The purpose of this paper is to develop a professional consensus that proposes, in the light of the current scientific evidence and the clinical experience of an expert panel, some clinical recommendations directed at the Orthopaedic and Trauma Surgery (OTS) specialist and with the aim of reducing the variability in the prophylactic management of venous thromboembolic disease in knee and hip arthroplasty in clinical practice. The Delphi method was used, which consisted of two rounds of an e-mail questionnaire. Of the 55 items considered, a consensus was reached in 37 (67.2%) of them. In 31 cases there was consensus with the formulation of the item, and in 6 cases there was no agreed consensus. It was observed that there was a consensus in multiple clinical recommendations that could help OTS specialists in the making of decisions in their clinical practice.
El objetivo de este documento es desarrollar un consenso profesional que proponga a la luz de la evidencia científica actual y de la experiencia clínica de un panel de expertos, unas recomendaciones clínicas dirigidas a especialistas COT y orientadas a reducir la variabilidad de la práctica clínica en el tratamiento de la profilaxis de la enfermedad tromboembólica venosa, en la cirugía protésica de rodilla y cadera. Se empleó el método Delphi, el cual consiste en 2 rondas de un cuestionario por correo electrónico. De los 55 ítems considerados se consiguió el consenso en 37 de ellos (67,2%). En 31 casos se consensuó según la formulación del ítem y en 6 casos se consensuó en desacuerdo. Se observa que existe un consenso en múltiples recomendaciones clínicas que pueden ayudar en la toma de decisiones del especialista de COT en su práctica clínica.
The objective of this work was to develop a professional consensus which proposed, in the light of current scientific evidence and the clinical experience accumulated by an expert panel, a series of professional standards and clinical guidelines for traumatology specialists aimed at reducing variability during clinical practice1 in the prophylactic treatment of venous thromboembolism (VTE) during prosthetic surgery of the knee and hip.
Knee and hip prosthetic surgery entails a high risk of VTE. In fact, it is the surgery with the highest incidence of VTE. These 2 types of surgery have been extensively studied – perhaps at the expense of other types of orthopaedic surgery, where studies regarding VTE are lacking – and routine thromboprophylaxis has been established for over 2 decades. Among those patients who do not receive thromboprophylaxis the figures of deep vein thrombosis (DVT) are around 40–60% for total venographic DVT and 10–30% for proximal venographic DVT.2
The administration of thromboprophylaxis has managed to reduce these incidences notably. However, the figures for symptomatic DVT remain at between 2% and 10% in the 3 months after surgery. VTE is the most common cause of hospital readmission after total knee arthroplasty (TKA). Some cases will not manifest until various years later, in the form of postphlebitic syndrome.
In patients undergoing total hip arthroplasty (THA) the likelihood of developing asymptomatic DVT is 40–60% if thromboprophylaxis is not administered. For this reason, prophylaxis is routinely recommended in THA. So far, low molecular weight heparins (LMWH) have been the most studied thromboprophylactic method. Fondaparinux is a synthetic pentasaccharide which selectively inhibits the Xa coagulation factor and has also shown effectiveness for this purpose. Warfarin (INR: 2–3) has also shown efficacy in THA. Other, orally administered, prophylactic drugs have been incorporated recently with good efficacy results: rivaroxaban and apixaban are inhibitors of factor Xa and dabigatran is a direct inhibitor of thrombin. Among the mechanical methods, intermittent pneumatic compression (IPC) has offered good results, although its effectiveness on proximal DVT is scarce. Pneumatic compression in the feet has also shown some efficacy, although the experience in this respect is very limited. Gradual compression stockings (GCS) have not shown any evidence of efficacy. The advantage of mechanical methods is an absence of haemorrhagic risk.
With respect to TKA, the risk of VTE is higher than that observed for THA. Nevertheless, this increased incidence is at the expense of distal DVT, since the incidence of proximal DVT is less in TKA. LMWHs have shown effectiveness in this regard. Fondaparinux is effective in TKA and warfarin (INR: 2–3) has also shown good results. Furthermore, some orally administered drugs have recently been employed in TKA prophylaxis with good efficacy results, including rivaroxaban, apixaban and dabigatran. Due to the design of their studies, the technical datasheets of these drugs only contemplate their administration until 10–14 days after surgery, although various guides advise maintaining VTE prophylaxis for up to 28–35 days. With regard to mechanical methods, the application of IPC during surgery or immediately after has shown efficacy. However, patient tolerance was scarce and its application at home is very difficult. Moreover, the optimum method of leg compression has not yet been established. The plantar venous pump (BVP) and GCS methods provide little or no protection.
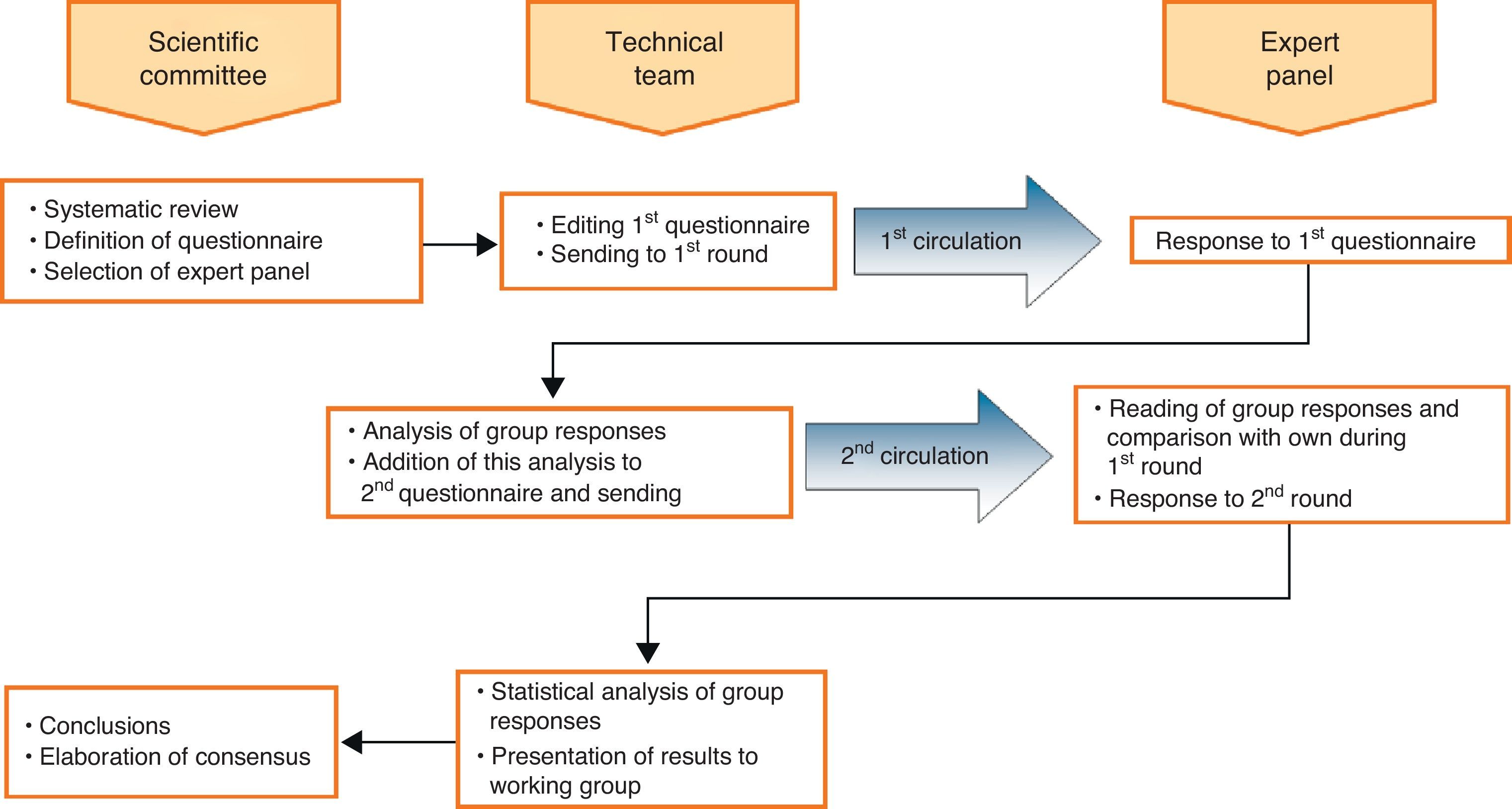
Material and methodsThe Delphi method3,4 consists in carrying out 2 successive rounds of structured surveys with a previously defined expert panel. During the second round, each expert knows the results of the responses from the previous round, thus it is possible to contrast personal opinions with those of other experts.
The advantages of this method are:
- •
anonymity of the views expressed by each expert.
- •
controlled interaction of each expert with the views of the rest of the group.
- •
the opportunity for each participant to reflect and reconsider personal opinions.
- •
statistical validation of the consensus achieved.
Three participation levels were defined for a correct application.
Firstly, a Scientific Committee consisting of 3 experts – the signatories of the study – was appointed. This group was responsible for the systematic review of the study topic, the definition of the contents of the questionnaires, the selection of the panel of experts and the final draft of the conclusions document. Secondly, the technical team responsible for the implementation of the method was selected (publication and distribution of the first questionnaire, analysis of responses to the first circulation, interim report and dissemination of the second questionnaire, analysis of the second questionnaire, statistical interpretation of the consensus achieved). Finally, the Scientific Committee appointed the Expert Panel (Appendix 1), which was the group of renowned panellists, experts on the subject, whose opinion was sought.
The criteria for the appointment of panellists required professionals belonging to SECOT who had previously participated in activities related to the study topic. The vast majority of them were members of the SECOT Study Group on thromboembolism. Only 2 members did not belong to SECOT, but their inclusion was accepted due to their ample experience on the subject of thromboembolic disease.
Fig. 1 summarises the operation of the system. Questions or items presented to panellists were drafted following 2 general principles: on the one hand, to confirm or invalidate commonly accepted views on VTE, sometimes with little or no scientific basis, and on the other, to test their opinion regarding recent developments, such as oral prophylaxis. We defined the following 5 topics, from which questions were developed:
- I.
Evidence.
- II.
Risk factors.
- III.
Mechanical methods.
- IV.
Pharmacological methods.
- V.
Anaesthesia.
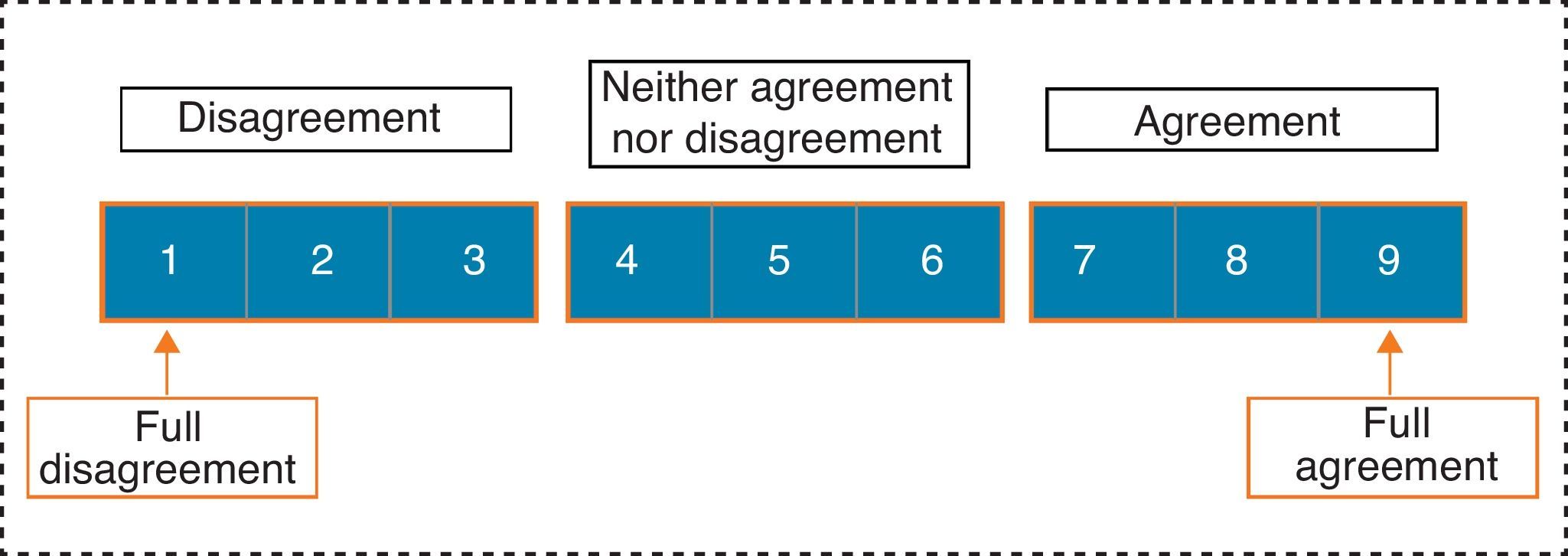
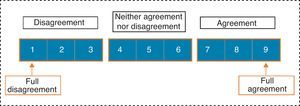
Panellists expressed their professional judgment on each item, selecting a single answer from among those displayed in Fig. 2.
In order to analyse the general opinion and the type of consensus achieved on each issue raised, we used the position of the median scores of the group and the “level of agreement” reached by the respondents, according to the following criteria:
- •
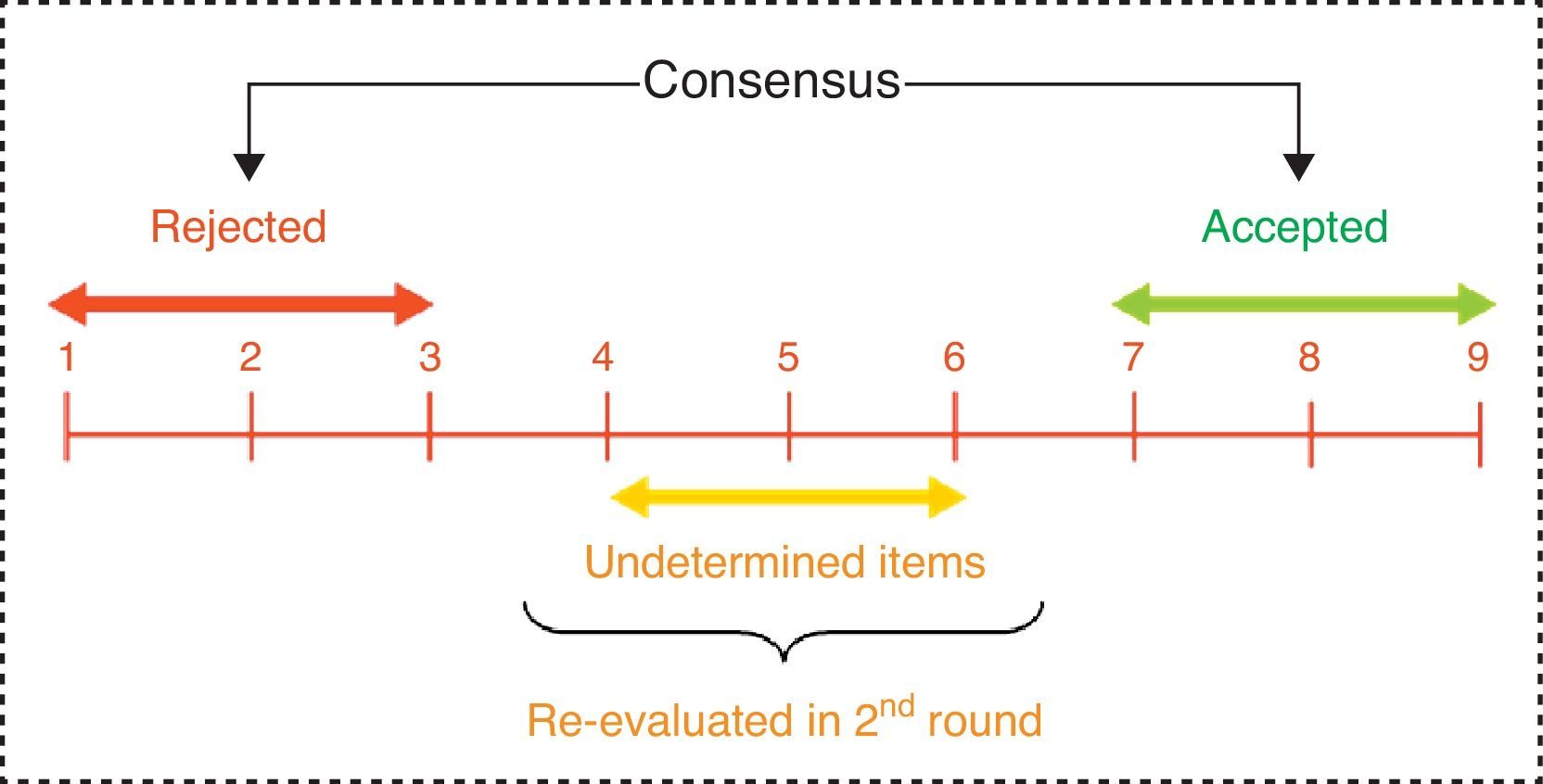
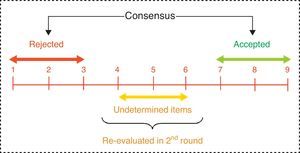
We considered consensus to be reached on an item when there was “agreement” of opinions within the panel, that is, when the number of experts who scored outside the 3-point region ([1–3], [4–6], [7–9]) containing the median were less than a third of total respondents. In such cases, the value of the median determined the consensus achieved by the group: general “disagreement” with the item if the median≤3, or general “agreement” with the item if the median≥7. Those cases in which the median was in the 4–6 region were considered as “doubtful” items for a representative majority of the group.
- •
Conversely, we established “disagreement” of opinions within the panel when the scores of one third or more of the panellists were in the [1–3] region, whilst another third or more were in the [7–9] region. The remaining items, in which no agreement or disagreement was observed, were considered to have an “unspecified” level of consensus (Fig. 3).
All items on which the group did not reach a clear consensus in favour or against the issue raised (doubtful items, those on which there was disagreement and those which showed an unspecified level of consensus) were proposed for reconsideration by the panel during the second Delphi round. Those items on which there was a high dispersion of opinions among respondents, with an interquartile range≥4 points (range of scores contained between the p25 and p75 values of the distribution), also underwent reassessment. Between both rounds, panellists were informed of the detailed distribution of the total responses in the first survey (using bar charts), thus enabling comments and clarifications to be provided by each participant. After reviewing this information, they were asked for a new, personal reassessment of those items on which no consensus was reached during the first round.
Statistical analysisIn order to analyse the opinion of the group and the type of consensus reached on each issue considered, we used the position of the median scores of the group and the “level of agreement” reached by the respondents, according to the criteria detailed below.
Items were considered consensuated when there was “agreement” in the opinion of the panel. That is, when the experts who scored it outside the 3-point region ([1–3], [4–6], [7–9]) containing the median were less than a third of respondents. In this case, the median value determined the consensus reached by the group: general “disagreement” with the item, if the median was less than or equal to 3, or general “agreement” with the item if the median was more than or equal to 7. Those cases in which the median was in the 4–6 range were considered as “doubtful” items for a representative majority of the group.
On the other hand, it was established that there was “disagreement” of judgment among the panel when the scores of a third or more of the panellists were in the [1–3] region, whilst another third or more were in the [7–9] region. The remaining items, in which neither agreement nor disagreement was observed, were considered to have an “unspecified” level of consensus.
All items on which the group did not reach a clear consensus in favour or against the issue considered (doubtful items, those in which discordance was observed and those showing an unspecified level of consensus), were proposed for reconsideration by the panel in the second Delphi round. Items on which there was a high dispersion of opinions among respondents, with an interquartile range higher than or equal to 4 points (range of scores contained between p25 and p75 values of the distribution) also underwent re-evaluation.
Between both rounds, panellists were informed of the detailed distribution of the responses of the group in the first survey (using bar charts), thus facilitating comments and clarifications by each participant. After reviewing this information, they were asked for a reassessment of the items with no consensus in the first round.
After the second round of the survey, the same criteria were applied to discriminate those items with a definite agreement from those on which it was not possible to unify the criteria of the panel. In order to establish a graphical comparison between items, we calculated the mean score of the panellists on each issue, with a confidence interval of 95%. The more extreme the mean score of an item (closer to 1 or 9), the higher the level of consensus reached, either in disagreement or agreement, respectively, with the proposal of each item. Those items on which no consensus was reached after completing the process described were analysed descriptively in order to distinguish whether this situation was due to persisting disagreement or to a majority of the panel being in the region of doubt regarding the item, when most of the group declared not having a definitive criterion (score between 4 and 6).
ResultsOf the 55 items considered, a consensus was reached for 37 of them, that is, in 67.2% of cases. In 31 cases, a consensus was reached according to the formulation of the item, whereas in 6 cases a consensus of disagreement was reached. Consensus was obtained in 20 cases in the first round and in 17 cases in the second round. Detailed results for each item are described in Table 1.
Results of the items presented to the expert panel.
| Median | Percentage against | Mean | |
| Block 1: evidence | |||
| Clinical trials and epidemiological data from large populations provide different evidence on thromboembolic prophylaxis. Nevertheless, there is certain controversy regarding the importance to be given to each of them: | |||
| 1. Information from epidemiological studies conducted on large patient populations (with a different design from randomised clinical trials) should be taken more into account when preparing Recommendation Guidelines on thromboprophylaxis | 7.00 | 29.40 | 6.88 |
| 2. Randomised clinical trials are the only studies which should be taken into account when developing Recommendation Guidelines on thromboprophylaxis | 5.00 | 58.80 | 5.41 |
| Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE). Prevention studies on VTE use multiple variables as “end-points” to measure the effectiveness of preventive methods (venographic DVT, PE, any manifestation of pulmonary embolism, death from any cause, etc.): | |||
| 3. Asymptomatic VTE findings have the same relevance as symptomatic ones to asses preventive efficacy | 7.00 | 23.50 | 6.65 |
| 4. Venographic DVT (identified by venography) on all the patients in a given therapeutic series is a good indicator of the relevance of VTE in such patients | 8.00 | 11.80 | 7.88 |
| On the perception of PE risk in prosthetic knee and/or hip surgery, and its clinical significance: | |||
| 5. The clinical relevance of VTE in orthopaedic surgery is adequately dimensioned | 7.50 | 16.70 | 7.33 |
| 6. The real risk of VTE in orthopaedic surgery is exaggerated by the commercial interests of pharmaceutical laboratories | 3.00 | 11.80 | 2.94 |
| On the perceived risk of bleeding by thromboembolic prophylaxis in knee and/or hip replacement surgery: | |||
| 7. Recommendation Guidelines underestimate the risk of bleeding in relation to knee surgery | 7.00 | 35.30 | 5.88 |
| 8. Recommendation Guidelines underestimate the risk of bleeding in relation to hip surgery | 7.00 | 41.20 | 5.82 |
| On the representation of orthopaedic surgeons in the development of International Recommendation Guidelines on thromboprophylaxis: | |||
| 9. Orthopaedic surgeons are adequately represented in the development of International Recommendation Guidelines | 5.00 | 41.20 | 4.82 |
| On the adequacy, apart from national and international guidelines, of developing local protocols (at each hospital) for thromboprophylaxis: | |||
| 10. Despite the existence of numerous guides and based on them, it is desirable that each hospital elaborates an action protocol for prophylaxis in prosthetic surgery which is appropriate to their specific clinical practice and available drugs | 8.00 | 17.60 | 7.18 |
| Block 2: risk factors | |||
| Indicate the degree of relative importance, in your view, of the following risk factors for VTE in knee and/or hip prosthetic surgery: | |||
| 11. A history of thromboembolic disease | 9.00 | 0.00 | 8.67 |
| 12. Obesity | 7.00 | 17.60 | 7.12 |
| 13. Advanced age | 8.00 | 11.80 | 7.24 |
| 14. Varicose veins | 7.00 | 47.10 | 6.47 |
| 15. Discharge on the operated limb | 7.00 | 31.30 | 6.94 |
| On the need to personalise the prophylactic dose to administer, according to a particularly high thromboembolic risk: | |||
| 16. Thromboprophylaxis is well defined for patients with a high thrombotic risk | 8.00 | 17.60 | 7.41 |
| 17. In some cases, a high thromboembolic risk may make the surgical intervention unadvisable | 4.00 | 82.30 | 5.00 |
| Block 3: Mechanical methods | |||
| In connection with the advice contained in guides on the application of mechanical methods for prophylaxis in hip and knee replacement surgery, on candidate patients: | |||
| 18. Guidelines give adequate advice on the implementation of mechanical prophylactic methods with regard to the type and mode of application | 3.00 | 35.30 | 3.71 |
| 19. Patients with higher thrombotic risk should add some kind of mechanical compression method to pharmacological prophylaxis | 7.50 | 27.80 | 7.06 |
| 20. There is a clear criterion on which type of patients will benefit from the application of a mechanical method | 3.00 | 23.50 | 3.53 |
| Regarding the different mechanical methods available: | |||
| 21. Elastic compression stockings should be used routinely in knee arthroplasty surgery | 6.00 | 58.90 | 6.18 |
| 22. Elastic compression stockings should be used routinely in hip arthroplasty surgery | 8.00 | 29.40 | 7.06 |
| 23. The plantar venous compression pump is a useful mechanical method in prosthetic knee surgery in certain patients | 7.00 | 23.50 | 6.47 |
| 25. The plantar venous compression pump is a useful mechanical method in prosthetic hip surgery in certain patients | 6.00 | 53.00 | 6.06 |
| Block 4: pharmacological methods | |||
| Regarding the indication, timing and duration of prophylaxis after hip or knee arthroplasty: | |||
| 27. All patients undergoing knee or hip prostheses should receive pharmacological prophylaxis (regardless of their risk) | 9.00 | 11.80 | 8.35 |
| 28. The start of prophylaxis may take place either before or after surgery indistinctly | 9.00 | 5.90 | 8.24 |
| 29. Each drug should define when to start its administration according to its own balance of efficacy and safety | 9.00 | 0.00 | 8.50 |
| 30. Pharmacological prophylaxis should always be maintained until 28–35 days in hip arthroplasty | 9.00 | 11.80 | 7.88 |
| 31. Pharmacological prophylaxis should always be maintained until 28–35 days in knee arthroplasty | 8.00 | 17.60 | 7.41 |
| 32. Thromboembolic prophylaxis should be increased in prosthetic replacements (in relation to primary surgery) | 3.00 | 35.30 | 4.06 |
| Regarding the professionals involved in the surgical process of arthroplasties: | |||
| 33. Following the protocol of the centre, the surgeon must decide the type of prophylaxis to employ | 8.00 | 22.20 | 7.22 |
| 34. Following the protocol of the centre, the anaesthesiologist must decide the type of prophylaxis to employ | 2.00 | 11.80 | 2.53 |
| On new oral anticoagulants: | |||
| 35. New oral anticoagulants may be administered routinely in prosthetic surgery, regardless of whether or not there is a contraindication against subcutaneous agents | 9.00 | 17.60 | 7.71 |
| 36. Unless otherwise indicated in the datasheet, the new oral anticoagulants are applicable to all patients undergoing hip arthroplasty | 8.00 | 27.80 | 7.39 |
| 37. Unless otherwise indicated in the datasheet, the new oral anticoagulants are applicable to all patients undergoing knee arthroplasty | 8.00 | 27.80 | 7.39 |
| 38. The new oral anticoagulants should be administered with particular caution in case of bleeding risk | 7.50 | 31.30 | 6.69 |
| 39. The new oral anticoagulants should be used with particular caution in case of high thrombotic risk | 5.00 | 68.90 | 5.13 |
| 40. It is appropriate to extend prophylaxis with the new oral anticoagulants up to 28–35 days postoperatively in knee arthroplasty (although there are no studies longer than 10 days) | 8.00 | 23.50 | 7.59 |
| Regarding the administration method of anticoagulant treatments: | |||
| 41. Oral administration of anticoagulant therapy facilitates proper compliance with the treatment | 8.00 | 29.40 | 7.53 |
| 42. It is appropriate to change from subcutaneous prophylaxis to oral prophylaxis after the immediate postoperative period (24–48h) | 8.00 | 31.30 | 6.88 |
| 43. It is appropriate to change from subcutaneous prophylaxis to oral prophylaxis after hospital discharge | 6.50 | 62.50 | 6.44 |
| Regarding the usefulness of aspirin for prophylaxis: | |||
| 44. ASA can be effective if combined with other mechanical and/or anaesthetic prophylactic methods | 2.00 | 47.10 | 3.76 |
| 45. Aspirin has a much lower efficacy than LMWH in the prophylaxis of postoperative VTE | 8.50 | 27.80 | 7.44 |
| Regarding the risks of the various preventive drugs available: | |||
| 46. LMWH provide the best efficacy–safety balance | 8.00 | 22.20 | 7.44 |
| 47. Fondaparinux provides the best efficacy–safety balance | 3.00 | 23.50 | 3.47 |
| 48. The new oral anticoagulants provide the best efficacy–safety balance | 6.00 | 41.20 | 6.18 |
| 49. The type of prophylaxis should be varied in patients under treatment with low doses of antiaggregants (100mg ASA) | 2.00 | 23.50 | 2.82 |
| 50. Heparin-induced thrombocytopenia is a relevant complication in prophylaxis using heparin, which sometimes motivates its withdrawal | 7.00 | 47.10 | 5.71 |
| 51. The availability of an antidote is a relevant factor in the decision to administer a particular type of thromboprophylaxis | 7.00 | 47.10 | 5.76 |
| Regarding the opinion of experts about thromboprophylaxis: | |||
| 52. Given that the datasheets only reflect the conditions of available clinical trials, the existence of expert opinions which advise on situations which have not been evaluated previously is adequate | 8.00 | 11.10 | 7.61 |
| Block 5: Anaesthesia | |||
| On the implications of the use of epidural catheters in the treatment of postoperative pain: | |||
| 53. Placement of an epidural catheter conditions the type of thromboprophylaxis (drug) to be administered after orthopaedic surgery | 8.00 | 16.70 | 7.44 |
| 54. Carrying out a peripheral nerve block determines the type of thromboprophylaxis (drug) to be administered after orthopaedic surgery | 3.00 | 31.20 | 3.50 |
| On the implications of the thromboembolic risk of a patient in the type of anaesthesia to be administered in traumatology interventions: | |||
| 55. The thrombotic risk of a patient should be taken into account when deciding on the type of anaesthesia to employ | 7.00 | 27.80 | 6.67 |
Those values on which a consensus was obtained are presented in bold.
Regarding prosthetic knee and hip surgery, there are some discussion points related to VTE which arise from the manner in which the evidence was obtained: clinical trials traditionally regarded as the only source of evidence, the effectiveness indicator thereof, the assessment of bleeding risk in them, etc. With regard to mechanical methods there is some confusion about their indication and application. Pharmacological methods are being revolutionised by the development of new oral anticoagulants. Thus, there is a reason for examining the opinions of experts who, through their consensus, could provide assistance in this field of orthopaedic surgery.
EvidenceIn relation to acquiring evidence, we obtained an interesting consensus regarding the need to take the information from epidemiological studies (with a different design from randomised clinical trials) conducted on large populations of patients more into account when developing Recommendation Guidelines for thromboprophylaxis. To date, Recommendation Guidelines are based almost exclusively on clinical trials. Increasingly, there is data available from Health Records and/or Arthroplasty Registries containing information on large populations.5 These studies complement the recognised limitations of clinical trials.
Randomised clinical trials are no longer considered unanimously as the only studies to be taken into account when developing guidelines on thromboprophylaxis. This loss of unanimity was reflected in the study.
There is an ongoing debate regarding the most reliable indicator of VTE.6,7 Fundamentally, there are doubts that DVT observed through venography – mostly asymptomatic – is as clinically relevant as the symptomatic type. Therefore, its reliability as an indicator of the incidence of VTE is also questioned. The consensus of the experts was that it is a good indicator.
Occasionally, some opinions are expressed in the sense that VTE is overestimated in trauma surgery –partly through the influence of the pharmaceutical industry – and is being given undue importance. Hence, patients are being treated excessively, which places them in a situation of haemorrhagic risk. However, there was a broad consensus that VTE is adequately dimensioned in our field and that commercial interests do not have an undue influence on the estimation of the problem.
Some criticisms of clinical trials highlight the possibility that bleeding is underestimated, since not enough information is collected in the studies. However, the majority of experts consulted did not share the idea of an underestimation of bleeding in this type surgery.
Some voices5 have denounced the fact that orthopaedic surgeons are not adequately represented in certain international guidelines with a broad impact on our specialty, and that this could lead, for example, to an underestimation of the risk of administering thromboprophylaxis. The statement that the representation is adequate did not obtain a consensus or, in other words, the current representation of orthopaedic surgeons raises some concerns. There was no consensus regarding the assertion that traumatologists are sufficiently represented. There was a certain level of dissatisfaction with said representation.
Despite the existence of numerous guidelines from national and international agencies based on extensive evidence reviews, there is a need for each hospital to elaborate an action protocol for prophylaxis which is appropriate to their specific everyday clinical practice and available drugs. International guidelines are based solely on data drawn from the evidence and, therefore, do not provide guidance on clinical situations for which no evidence has been obtained, but which physicians must resolve regardless. Consensus guidelines with local experts should help to resolve those cases which general recommendations may define ambiguously.8
Risk factorsExperts have confirmed the classical risk factors including history of thromboembolic disease (the highest level of consensus), obesity and advanced age.9
It is interesting that discharge of the affected limb appears as a risk factor, as it does not always appear in such lists and causes doubts for surgeons about when and for how long to prescribe prophylaxis. Another interesting fact is that the presence of varicose veins, commonly considered as a risk factor, was unanimously not considered as such by the experts consulted, according to some studies which do not relate them to thrombotic risk.10
Mechanical methodsMechanical methods represent the aspect of prophylaxis requiring the most help from the experts, as they are the least studied. A number of statements have been made, bearing in mind the confusion regarding this issue. It has been stated that the guidelines do not properly report on their implementation. This is one of the main drawbacks for their use. Moreover, there was a consensus among the panellists – and this was an important outcome of this study – in the belief that patients with a higher thrombotic risk should receive a mechanical method of prophylaxis in addition to pharmacological prophylaxis. However – and here the confusion is confirmed – there was also a consensus regarding the fact that there are no clear criteria defining which patients could benefit from this application.11,12
Despite the confusion regarding mechanical methods, a consensus was achieved on 2 issues which are not minor: on the routine use of elastic compression stockings in hip surgery and on a role for the application of the plantar venous pump in knee replacement surgery. Elastic stockings in knee surgery interfere with the surgical site and are not recommended for routine use. The plantar pump is considered ineffective in proximal DVT, which is typical of hip surgery.
Pharmacological methodsRegarding the duration of thromboprophylaxis, there are strong recommendations for its administration during the first 14 days and weaker recommendations up to 35 days. There was a broad consensus in supporting that all patients undergoing knee and hip prosthetic surgery should receive pharmacological prophylaxis, and that this should be maintained for up to 28–35 days.
The beginning of prophylaxis13,14 was a highly debated topic. There was a consensus on the statement that starting before or after surgery is indistinct. Although the technical datasheets of some drugs recommend starting their use in the preoperative period, it was also accepted that it is not incorrect to do so postoperatively. Regarding the precise moment for a postoperative start of prophylaxis, the consensus was that each drug should define its own ideal moment, in line with the recent emergence of anticoagulants which define this time according to their pharmacodynamic characteristics and clinical trials conducted.15
Another question that arises is whether prophylaxis should be increased for review surgery. Few patients undergoing such surgery have been included in the clinical trials of these drugs. No consensus was obtained on the decision to increase thromboprophylaxis among patients undergoing prosthetic replacements. There is no data pointing to a higher thrombotic risk despite a previous surgery.
Confidence in the new oral anticoagulants was made clear by a consensus on their routine use in hip and knee surgery, exercising caution with those patients with a particular risk of bleeding. However, no unanimity was obtained on the need for special vigilance among patients with particular risk of thrombosis. One possible explanation is that there is confidence in the efficacy of oral drugs, although this confidence would be less in connection with the possibility of bleeding. This last concern is considered logical due to the novelty of the drugs and is expected to disappear with the progressive incorporation of these drugs into routine clinical practice.16–23
One issue that has aroused controversy is the extension of prophylaxis in knee surgery up to 35 days by the new oral anticoagulants. According to the technical datasheet, based on studies conducted, this is limited to the first 10–14 days postoperatively. However, in this consensus, the opinion of the experts went beyond the studies and – similarly to hip surgery – they considered that in knee surgery it is appropriate to extend prophylaxis up to 28–35 days, whether it is performed with oral anticoagulants or with LMWH, despite the lack of specific studies.
There is a widespread idea that oral treatment would facilitate compliance, although the few studies conducted in this respect do not support this assertion. In this regard, it is significant that the panellists did not reach a consensus.
On the other hand, a change from the oral to the subcutaneous route and vice versa (mainly in the postoperative period and upon hospital discharge) was also defended – too easily. This readjustment does not only involve a change in the route of administration but also a change of drug. There are no studies in connection to the guidelines to be imposed, and this was reflected by the lack of consensus of the panellists when asked about these possibilities as a therapeutic alternative.
The administration of ASA as a prophylactic method24 is vigorously defended, mainly in the USA. A broad consensus was achieved when affirming that aspirin has a much lower efficacy than LMWH and also when affirming that LMWHs are the drugs which provide the greatest confidence.
Finally, with regard to pharmacological prophylaxis, considering that there are numerous clinical situations which are not covered in the datasheets of drugs, there was consensus regarding the importance of expert opinion to fill these gaps. The reason for the present document is very much in this line.
AnaesthesiaRegarding anaesthesia, there was consensus in affirming that placement of an epidural catheter determines the type of thromboprophylaxis to administer,25 and also in denying that such prophylaxis is constrained by increasingly present peripheral nerve blocks. From this point of view, it would be advisable to progressively abandon the use of epidural catheters.
Lastly, there was consensus in taking thrombotic risk into account when deciding on the type of anaesthesia. Spinal anaesthesia has been associated with a decrease in the risk of thrombosis.
Level of evidenceLevel of evidence V.
Conflict of interestsThe authors E.C. and LL.P. have worked at some point as consultants for Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Pfizer, Rovi and Sanofi. The author R.O. has worked at some point as consultant for Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Pfizer and Rovi.
Arcelus Martínez, Juan Ignacio; Cáceres Palou, Enric; Castellet Feliu, Enric; De Frías González, Mariano; Delgado Martínez, Alberto; Durán Giménez-Rico, Lourdes; Gil Garay, Enrique; Gomar Sancho, Francisco; Gómez Barrena, Enrique; Hinarejos Gómez, Pedro; Lecumberri Sagües, Ramón; Llau Pitarch, Juan Vicente; López-Oliva Muñoz, Felipe; Montáñez Heredia, Elvira; Moreno García, Alonso Carlos; Otero Fernández, Rafael; Pérez Caballer Pérez, Antonio; Pino Mínguez, Jesús; Resines Erasun, Carlos; Rodríguez Altonaga, José Ramón; Rocha Pérez, Eduardo; and Ruiz Iban, Miguel Ángel.
Please cite this article as: Castellet E, et al. Consenso SECOT sobre tromboembolismo en la cirugía protésica de rodilla y cadera. Rev Esp Cir Ortop Traumatol. 2013;57:150-9.