Different authors have demonstrated the usefulness of the histological analysis in the diagnosis of prosthetic joint infection; however, its clinical validity is still controversial. The aim of this article is to describe and analyse the clinical validity of histological analysis in the diagnosis of prosthetic infection in patients undergoing hip or knee prosthetic replacement.
Material and methodsWe present a retrospective study including 133 hip and knee prosthetic replacements performed in our centre between 2008 and 2020. A descriptive, bivariate statistical analysis was performed and the clinical validity of the histological analysis was determined.
OutcomesThe clinical validity of the intraoperative histology offered a sensitivity of 48%, a specificity of 91%, a positive predictive value of 55% and a negative predictive value of 88%.
ConclusionsThe determination of the clinical validity of histological analysis shows a high specificity. This analysis is an appropriate diagnostic tool for detecting healthy patients, with no infection.
Diferentes autores han puesto de manifiesto la utilidad del análisis histológico en el diagnóstico de la infección protésica; sin embargo, todavía hoy, su validez clínica es motivo de controversia. El objetivo del presente manuscrito es describir y analizar la validez clínica del análisis histológico en el diagnóstico de infección protésica en el paciente sometido a un recambio protésico de cadera o rodilla.
Material y métodosSe presenta un estudio retrospectivo que incluye 133 recambios protésicos de cadera y rodilla realizados en nuestro centro entre 2008 y 2020. Se realizó un análisis estadístico descriptivo, bivariado y se determinó la validez clínica del análisis histológico.
ResultadosLa validez clínica del análisis histológico ofreció una sensibilidad del 48%, una especificidad del 91%, un valor predictivo positivo del 55% y un valor predictivo negativo del 88%.
ConclusionesLa determinación de la validez clínica del análisis histológico pone de manifiesto una especificidad elevada. Dicho análisis supone una herramienta diagnóstica apropiada para detectar pacientes sanos, con ausencia de infección.
Joint replacement surgery is one of the most successful and cost-effective surgical procedures in orthopaedic surgery, as it has been shown to decrease pain and restore function in patients with advanced osteoarthritis.1 The ageing population and progress in surgical technique, which is becoming increasingly refined, has led to a significant increase in the number of prostheses implanted.2,3 Despite its many benefits, prosthetic surgery is not free of complications, including infection.
In the case of prosthetic infection, correct diagnostic guidance is essential to enable the most appropriate treatment. Diagnosis is based on high clinical suspicion, adequate physical examination, blood tests including complete blood count and acute phase reactant testing, including C-reactive protein (CRP), radiological studies, and diagnostic arthrocentesis. However, in many cases, these tests do not provide a high diagnostic yield for early and accurate diagnosis.4
Due to the disadvantages of currently available preoperative diagnostic tests, the histological analysis described by Mirra et al.5 is still performed today. Since the early work presented by these authors, numerous manuscripts have been published highlighting the benefits of histological analysis in the diagnosis of prosthetic infection. However, its clinical validity remains controversial.
The aim of the present study is to describe and analyse the clinical validity of intraoperative histology in the diagnosis of prosthetic infection in patients undergoing hip or knee replacement.
Material and methodsStudy designWe present a retrospective study that includes 147 hip and knee prosthetic replacements performed in our hospital between 2008 and 2020.
ParticipantsStudy participants were patients who underwent single or double hip or knee arthroplasty replacement. Cases in which intraoperative culture results were not accessible and replacements where the Myrrh test was not performed were excluded. All participants were classified as infection likely and infection unlikely according to the criteria described by McNally et al.6
Surgical techniqueAll procedures were performed by experienced surgeons from the hip and knee unit of the orthopaedic surgery and traumatology department of Malaga's Hospital Universitario Virgen de la Victoria. A lateral Hardinge approach was used for the hip replacement and an anterior longitudinal approach was used for the knee revision. Spinal anaesthesia was used in most patients, except those with contraindications for spinal anaesthesia, in which case general anaesthesia was used. Preoperative antibiotic prophylaxis was used in all cases with intravenous cefazolin one hour before the procedure, except in patients with allergies, who received vancomycin. A thigh root ischaemia cuff was used for prosthetic knee replacements.
During the procedure, at least 3 tissue samples were sent to anatomical pathology for intraoperative analysis, which was extracted from the interface membrane in intimate contact with the prosthetic implant.7
Traditionally, histological analysis has been as per the criteria described by Mirra et al.,5 who recorded the number of polymorphonuclear cells (PMN) in 5 different microscopic fields, which they classified as follows: absent, 1+ (1 to 5 cells per field), 2+ (6 to 49 cells per field) and 3+ (more than 50 cells per field). However, in our hospital, this analysis was performed according to the adaptation described by Feldman et al.,8 >5PMN per high-magnification field (40×) on average, in 5 separate fields, being considered pathological. PMN were identified and counted on frozen sections, avoiding areas with a fibrous appearance, after staining with haematoxylin–eosin, based on their lobulated nuclei and sparse cytoplasmic rim (avoiding fragmented nuclei). The pathology department informed the surgeons of the number of PMNs per field during the operation.
In addition, at least 5 intraoperative samples were collected in all cases and sent to microbiology for culture and antibiogram.
VariablesDemographic variables were collected and analysed (sex, age, body mass index [BMI]), toxic habits (smoking), comorbidities (diabetes mellitus [DM], rheumatological disease [RD]), presence of clinical signs of infection prior to replacement (fever, exudate through the wound, fistula), preoperative analytical parameters (CRP value, arthrocentesis culture result), radiographic parameters (signs of loosening on plain radiography, result of scintigraphy), diagnosis motivating hip arthroplasty in the initial surgery (coxarthrosis or fracture), type of fixation (cementation), number of PMN per field, result of histological analysis (< or >5 PMN per field), result of intraoperative cultures (considered representative in the presence of at least 2 samples with the same microorganism),6 and whether the replacement was performed in one or two stages.
Statistical analysisThe data collected were entered into an Excel database and analysed with Statistical Package for Social Sciences (SPSS®) software. In the descriptive analysis, quantitative variables were expressed with measures of central tendency (arithmetic mean, median, and mode) and measures of dispersion (range and standard deviations). Qualitative variables were expressed as percentages. The data were represented graphically to facilitate the reading and interpretation of the variables analysed.
Considering the sample size, the normality of the quantitative variables collected in the study was assessed with the Kolmogorov–Smirnov test. Regarding quantitative variables, a hypothesis test for independent samples was performed, using the Student's t-test for variables with a normal distribution and the non-parametric Mann–Whitney U and Kruskal–Wallis tests for variables that did not follow a normal distribution. Qualitative variables were analysed using Pearson's χ2 test. Correlation analysis between the preoperative CRP value and the number of PMNs seen in the Mirra test was also made using Spearman's non-parametric Rho coefficient.
To determine the clinical validity of the Mirra test in the diagnosis of prosthetic infection, the main objective of this study, the following parameters were calculated: sensitivity (sen), specificity (spe), positive predictive value (PPV), negative predictive value (NPV), pretest probability (prevalence), positive posttest probability and negative post-test probability.
There are no funding sources or other conflicts of interest directly or indirectly related to the content of the study. Inclusion in the study was not harmful or detrimental to the patients included in the study. All ethical principles as laid down in the Declaration of Helsinki, as last revised in Fortaleza, Brazil 2013, were carefully followed throughout the work. The provisions of Organic Law 3/2018, of 5 December, on Personal Data Protection and Guarantee of Digital Rights were respected in all phases of the study.
ResultsThe preliminary sample included 147 prosthetic replacements; however, 14 cases were excluded from the original database due to inability to access intraoperative culture results, and therefore the final statistical analysis included a total of 133 prosthetic replacements. The mean follow-up was 6 years (1–12 years).
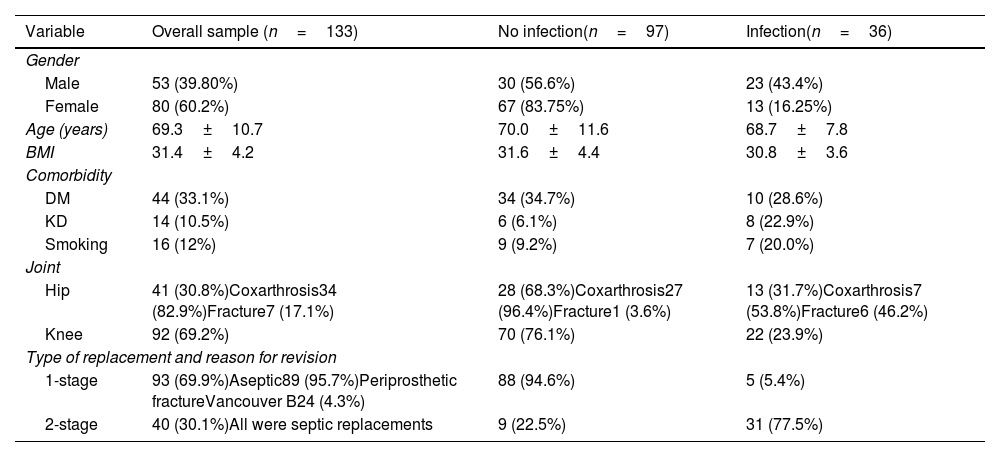
Demographic variables, type of joint, type of replacement and reason for revision for the overall sample, for the group classified as infected (infection confirmed), and for the non-infected group are presented below (Table 1).6
Distribution of demographic variables, joint, type of replacement, and reason for revision.
| Variable | Overall sample (n=133) | No infection(n=97) | Infection(n=36) |
|---|---|---|---|
| Gender | |||
| Male | 53 (39.80%) | 30 (56.6%) | 23 (43.4%) |
| Female | 80 (60.2%) | 67 (83.75%) | 13 (16.25%) |
| Age (years) | 69.3±10.7 | 70.0±11.6 | 68.7±7.8 |
| BMI | 31.4±4.2 | 31.6±4.4 | 30.8±3.6 |
| Comorbidity | |||
| DM | 44 (33.1%) | 34 (34.7%) | 10 (28.6%) |
| KD | 14 (10.5%) | 6 (6.1%) | 8 (22.9%) |
| Smoking | 16 (12%) | 9 (9.2%) | 7 (20.0%) |
| Joint | |||
| Hip | 41 (30.8%)Coxarthrosis34 (82.9%)Fracture7 (17.1%) | 28 (68.3%)Coxarthrosis27 (96.4%)Fracture1 (3.6%) | 13 (31.7%)Coxarthrosis7 (53.8%)Fracture6 (46.2%) |
| Knee | 92 (69.2%) | 70 (76.1%) | 22 (23.9%) |
| Type of replacement and reason for revision | |||
| 1-stage | 93 (69.9%)Aseptic89 (95.7%)Periprosthetic fractureVancouver B24 (4.3%) | 88 (94.6%) | 5 (5.4%) |
| 2-stage | 40 (30.1%)All were septic replacements | 9 (22.5%) | 31 (77.5%) |
Second septic replacement times, partial replacements, and acute infections treated with cleaning, debridement, and polyethylene replacement were not included.
Prior to the prosthetic replacement, diagnostic arthrocentesis was performed in 22 patients (16.5%). The culture of the extracted specimen was positive in 14 patients (10.5%). Similarly, all patients underwent a blood test to assess acute phase reactants. A mean CRP value of 19.2±29.6mg/l was observed. At the time of prosthetic replacement only 2 patients (1.5%) had fever and 15 (11.3%) had exudate through the wound.
On preoperative conventional radiology, up to 111 patients (83.5%) showed signs of prosthetic loosening. In addition, scintigraphy was performed in 54 patients, which was positive in 50 (92.6%).
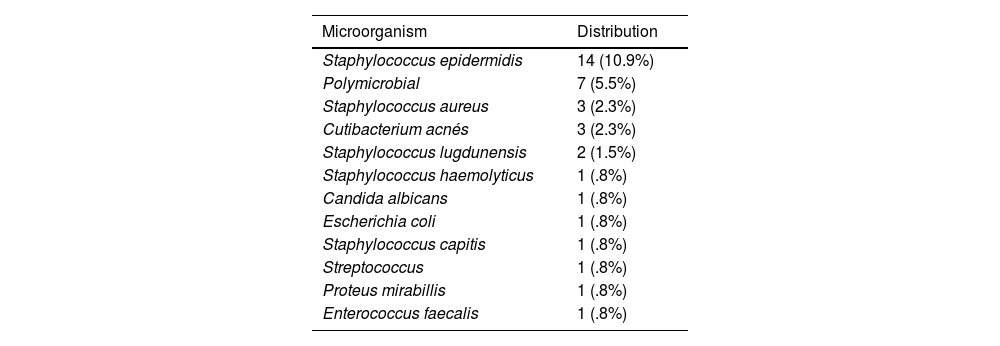
The intraoperative culture result was negative in 97 replacements (72.9%), compared to 36 replacements (27.1%) in which the result was positive (at least 2 positive samples).6 Of the 36 positive replacements, 5 (13.9%) were 1-stage replacements and 31 (86.1%) were 2-stage replacements. The microorganisms isolated in the intraoperative cultures are described in Table 2.
Microorganisms isolated in intraoperative cultures. Frequencies and percentages.
| Microorganism | Distribution |
|---|---|
| Staphylococcus epidermidis | 14 (10.9%) |
| Polymicrobial | 7 (5.5%) |
| Staphylococcus aureus | 3 (2.3%) |
| Cutibacterium acnés | 3 (2.3%) |
| Staphylococcus lugdunensis | 2 (1.5%) |
| Staphylococcus haemolyticus | 1 (.8%) |
| Candida albicans | 1 (.8%) |
| Escherichia coli | 1 (.8%) |
| Staphylococcus capitis | 1 (.8%) |
| Streptococcus | 1 (.8%) |
| Proteus mirabillis | 1 (.8%) |
| Enterococcus faecalis | 1 (.8%) |
Microorganisms isolated in the subgroup classified as polymicrobial infection:
- •
Staphylococcus epidermidis and Staphylococcus aureus: 3 patients
- •
S. aureus and Pseudomona aeruginosa: 3 patients
- •
S. epidermidis and Candida albicans: 1 patient
Regarding histological analysis, the mean PMN count was 4.68±9.36. Therefore, the result was positive in 22 patients, of whom 4 (18.2%) had been treated with a one-stage replacement and 18 (81.8%) with a two-stage (first stage) replacement (p<.001). A positive result was more frequent among male patients (13 [24.5%] vs. 9 [11.3%], p=.029). Also, of the 22 patients with positive histological analysis, a total of 12 (54.5%) had positive intraoperative cultures. In contrast, histological analysis was negative in 111 patients, of whom 98 (88.3%) had negative intraoperative cultures (p<.001).
Although all 4 patients with Vancouver B2 periprosthetic fracture treated with one-stage replacement had negative intraoperative cultures, 2 (50%) had a positive result on histological analysis.
It was observed that the positive result in histological analysis was more frequent in the presence of microorganisms such as S. epidermidis, Staphylococcus lugdunensis, Staphylococcus capitis, Streptococcus, Enterococcus faecalis, or polymicrobial infection (p<.001).
Along the same lines, higher preoperative CRP values were seen (29.1±27.1mg/l vs. 9.0±11.7mg/l, p<.001) in the patients with a positive histological analysis. The CRP value presented a statistically significant correlation with respect to the number of PMNs observed in the histological analysis (Spearman's Rho=.272, p=.045).
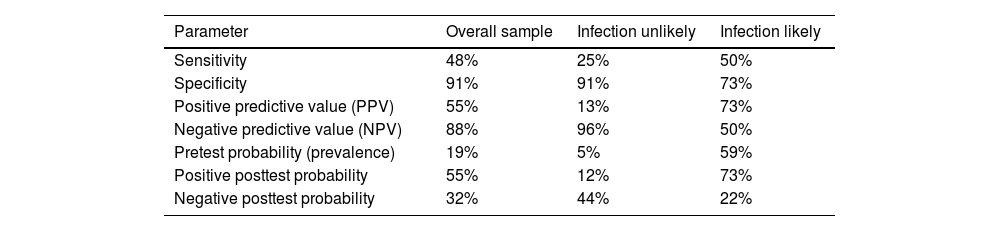
The clinical validity of the Mirra test in our hospital was calculated for the overall sample (133 replacements), and for patients classified as infection unlikely (82 replacements) and infection likely (51 replacements) according to the criteria described by McNally et al.6 (Table 3).
Clinical validity for overall sample, for patients classified as infection unlikely and infection likely.
| Parameter | Overall sample | Infection unlikely | Infection likely |
|---|---|---|---|
| Sensitivity | 48% | 25% | 50% |
| Specificity | 91% | 91% | 73% |
| Positive predictive value (PPV) | 55% | 13% | 73% |
| Negative predictive value (NPV) | 88% | 96% | 50% |
| Pretest probability (prevalence) | 19% | 5% | 59% |
| Positive posttest probability | 55% | 12% | 73% |
| Negative posttest probability | 32% | 44% | 22% |
Histological analysis, described in 1976 by Mirra et al.,5 is a useful tool in the diagnosis of prosthetic infection. Several studies have demonstrated its advantages, but it also has some drawbacks, including its clinical validity, which remains controversial.
Several authors have described histological analysis as having appropriate clinical validity,9 with sensitivity and specificity figures that could reach 100% and 98%, respectively.10 In our series, the specificity was high (91%), in contrast to the sensitivity, which was lower than that described in the literature. In this regard, in our setting, improvement actions based on standardisation of sample collection and processing have been implemented to increase the clinical validity of the test.
In the published literature, in most cases NPV gives better results than PPV, which is consistent with our series for the overall sample and the infection unlikely group. However, in our registry it can also be observed that PPV increases its value in the group of patients classified as infection likely, according to the classification described by Mcnally et al.,6 from 55% to 73.11,12
In relation to rheumatological disease, and specifically rheumatoid arthritis (RA), some authors suggest that it could be a cause of false positives when performing histological analysis as a diagnostic tool in patients with suspected prosthetic infection, with a PPV of around 25%.13 In this regard, our series included 14 cases with rheumatological disease, 5 of whom had RA. Of these 5 patients, 3 had a positive histological analysis result, however, only one patient had positive intraoperative cultures (66.7% of false positives).
Along the same lines, in line with that described by Muñoz-Mahamud et al.,14 our sample showed a high percentage of false positives in the histological analysis performed in patients undergoing prosthetic replacement for a Vancouver B2 periprosthetic fracture.
Traditionally, the most frequent microorganism in prosthetic infection is S. epidermidis, followed by S. aureus, an aspect that coincides with that observed in our study, in which the most frequent microorganism was S. epidemidis (14 cases, 10.9%), followed by polymicrobial infection (7 cases, 5.5%), and S. aureus (3 cases, 2.3%). Although there is little literature on polymicrobial infection, some authors warn that it could be responsible for up to 15% of prosthetic infections, which is consistent with our registry, where it is the second most frequent (7 cases, 5.5%).15,16
Among the results of the present study is the correlation between the preoperative CRP value and the histological analysis result. Different authors warn that the decision to perform a one- or two-stage replacement should not be based solely on the result of the histological analysis, but that additional diagnostic tools should be used, of which preoperative CRP stands out.17 However, CRP should not be used in isolation either as a screening test for prosthetic infection, especially in chronic infections.18
The limitations of the present study include the absence of a control group and its retrospective nature, as the results come from the analysis of a database of medical records. Furthermore, the sample is heterogeneous, as it includes both hip and knee prosthetic replacements.
ConclusionAnalysis of the clinical validity of histological analysis shows high specificity. In our study, histological analysis is an appropriate diagnostic tool to detect healthy patients in the absence of infection. The use of additional diagnostic tests, including preoperative CRP, is also essential.
Level of evidenceLevel of evidence III.
Transparency statementThe corresponding author, on behalf of the undersigned, guarantees the accuracy, transparency and honesty of the data and information contained in the study; that no relevant information has been omitted; and that all discrepancies between authors have been adequately resolved and described.
FundingThis research study received no specific funding from public sector agencies, commercial or non-profit organisations.
Authors’ contributionsAll authors contributed to the design of the study, data collection and analysis, interpretation of the results, and drafting and revising the final manuscript.
Conflict of interestsThe authors have no conflict of interests to declare.