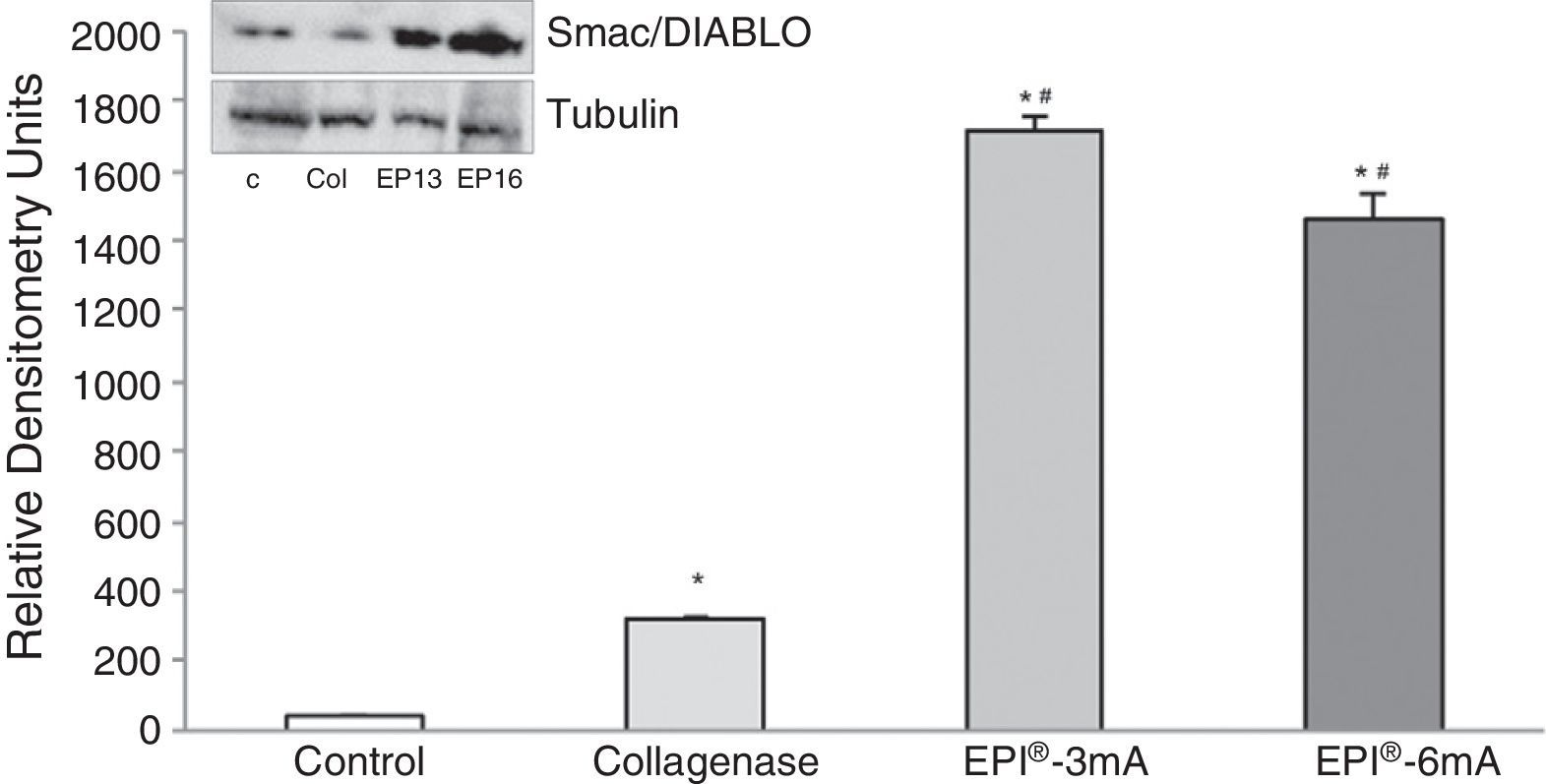
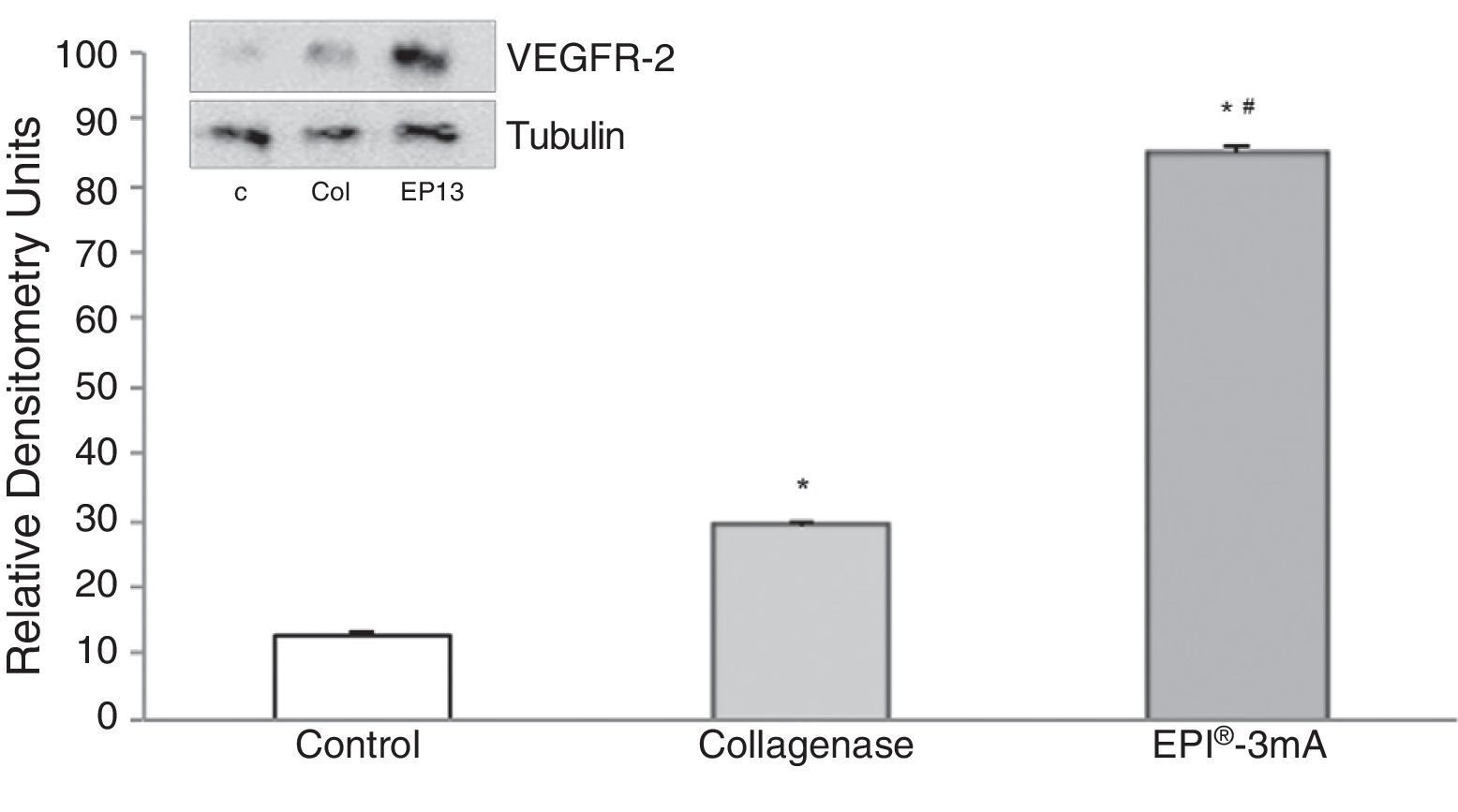
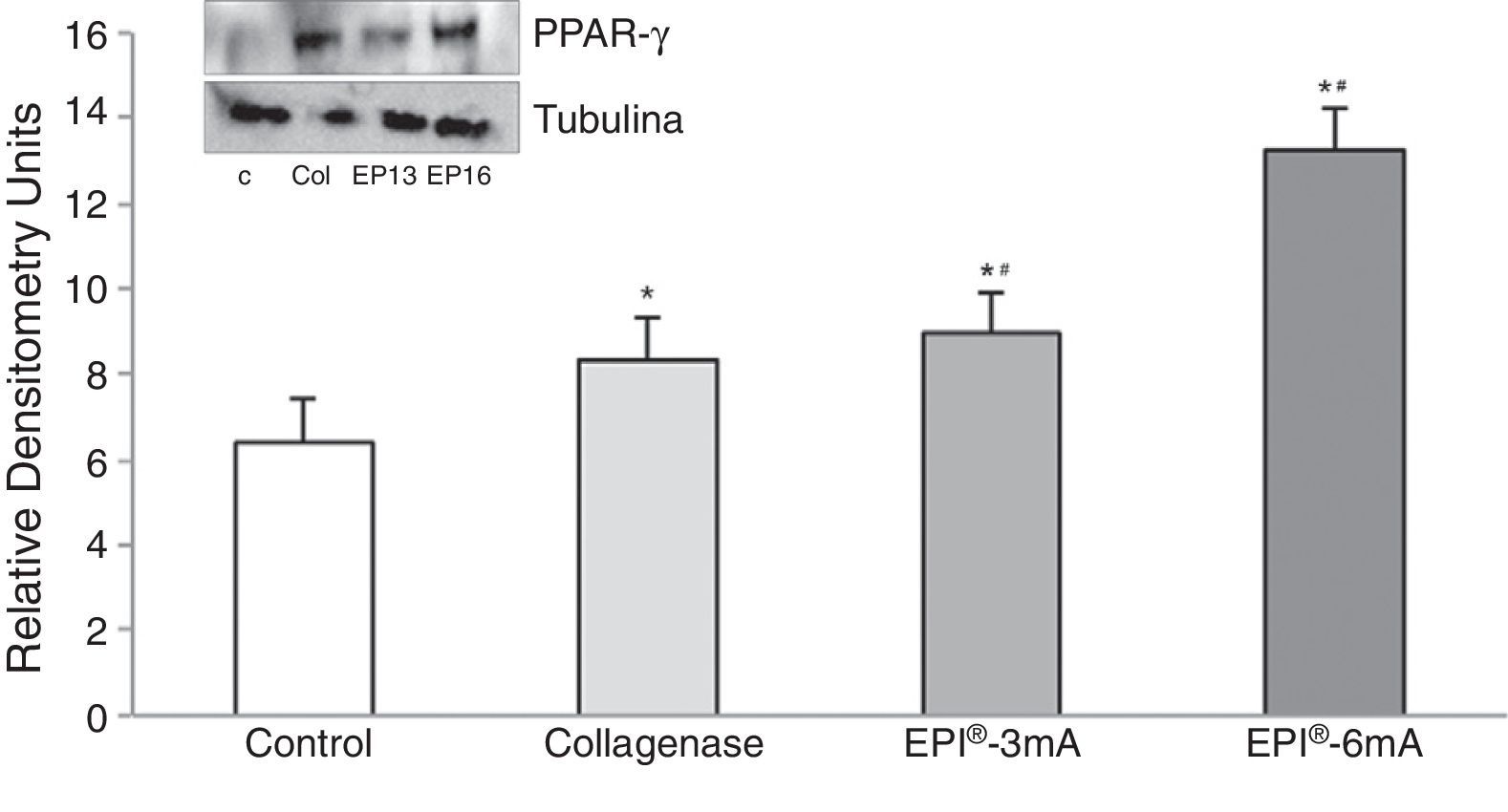
ResultsA statistically significant increase, compared to the control group, was observed in the expression of cytochrome C, Smac/Diablo, vascular endothelial growth factor, its receptor 2 and peroxisome proliferator-activated receptor gamma in the groups in which the EPI® technique was applied.
ConclusionsEPI® technique produces an increase in anti-inflammatory and angiogenic molecular mechanisms in collagenase-induced tendon injury in rats.
Investigar los mecanismos moleculares de respuesta tisular tras el tratamiento con la técnica Electrólisis Percutánea Intratisular (EPI®) en la tendinosis inducida por colagenasa tipo i en ratas Sprague Dawley.
MétodosEn una muestra de 24 ratas Sprague Dawley de 7 meses de edad y 300g se indujo tendinosis mediante la inyección en el tendón rotuliano de 50μg de colagenasa tipo i. Se procedió a dividir la muestra en 4 grupos: un grupo control, un grupo colagenasa y 2 grupos de tratamiento con técnica EPI® a 3 y 6mA, respectivamente. Se aplicó una sesión de tratamiento EPI® y tras 3 días se procedió al análisis de los tendones mediante técnicas de inmunodetección y electroforesis. Se analizaron las proteínas citocromo C, Smac/Diablo, factor de crecimiento endotelial vascular y su receptor 2. También se analizó el factor de transcripción nuclear peroxisoma proliferador activado del receptor gamma.
ResultadosSe observó un aumento estadísticamente significativo en la expresión del citocromo C, Smac/Diablo, factor de crecimiento endotelial vascular, su receptor 2 y peroxisoma proliferador activado del receptor gamma en los grupos a los que se les aplicó la técnica EPI® respecto al grupo control.
ConclusionesLa técnica EPI® produce, en la lesión tendinosa inducida con colagenasa tipo i en ratas, un aumento de los mecanismos moleculares antiinflamatorios y angiogénicos.
Patellar tendonitis affects a significant number of athletes whose common characteristic is performing jumps or ballistic movements.1 At present, tendonitis is considered as a degenerative, rather than an inflammatory process. Despite the fact that several therapeutic options have been described, none has become established as the standard.2,3
The use of experimental models based on the induction of tendonitis through collagenase (a metalloproteinase capable of breaking down the peptide links of collagen) has been applied in the past.4 The experimental study of tendonitis has previously been based on the assessment of proteins such as cytochrome C, Smac/Diablo, vascular endothelial growth factor (VEGF), its receptor 2 (VEGFR-2) and peroxisome proliferator-activated receptor gamma (PPAR-γ) nuclear transcription factor. Cytochrome C is a monomeric protein capable of activating the caspases which trigger the last phases of apoptosis in tendinopathies.5 Smac/Diablo is a mitochondrial protein whose release into the cellular cytosol induces apoptosis, presumably following the same routes as cytochrome C.6 VEGF is a signal protein involved in angiogenesis and vasculogenesis which has shown a capacity to stimulate division and migration of endothelial cells in vitro.7 VEGFR-2 is a tyrosine-kinase receptor which acts as the most important mediator of the angiogenic response of VEGF.8 Lastly, PPAR-γ, which belongs to the family of nuclear transcription factors (superfamily of steroid receptors), has been shown to cause a reduction of the inflammatory response.9
The Intratissue Percutaneous Electrolysis (EPI®) technique causes non-thermal, electrolytic ablation which induces a controlled inflammatory response, enabling the activation of cellular mechanisms involved in phagocytosis and regeneration of damaged soft tissue.10
Since recent works have shown good clinical results using the technique under study,11 the objective of the present analysis was to employ immunodetection and electrophoresis techniques to investigate the molecular mechanisms of tissue response involved in the treatment with the EPI® technique, following induction of tendonitis in Sprague Dawley rats through the use of collagenase.
Materials and methodThe study was conducted using 24 female Sprague Dawley rats, aged 7 months and weighing approximately 300g. The study fulfilled the ethical requirements of and was approved by the Bioethics Committee of the Medical School (A-1301314899794). The study followed the regulations contained in Royal Decree 1201/2005, from October 10th, on the protection of animals used for experimentation (BOE n.° 252. p. 34367–34391).
The animals were distributed into 4 groups: 6 control rats which did not undergo any intervention (control group), 6 rats injected with collagenase which did not undergo treatment with the EPI® technique (collagenase group), 6 rats injected with collagenase which were treated with the EPI® technique at 3mA intensity (EPI®-3mA group) and 6 rats injected with collagenase and treated with the EPI® technique at 6mA intensity (EPI®-6mA group).
The EPI® technique consisted in the ultrasound-guided application by a 0.32mm needle of a continuous current through a specially designed device certified for that purpose (EC Directive 93/42/EEC. EPI Advanced Medicine®, Barcelona, Spain).
Experimental modelWe injected 50μg of type I collagenase (Sigma–Aldrich Laboratories, St. Louis, MO, US) into the proximal region of the patellar tendon of the rats, causing tendonitis verified by ultrasound, following the protocol defined by the European Society of Musculoskeletal Radiology for the study of tendinopathies.12
In order to apply the EPI® technique, we carried out 3 ultrasound-guided punctures of 4s duration each in the proximal region of the patellar tendon of the rats, at an intensity of 3 or 6mA, depending on the study group. The rats were sacrificed after 7 days and a sample of the tendon was surgically extracted following the standard procedure.
We followed the Lowry13 method to determine protein concentrations in the tissue samples within ranges of 0.01–1mg/ml, and analyzed the samples through immunodetection and spectrophotometry (λ=660nm). We analyzed cytochrome C, Smac/Diablo, VEGF and VEGFR-2 proteins and also studied the PPAR-γ nuclear transcription factor. The results were validated by means of western blot studies versus tubulin and expressed as relative densitometry units.
Statistical analysisThe results were expressed as mean±standard deviation. The statistical analysis was conducted through the Student t test. We carried out an ANOVA test to asses the relationships between the variables, as well as post hoc and Dunnett tests to compare the different groups with the control group and the Scheffé test to compare all the groups with each other. The level of statistical significance was set at 5% (P<.05). The statistical analysis was conducted using the software package SPSS® version 17 (SPSS Inc., Chicago, IL, US).
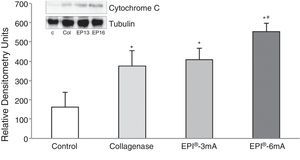
ResultsThe study of cytochrome C (Fig. 1) revealed elevated levels of this protein in all the groups being compared with the control group (P<.001). We observed statistically significant differences when comparing the EPI®-3mA group and the EPI®-6mA group (P<.013), and also when comparing the EPI®-6mA group and the collagenase group (P=.002).
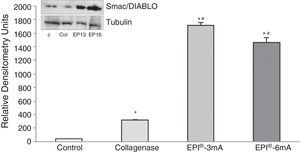
We also observed an overexpression of Smac/Diablo protein (Fig. 2) (P<.001), and detected statistically significant differences when comparing the 2 treatment groups (EPI®-3mA and EPI®-6mA) with the collagenase group (P<.001).
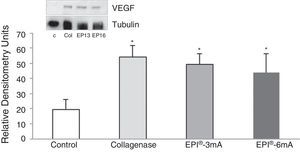
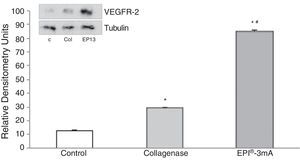
The analysis of VEGF (Fig. 3) revealed a significant increase (P<.001) in all the studied groups. A significant increase (P<.001) of VEGFR-2 was also detected (Fig. 4).
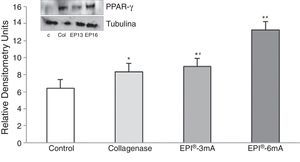
Lastly, there was a significant increase in PPAR-γ (Fig. 5) levels compared to the control group (P<.001), with statistically significant differences being observed when comparing the EPI®-3mA (P=.009) and EPI®-6mA (P<.001) groups with the collagenase group.
DiscussionThe main finding of this work was that the EPI® technique produced an overexpression of cytochrome C, Smac/Diablo, VEGF and VEGFR-2 proteins, as well as the nuclear transcription factor PPAR-γ.
Although several techniques have been described for the treatment of tendonitis, none is currently considered as the standard. Such techniques include excentric exercise, surgery (open or arthroscopic), shock waves, sclerosis of neovascularizations, non-steroidal anti-inflammatory drugs (NSAIDs) and the application of platelet rich plasma or aprotinin, among others.2,3
The EPI® technique consists in a non-thermal electrical current which induces a regenerative response in the damaged tissue.10 The ionic instability leads to formation of sodium hydroxide molecules, thus modifying the pH and increasing oxygen under the active electrode or cathode needle. This in turn enables phagocytosis and biological activation of tendon repair, which was altered by the chronic degenerative process.10,11
Previous works with electrolytic therapy, such as that by Gravante et al.14 revealed the effects of these techniques on the inflammatory response. A metaanalysis conducted by Gardner et al.15 showed that electrical stimulation in chronic wounds and decubitus ulcers led to a faster healing, whilst Zhao et al.16 observed how electrical fields applied to endothelial cell cultures stimulated the production of VEGF, as well as cellular migration and elongation. These results are in accordance with those observed in the present work. Subsequently, Yang et al.17 reported that the application of direct currents in damaged soft tissues was essential to epithelial cell migration and management in the scarring response.
The theory of tendon lesions secondary to overuse seems to be the most widely accepted.1 Coinciding with authors such as Alfredson et al.18 and Tan and Chan,19 we consider tendonitis as a degenerative process, more than an inflammatory process. In agreement with Fu et al.20 an increase in VEGF, Smac/Diablo, cytochrome C and VEGFR-2 proteins and anti-inflammatory protein PPAR-γ is related to the inflammatory response and tissue repair. Since tendonitis is a degenerative process, treatment with the EPI® technique could be justified.10,11,21–23The present study revealed a greater overexpression capacity of cytochrome C, an apoptosis marker related to tendonitis,5 following application of the EPI® technique. The Smac/Diablo protein was exported to the cytosol from the mitochondria, causing apoptosis through the activation of caspases6 and DNA damage resulting from binding to the CD95 receptor.24 The data obtained shows how the groups receiving treatment with the EPI® technique presented an increase in the expression of this protein. As previously described by Huang et al.25 the increase of apoptosis via Smac/Diablo and induction of VEGF through VEGFR-2 is probably due to the increase of B cell inhibition in the development of the bone marrow and differentiation of T cells from the thymus.
An increase of anti-inflammatory proteins, like PPAR-γ,9 has been observed after treatment with the EPI® technique. These proteins play a key role in the inhibition of expression of proinflammatory molecules secreted by macrophages, such as TNF-α, IL-6 and IL-1β,26 thus producing in the treated tissue a highly beneficial molecular response during tendonitis. This, in turn, results in an increase of the expression of VEGF and VEGFR-2, mediators responsible for angiogenesis anti-inflammatory response.7,27 The literature identifies the VEGFR-1 and VEGFR-2 receptors as the best expressed in the human Achilles tendon.8 Our results show an increase of VEGFR-2 following treatment with the EPI® technique, showing a modification of the cellular apoptosis pathway and increase of angiogenesis.
A limitation of this study was the use of experimental models in animals, as the results obtained may not be fully extrapolated to humans.28 However, the results of this study are promising and highlight the need to conduct additional studies which include molecular microdialysis and histological studies of the treated tissue.18,29 It is worth pointing out the discreet number of experimental animals, although the results showed adequate statistical power. Another limitation could be the study of 6 molecular alterations in such a complex and poorly-known pathology.
ConclusionsIn tendon lesions induced through type I collagenase in rats, the EPI® technique produces an increase of anti-inflammatory and angiogenic molecular mechanisms.
Level of evidenceLevel of evidence i.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe author J.M. Sanchez-Ibáñez holds the patent for the EPI® devices. He has taken part in the application of treatment, as well as in the drafting of the manuscript, but has not participated in sample collection, molecular analysis or the statistical study of the data obtained.
Please cite this article as: Abat F, Valles S, Gelber P, Polidori F, Stitik T, García-Herreros S, et al. Mecanismos moleculares de reparación mediante la técnica Electrólisis Percutánea Intratisular en la tendinosis rotuliana. Rev Esp Cir Ortop Traumatol. 2014;58:201–205.