To achieve bone continuity in an experimental model of segmental resection of femur bone by applying a treatment with committed to osteogenic bone linage mesenchymal stem cells.
Material and methodBone marrow mesenchymal stem cells, obtained from syngeneic Wistar murine, were committed into osteogenic lineage and embedded within a hydroxyapatite block. They were implanted in an experimentally created diaphyseal femur resection model. The diaphysis was synthetised with a 1.5mm thick plate. In order to calculate binomial distributions, we stablished one experimental and 3 control groups of 8 elements each: Group I, filling the gap with allograft; group ii, filling with a hydroxyapatite block without cells; group iii, filling with the hydroxyapatite block embedded with committed cells, and group iv, with the hydroxyapatite embedded with osteoinduced cells in a 3 dimensions TRAP culture. Descriptive analysis was performed by frequency distribution and Fisher statistic test. Level of statistical significance was considered at p<0.05.
ResultsGroup I presented good bone consolidation and no plate breakage. Group II showed fibrous but non-bone tissue, with rupture of all plates. Group III showed bone tissue in all cases, but the plates broke in all of them, while in group iv bone consolidation was achieve without any plate rupture.
ConclusionCell therapy with mesenchymal stem cells, trained in a 3 dimensions cell culture, produces bone tissue and ensures the permanence of the mechanical stabilisation performed in a segmental resection model. Limitations: A study with a larger sample size is necessary before planning the human inference.
Obtener continuidad ósea en un modelo experimental de resección segmentaria en la diáfisis del fémur mediante tratamiento con células mesenquimáticas indiferenciadas comprometidas al linaje osteogénico.
Material y métodoSe obtuvieron células mesenquimáticas indiferenciadas a partir de médula ósea de ratas Wistar singénicas, se diferenciaron al linaje osteogénico y se embebieron en bloques de hidroxiapatita. Se implantaron en una resección segmentaria en la diáfisis del fémur, que se sintetizó con una placa de 1,5mm de grosor. Se calcularon distribuciones binomiales estableciéndose un grupo experimental y 3 de control, constituidos por 8 elementos cada grupo. Grupo I, relleno con aloinjerto; grupo ii, con hidroxiapatita; grupo iii, con hidroxiapatita embebida con células osteocomprometidas; grupo iv con células osteocomprometidas mediante cultivo tridimensional. Se realizó estadística descriptiva con distribución de frecuencias mediante la prueba de Fisher, considerándose significativo el valor de p<0,05.
ResultadosEl grupo i presentó buena consolidación, sin rotura de placas. El grupo ii mostró tejido fibroso y rotura de todas las placas. El grupo iii mostró tejido óseo, pero en todos los casos se rompieron las placas. El grupo iv mostró consolidación sin rotura de placas.
ConclusiónLa terapia mediante células mesenquimáticas indiferenciadas en cultivos tridimensionales produce tejido óseo y asegura una estabilización mecánica permanente. Limitaciones: antes de la inferencia humana es necesario realizar el experimento en grupos con más elementos.
There are more than 200,000 annual cases using allografts in the United States.1 Lack of donor site morbidity, the overall success of outcomes and reduced surgical times make the allograft an attractive alternative to the autograft.2,3 However, high costs and risks such as viral transmission make the allograft an imperfect substitute.4
Tissue engineering is presented as an alternative to autografts and allografts for bone harvesting with the aim of some therapeutic action taking place.5
The development of a bone marrow (BM) cell culture system in a collagen gel allows the isolation, amplification and induction towards chondro-osteogenic lineage of a population of cells that express markers of these tissues in vitro and form ectopic cartilage and bone when implanted in vivo.6
In animal models of osteogenesis, it has been shown that the administration of transforming growth factor-beta 1 (TGF-β1) stimulates the activity of osteoblasts, triggering the formation of new bone tissue, and that they are influenced by the commitment status of the target cells, the cytokines of the microenvironment and the specific culture conditions.6
Undifferentiated or pluripotent mesenchymal stem cells (MSCs) are used as a cell source for the formation of osteocartilaginous tissue.7 These cells, by virtue of certain stimuli with molecular mediators, can commit into an osteochondroid phenotype, which includes the use of a serum free culture medium or the addition of dexamethasone, ascorbate, TGF-β or bone morphogenetic proteins (BMP): the three-dimensional high-density culture method being one of the most widely used for this purpose.8,9
MSCs must meet a number of conditions: they must be adherent in plastic culture, be able to differentiate to bone, cartilage and fat, and be phenotypically positive for CD105, CD73 and CD90, and negative for CD45, CD34, CD11b, CD14, CD79a and HLA-DR.10
BMPs were originally identified by Urist as organic components found in the bone matrix that could induce ectopic bone formation.11 BMP-2 is one of the most potent osteo-inducing cytokines and has been shown to increase cartilage and bone formation. TGF-β1, in combination with BMP-2, strongly enhances ectopic bone formation, with resulting bone volume 5 times greater than that induced by BMP-2 in animal models.12
The use of factors with collagen-binding domains in experimental cultures enables us to selectively control the cell culture process and, specifically, the chondro-osteogenic line.13
A cell population expressing markers of osteogenic differentiation and capable of forming ectopic bone in vivo can be selected in murine BM culture by the appropriate addition of rhTGF-β1-F2 and rhBMP-2. To our knowledge, there are no studies published in the international literature that have used the cell training set out here, or its combination with a biomaterial such as hydroxyapatite (HA), as a therapeutic element in long bone fracture repair.14
Ceramic biomaterials are among the most interesting in reconstructing bone defects, due to their recognised osteoconductive properties, biocompatibility, biodegradation capacity, unlimited quantitative availability and appropriate conditions for use as moulds for tissue engineering. Coralline HA belongs to this group.15
The aim of this study is to evaluate, by means of a prospective study, the usefulness of MSCs, from BM, trained in vitro and adsorbed onto HA pieces, after their implantation in segmental resections of femur. This general objective is specified as a possible inference for human populations in clinical situations that require osteogenesis promotion.
Material and methodsOur aim was to use cells trained in vitro to replace significant bone loss, particularly in segmental resections of the femur. We intended to use a tissue engineering technique, with MSC cultured and osteo-induced in a biomechanical fracture model with a 5mm bone defect, in a murine femur, stabilising the fracture according to the principle of neutralisation, with an osteosynthesis plate and filling the defect alternatively with allograft, HA block or HA with osteo-induced MSC in conventional culture and in three-dimensional culture with collagen gel.
We used the rat because it is a viable model in which to implant osteosynthesis plates, with a 5mm femoral resection model, which has already been shown not to consolidate spontaneously in the literature.
Bone marrow harvesting and processingBM obtained from 8-week-old female Wistar rats (Charles River, Barcelona, Spain), sacrificed to remove their femurs, was used as the source of MSC. When in the cell culture laboratory, the samples in sterile and cold conditions were transferred to a new alpha-MEM culture medium with antibiotics (100 μg/ml penicillin G [Sigma], 50 μg/ml of gentamicin [Sigma] and 0.3 μg/ml of amphotericin B [Flow-ICN]). The BM was then broken down by repeated pipetting, centrifuging at 1800rpm for 5min.
Isolation and culture of the cellsThe cultures were made on 48-well plates (Falcon®) at a concentration of 2 × 106 cells/well/150ml of collagen. The culture plates were put into the incubator (37°C) for 30min to allow the formation of collagen gels. Finally, 200μl of low serum culture medium per well, containing the growth factor (this was omitted in the control situation) was added over the gels. The culture medium was renewed every 3–4 days, adding fresh growth factor. All the cultures were kept in an atmosphere with 95% air and 5% CO2, at a temperature of 37°C and 100% relative humidity.
In the case of the three-dimensional cultures, for the first 10 days the cells were cultured at very low serum concentration (0.5% FBS), in the so-called selection period, to eliminate most of the cells from the haematopoietic line. The presence of rhTGF-β1-F2 allows the osteoprogenitor stem cell population intended for selection to be kept alive selectively.
On day 11 of the culture, the amplification and induction period began, which consists of changing to full culture medium (10% FBS) for 7 days. On the last 3 days of this period, rhBMP-2 (50ng/ml, R&D Systems) was added instead of rhTGF-β1-F2, to take advantage of the osteogenic induction capacity of that factor. Likewise, dexamethasone 10-8M (Sigma) and beta-glycerophosphate 2mM (Sigma) were added (Fig. 1). Flow cytometry identified the different surface markers that allowed the identification and isolation of MSC, such as CD29, CD44, CD73, CD90, CD105, CD166, HLA-I and STRO-1 (Fig. 2).
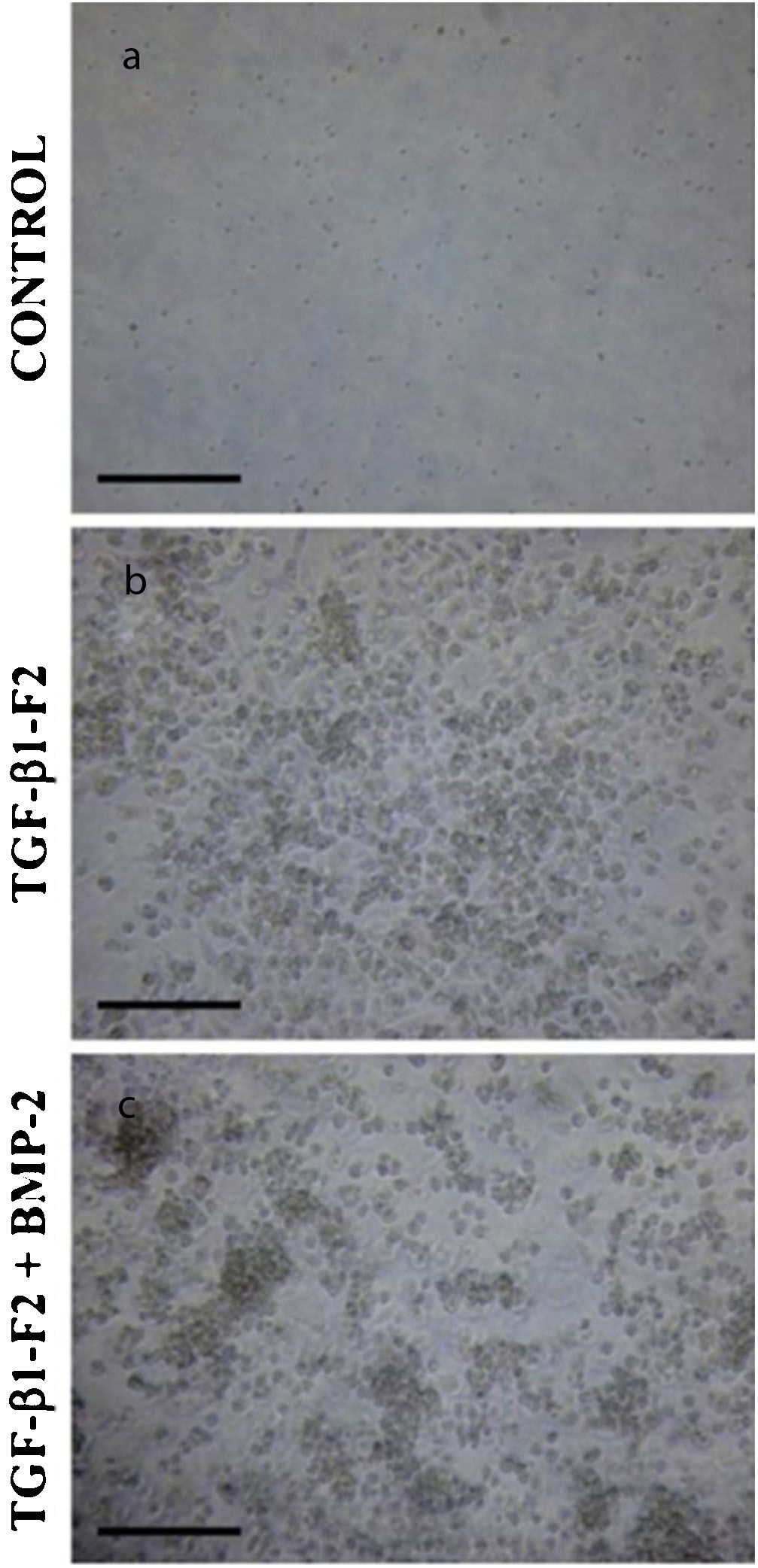
Three-dimensional cell morphology of the collagen matrix at the end of the differentiation period (day 16). (a) Control cultures in the absence of growth factor. (b) The cell cultures in the presence of rhTGF-β1-F2. (c) Cell cultures in the presence of rhTGF-β1-F2 and rhBMP-2 pulse.
At the end of the selection period (10 days of culture) a biochemical analysis was performed to determine DNA in the collagen gels, in order to determine the number of cells and the measurements of alkaline phosphatase, an enzyme of mineralisation of the pre-osteoblast differentiation pathway, as well as osteocalcin, a specific enzyme of osteoblast differentiation.
After the amplification and induction period (7 more days of culture) a biochemical analysis of DNA and alkaline phosphatase in the collagen gels was again performed. Osteocalcin was measured in the gels and also in the culture medium, since at this point the level of osteocalcin, as a soluble protein released into the medium, had to be sufficiently high to be detected.
For the samples coming from the TRAP it was necessary to break down the collagen gel to release the cells. This was done by incubating with the same volume of collagenase at 0.05% for 10min at 37°C. After that amount of time the reaction was stopped with DMEM, adding twice the volume of collagenase used (Gibco®). At this point, samples can be frozen at −20°C until the time of analysis.
Cell implantationIn the case of adherent cultures, and once the indicated semi-confluence was reached, the cells were detached and transferred to other culture vials in identical conditions as those described above, until a culture pass 1 (primary culture) was reached, which is used for implantation.
With respect to the three-dimensional cultures, after 17 days of culture, the cells were implanted in the murines (period of implantation). Once the cells were released from the collagen gels, they were centrifuged at 500rpm for 10min, counted and resuspended in serum-free culture medium.
Experimental groupsWistar rats (Charles River, Barcelona, Spain) 15 weeks old and 300g in weight were used, distributed into 4 experimental groups of 8 animals. The surgical procedure was performed using, to replace the resected segment, equivalent segments of allogeneic bone (from another syngeneic element), kept at −80°C.
Group I: the surgical procedure was performed using, to replace the resected segment, equivalent segments of allogeneic (from another syngeneic mouse), maintained at −80°C.
Group II: the surgical procedure was performed using equivalent HA segments of 2 × 5mm (Prosteon® 500R, Interpore, Zimmer Biomet, Warsaw, IN, USA), and a pore size of 500 μm.
Group III: the surgical procedure was performed using equivalent HA segments, the MSC previously adsorbed in primary culture, there being 2.4 × 106 cells per block of biomaterial.
Group IV: the surgical procedure was performed using equivalent HA segments of 2 × 5mm, previously adsorbed from cells trained by synergistic TRAP (rhTGF-β1-F2+rhBMP-2), 2.4 × 106 cells per biomaterial block.
In the 4 groups, the elements were identified by marking the right or left ear, making from one to 4 holes.
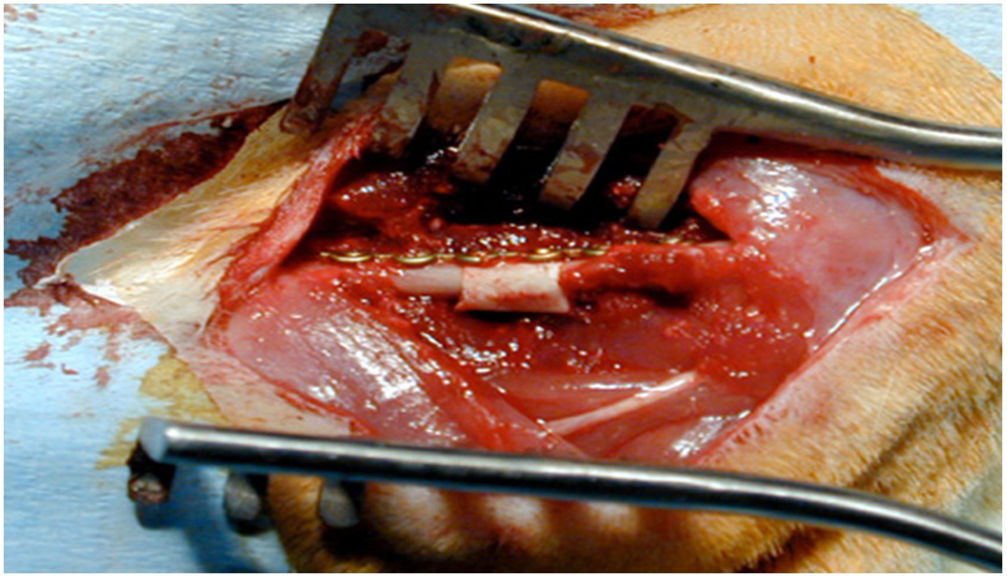
Surgical techniqueOnce the animals had been anaesthetised, they were placed in the right lateral position and the area to be incised was shaved—laterally to the left femur—to make anterolateral access between the vastus lateralis and the biceps femoris, with dissection by layers and haemostasis, reaching the femoral diaphysis. Using an oscillating micro-saw with 2 cuts, a 5mm segment of bone was extracted. The previously carved allograft segment or HA block, as appropriate, was then implanted. The system was then stabilised with a 1.2mm osteosynthesis plate and 4 screws (TIT Profyle®, Stryker Orthopaedics, Mahwah, NJ, USA). The wound was then closed in layers, using sterile skin staples. The test animals were given metamizole in their drinking water for pain management in the following days and housed for 12 weeks under standard conditions and nutrition. They were then sacrificed, and all femurs were harvested for histological study in the laboratory.
Radiographic studyAfter the surgical procedure, X-rays were taken at 4-week intervals, up to a total of 12 weeks, which is considered sufficient time to evaluate possible bone regeneration in this animal model. It was noted in all the rats whether or not the osteosynthesis plate was broken.
Data processing and statistical analysisThe Binomial Power Calculations of the University of California, Los Angeles, was used to calculate the sample size through their Internet portal (http://ebook.stat.ucla.edu/calcual.../b-2arcsine/b-2arscine-samp.htlm). A power of .80 (probability of acceptance of the alternative hypothesis being true) was accepted for different success rates of the experimental procedure. A descriptive analysis was carried out using frequency distribution. The groups were compared (i vs. ii, i vs. iii, i vs. iv) according to variables of bone formation, consolidation and type of consolidation, using the Fisher statistic, and establishing the level of statistical significance at p < 0.05.
StainsAll the histological samples were treated with these stains to identify cartilage and bone.
- -
Haematoxylin and eosin.
- -
Picrosirius red.
- -
Goldner's trichrome.
No complications such as infection or skin necrosis were observed.
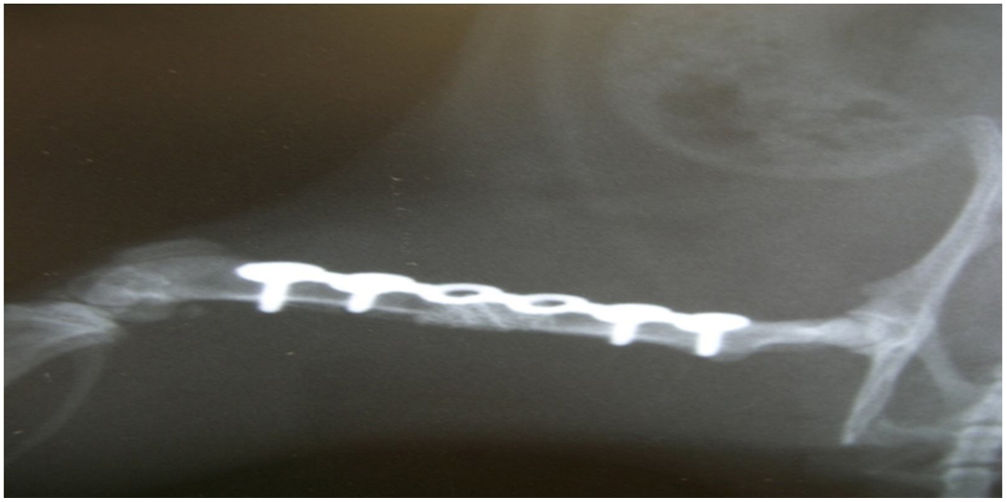
In group i good osseointegration was observed radiologically, with no biomechanical failure, at week twelve in all the elements. The descriptive statistics show 100% consolidation and 100% integrity of the osteosynthesis plate.
In group II, at the twelfth week there was a biomechanical failure in all cases, and all the osteosynthesis plates were broken. Histologically, fibrotic tissue was observed in all the samples, characteristic of non-consolidation (Fig. 3). There were significant differences between groups i and ii in relation to consolidation (Fisher's test, p = 0.002).
In group III, satisfactory biological behaviour was observed, with the formation of bone tissue interspersed with fibrous tissue in all the samples, unlike group II, where they all had fibrous tissue. Histologically, osteoid tissue was observed in all samples. However, the biomechanical results showed a failure of osteosynthesis, as all the plates were broken, without sufficient bone formation to achieve full consolidation of the bone defect; the biological response was not sufficient. In the descriptive statistical study, there were no differences between groups i and iii in relation to bone formation, but there were differences in relation to consolidation (Fisher's test, p = 0.002). Radiological imaging is poor in bone.
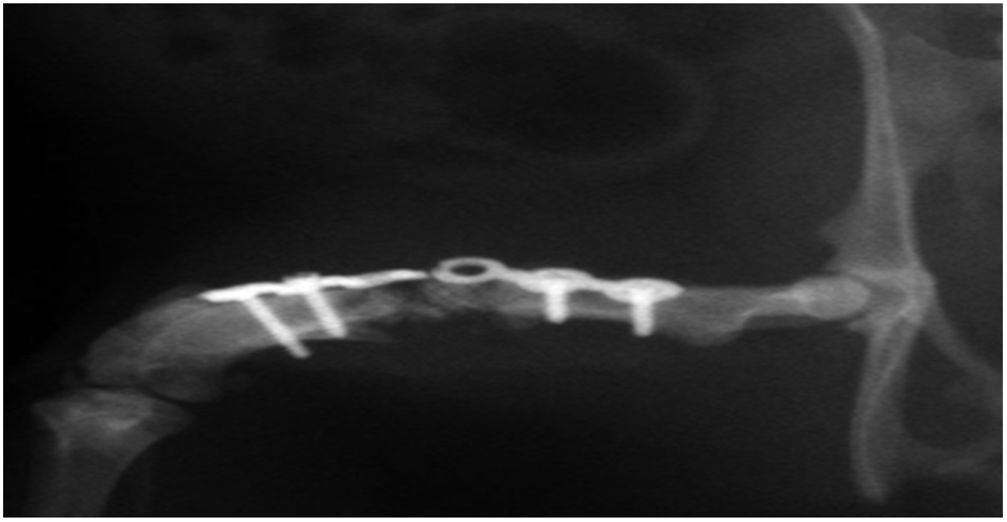
In group IV, histological consolidation of the defects was observed with both picrosirius red and Goldner's trichrome. Radiographically, at 12 weeks no breakage of the osteosynthesis plates was found, and therefore biomechanically it was considered a stable model, and histologically there was a greater amount of bone than in the other groups. No significant differences were found between group i and iv (Fisher's test, p = 0.002) (Figs. 4 and 5). Radiologically, it presents a bone-rich consolidation image.
Radiographic study of right murine femur at 12 weeks after femoral implant with HA+MSC trained by TRAP (group iv), and stabilisation by osteosynthesis plate and 4 screws. Consolidation is observed in the medial side of the HA junction-receptor of the host, and no consolidation in the lateral side (12×).
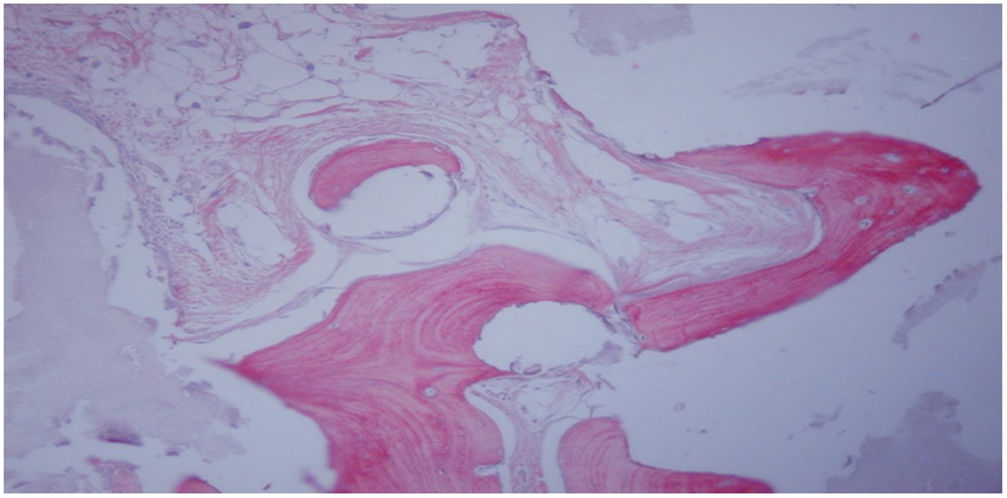
Histological study at 12 weeks after femoral implant with HA block + MSC trained by TRAP (group iv). Neoformed bone tissue with mature matrix (light red) flanked by evident cementing lines is observed, as well as osteoid laminae (intense red) with trapped osteocytes. Staining with Goldner's trichrome (200×).
There are bone defects after various surgical procedures and currently none of the regeneration methods are optimal.
HA has been effectively tested in a subcutaneous model in experimental rats. In this experimental study, all HA discs showed bone formation inside the pore areas, as demonstrated by decalcified histological sections and microtomography.16 According to our results, the combination of MSC and HA could be used as an excellent substitute for bone grafting due to its mechanical properties and bone-forming ability.17 Li et al.18 used a composite of nano-hydroxyapatite, collagen and calcium sulphate as a carrier. The construct was implanted together with rhBMP-2 in a critical defect in the femoral condyle of a rabbit. Radiological results and histological staining indicated that more new bone was produced in the rhBMP-2 group.
In our study we used a model of segmental resection in the rat femur, with a defect of 5mm, which has been shown to be a critical defect that does not consolidate spontaneously.19
Warzecha et al.,20 also using a model of segmental resection in stabilised femur with an 1.0mm osteosynthesis plate and 1.3mm screws, used calcium triphosphate and BMP-2, and obtained a good biomechanical model for the study of osteogenic stimulation.
Several fixation methods to stabilise a bone defect in the rat femur have also been described and are commonly used in clinical practice, including osteosynthesis plates, endomedullary nails and external fixation.21 The decision to stabilise the bone defect with an osteosynthesis plate is due to its greater stability and comfort.22,23 With this model, a 5mm bone defect can be filled with HA block with or without cells. Ferreira et al.,24 in a segmental resection model in mice femurs not treated with cells, observed that all cases resulted in non-consolidation.
MSCs have been isolated and characterised from BM samples from many species, including human, rabbit, rat, sheep, goat, monkey, dog and pig, through preferential binding to cell culture plastic surfaces.25 Friedenstein et al.26 set the standards for guinea pig MSC culture through the ability of MSCs to bind to plastic matrices, using the original method for other MSC cultures from BM from different animals, including humans. In our study, the BM cells were subjected to a 10-day culture period at very low serum concentration (selection period) in order to remove most of the cells from the haematopoietic line. The presence of rhTGF-β1-F2 allowed us to selectively keep alive the population of osteoprogenitor stem cells that we intended to select. The culture medium was renewed every 3–4 days, adding fresh factor. The phenotypic characterisation of the cells of the adherent or primary cell culture is achieved by flow cytometry, confirming that the cells are in active phase G0/G1, and CD29, CD73, CD105, CD166, HLA-I and STRO-1 are positive as phenotypic marker, and CD34, CD45 and CD44 are negative. This is constant in all primary cultures.27
TGF-β and its related factors, including BMP and activins, regulate various cell processes, such as proliferation, differentiation, apoptosis and extracellular matrix formation during embryogenesis. TGF-β signalling is mediated by 2 types of transmembrane serine/threonine kinase receptors, type i (ALK5) and type ii, that form a heteromeric complex. In this signalling complex, TGF-β binds to the type ii receptor (TGFβR2), phosphorylating the type ii receptor phosphorylating and activating the ALK5 receptor. The activated ALK5 induces signalling cascades through Smad-dependent and Smad-independent pathways. In the Smad-dependent pathway, the TGF-β-receptor complex activates Smad 2/3, while the BMP-receptor complex activates Smad 1/5/8.28
Chen et al.29 describe in a review the importance of TGF-β/BMP in signalling a large number of cell processes, that are crucial throughout life. TGF-β/BMP act in bone formation in mammals. Signal transduction by TGF-β/BMP is takes place specifically through a Smad-dependent pathway (TGF-β/BMP ligands, receptors and Smads) and another Smad-independent pathway (p38 mitogen-activated protein kinase pathway).
In this paper we use rhTGF-β1-F2; the particularity of the F2 molecule is the affinity to bind to collagen, and therefore its action in the environment is intensified. The cDNA sequence encoding the carboxy-terminal region of rhTGF-β1 was designed to include a high affinity collagen-binding decapeptide derived from von Willebrand factor.30
BMPs have been shown in animal and human studies to be capable of osseoinduction. In our model we use rhBMP-2. In addition, TGF-β1 in combination with BMP-2 has been published to strongly enhance ectopic bone formation, and the resulting bone volume is 5 times greater than that induced by BMP-2 alone.31
In this study we have attempted to further characterise BM-derived cells by isolating them in the three-dimensional collagen gel culture system with exposure to a novel recombinant human TGF-β1 fusion protein (rhTGF-β1-F2), designed to contain a collagen-binding auxiliary domain. The combination of rhTGF-β1-F2 and rhBMP-2 has improved the commitment of MSCs into the osteogenic lineage, and the complete culture or TRAP, taking into account that in the primary culture no pulses of rhBMP-2 are added. Use in the culture induction phase should also be considered, over the last 3 days, both in primary culture and in TRAP, of the addition of dexamethasone (10-8M) and beta-glycerol phosphate (2mM) as non-specific osteo-inducers. After 17 days the cells were detached, counted and implanted in groups iii and iv, respectively. In group iii, with segmental resection of femur stabilised by 1.5mm osteosynthesis plate, filling the bone defect with a 5mm HA block, with 2.4 million cells osteo-induced in primary culture. And in group iv, with 2.4 million cells osteo-induced in TRAP, that is, with the conjunction of rhTGF-β1-F2 and rhBMP-2.
ConclusionsMesenchymal stem cells trained in vitro in three-dimensional cultures with collagen gels, express typical osteogenic differentiation markers. Group i achieved consolidation in all femurs, and biomechanically it was stable, and no osteosynthesis plates were broken. Group ii behaved as non-union with fibrous tissue, with biomechanical failure through breakage of all the osteosynthesis plates. Group iii presented bone formation, but no consolidation of the defect, with biomechanical failure through breakage of all the osteosynthesis plates. Group iv developed consolidation favoured by the maturity of the neoformed osteochondral tissue. Biomechanically, the results were satisfactory, with a stable model and no broken osteosynthesis plates.
Tissue engineering with osteo-induced cell cultures offers us the possibility to obtain bone tissue for clinical practice in Traumatology, given the large amount of orthopaedic and traumatic diseases that demand bone tissue.
Limitations of the studyThe sample size is small, given the difficulty of the research and the high costs of the experimental study. The difficulty of extrapolating these results to human populations also needs to be considered and must be corroborated with prospective clinical studies.
Level of evidenceLevel of evidence x.
Conflict of interestsThe author has no conflict of interest to declare.
My most sincere thanks for their support and dedication to professors: Enrique Guerado Parra (Faculty of Medicine, Malaga), José Antonio Andrades Gómez and José Becerra Ratia (Faculty of Science: Cellular Biology, Malaga).
Please cite this article as: Godino Izquierdo M. Capacitación osteogénica in vitro de células madre mesenquimales de médula ósea para su aplicación en resecciones segmentarias de hueso. Rev Esp Cir Ortop Traumatol. 2020;64:236–243.