Osteochondral allografts (OA) kept fresh for a long time, are presented as a viable option for the treatment of large chondral and osteochondral lesions.
GoalsChondrocyte viability decreases substantially when allografts are stored for more than 15 days. The objective of this work is to validate the viability and clinical and functional results of OA transplantation stored at 37 °C in a cell culture medium, applied in cartilage defects of the knee, defining the means and limits of allograft storage, among 15 and 28 days after extraction.
Patients and MethodThis study presents the results of 20 consecutive patients, operated between 2003 and 2019, who underwent a fresh-preserved osteochondral allograft, implanted on cartilage defects of the femoral condyle and patella. The minimum follow-up time was 10 years and the maximum 17. The mean age of the patients was 29 (14−44).
The clinical control data were collected using IKDC, International Knee Documentation Committee (knee-specific), KOOS, Knee injury and Osteoarthritis Outcome Score protocols. Likewise, the degree of satisfaction was evaluated. Cartilage control was performed using the International Cartilage Repair Society Score (ICRS) and the Oswestry Arthroscopy Score (OAS). Radiological evaluations were performed using MRI and helical CT.
ResultsStatistically significant improvements, P < .0001, were observed for the (IKDC) (30–65); the 5 components of the KOOS protocol, (pain, 66–85); Specific symptoms, (72–82); Activities of daily living, 74–91; Sports and recreational function, from 37 to 70, related to the knee, Quality of life, (25–60). Regarding the degree of satisfaction, 89% answered being satisfied or very satisfied.
The ICRS and OAS results, all patients went from grade IV to grade I, except for one who is currently in IV.
The incorporation of the graft in the recipient area occurred at three months, verified by helical CT and MRI.
ConclusionOsteochondral allograft transplants stored fresh at 37 °C are established as a long-term solution for the treatment of localized osteochondral defects in the knee.
Los aloinjertos osteocondrales (AOC) conservados en fresco de forma prolongada en el tiempo, se presentan como una opción viable para el tratamiento de grandes lesiones condrales y osteocondrales.
ObjetivosLa viabilidad de los condrocitos disminuye sustancialmente cuando los aloinjertos son almacenados durante más de 15 días. El objetivo de este trabajo es validar la viabilidad y los resultados clínicos y funcionales del trasplante de AOC almacenados a 37 °C en un medio de cultivo celular, aplicado en defectos de cartílagos de la rodilla, definiendo los medios y límites del almacenamiento de los aloinjertos, entre 15 y 28 días después de la extracción.
Pacientes y MétodoEste estudio presenta el resultado de 20 pacientes consecutivos, operados entre el 2003 y 2019, a quienes se les practico a un aloinjerto osteocondral conservado en fresco, implantado sobre defectos de cartílago del cóndilo femoral y rotula. El tiempo de seguimiento mínimo fue de 10 años y máximo de 17. La edad media de los pacientes era de 29 (14−44) años.
Los datos de control clínico fueron recogidos usando protocolos IKDC, International Knee Documentation Committee (especifico de rodilla), KOOS, Knee injury and Osteoarthritis Outcome Score. Asimismo, se evaluó el grado de satisfacción. El control del cartílago se realizó mediante International Cartilage Repair Society Score(ICRS) y Oswestry Arthroscopy Score (OAS). Las evaluaciones radiológicas se realizaron mediante Rmn y Tac helicoidal.
ResultadosSe observaron mejoras estadísticamente significativas, P < .0001, para el (IKDC) (30 a 65); los 5 componentes del protocolo KOOS, (dolor, 66 a 85); Síntomas específicos, (72 a 82); Actividades de la vida diaria, 74 a 91; Función deportiva y recreativa, de 37 a 70, relacionado con la rodilla, la Calidad de vida, (25 a 60). En lo que respecta al grado de satisfacción el 89% respondía estar satisfecho o muy satisfecho.
Los resultados ICRS y OAS, todos los pacientes pasaron del grado IVº al grado Iº, menos uno que en la actualidad está en IVº.
La incorporación del injerto en la zona receptora ocurrió a los tres meses, comprobado mediante TAC helicoidal y RMN.
ConclusiónLos trasplantes de aloinjerto osteocondral conservados en fresco a 37ªC se establecen como una solución a largo plazo para el tratamiento de defectos osteocondrales localizados en la rodilla.
Osteochondral allografts (AOC) were first used by Lexer1 in 1908 to restore articular surfaces.
Later, in the 1970s, two schools had already emerged as leaders in the study of these procedures, one in Canada (Toronto), Gross et al. began their experience with OA in post-traumatic reconstructions and periarticular tumours.2,3
The other school based in San Diego, USA, Meyers et al. first used this technique specifically for the treatment of chondral and osteochondral diseases.4
Later, in the 1990s, the foundations were laid to treat osteochondritis dissecans5 and osteonecrosis6 joint defects using fresh osteochondral allografts.
This century, given the expectations created by this type of procedure, many scientific studies have been published in basic sciences on the preservation, immunogenicity and viability of allografts,7–10 as well as clinical reviews with a very long-term follow-up of up to 22 years.11
In grafting, as an alternative, it is recommended that transplantation should be performed with allografts that are either fresh, implanted immediately after extraction or after storage.12
The best way to preserve the graft prior to transplantation is currently a matter of debate. This should be either at 4 °C, using different methods to study the viability of the chondrocytes and clinical results,13,14 or at 37 °C, preserved in a cell culture medium in a CO2 incubator.15,16
Another concept to consider is the immune response to the allograft of the recipient, basically activated by cancellous bone.17
However, cartilage does not produce an immunogenic reaction, if it is intact, i.e., the chondrocytes are within the barrier of the extracellular matrix.18
No less important is the study of allograft viability prior to transplantation. Therefore, it is important to establish methods for evaluating the quality of the graft, which in this case depends on the viability of the chondrocytes.10,19–21
Patients and methodsDonors are obtained from within the Transplant Programme of the Marqués de Valdecilla University Hospital (HUMV) and the protocol for bone extraction from the Blood and Tissue Bank of Cantabria is applied specifically. In this case the femoral condyles and a patella which are obtained under strict aseptic conditions in the operating theatre, a maximum of 12 h after life support has been withdrawn from the donor.
Osteochondral specimens obtained from donors, are preserved in culture media. This is Dulbecco's Modified Eagle Medium (DMEM) supplemented with 20% of the patient's own serum, l-glutamine 20 Mm, antibiotics and antifungals (penicillin 100 U/mL, streptomycin 100 μg/mL and amphotericin B 2.5 μg/mL), and incubated at 37 °C in a CO2 incubator at 5%. The culture medium should be replaced twice a week, in a laminar flow hood, at which time a microbiological control is performed. Cell viability controls are also carried out using tetrazolium salts during the storage period.20,21
After a minimum period of 15 days in in vitro culture, the femoral condyles are safe to be used as osteochondral grafts.
All donors are screened for bacterial and viral disease. Special care must be taken with diseases with a window period such as HIV and HCV.
The very low incidence of rejection is remarkable in this type of graft, as neither HLA or ABO typing of the donor or recipient is necessary, nor is preventive immunosuppression.
The study was approved by HUMV’s clinical research ethics committee, Santander, Cantabria.
Selection of recipientsPreferably young patients, with a mean age of 28 years (14 ± 44), with osteochondral lesions. Twenty patients participated in the study, with a follow-up of more than 10 years. In terms of gender distribution, we found 50% males (N = 10) and 50% females (N = 10).
Regarding the aetiology of the lesions, 90% of the cases were osteochondritis dissecans, 5% of the lesions were caused by a firearm and the remaining 5% were grade 4 chondromalacia patellae.
Of the patients, 90% had undergone one or more interventions previously (including arthroscopy, AK fixation, Herbert screws and Orthosorb pins, as well as microfracture and Pridie drilling).
The indications for these procedures in our series were grade IV traumatic OC lesions, severe osteochondritis dissecans with devitalised or loose fragment (grades III and IV) of femoral condyle, International Cartilage Research Society (ICRS) classification, the minimum size for inclusion being 1.8 cm2, and no other type of surgical procedure was assessed in this series.22 In our series, the primary lesion was a mean 3.9 cm2 (range: 1.8 cm2–6 cm²). The graft size was 3.75 cm2 (range: 1.5 cm2–6 cm²). The graft age from harvesting to implantation ranged from 15 to 28 days (Table 1).
Characteristics of the patients and details of the allograft.
| Total (N = 20) | |||
|---|---|---|---|
| Gender | |||
| Female | 10 | (50) | |
| Male | 10 | (50) | |
| Age | 29 | (14−44) | |
| Diagnosis | |||
| Osteochondritis dissecans | 18 | (88.89) | |
| Traumatic | 1 | (5.56) | |
| Degenerative | 1 | (5.56) | |
| Location | |||
| Internal femoral condyle | 18 | (88.89) | |
| Internal femoral condyle | 1 | (5.56) | |
| Patella | 1 | (5.56) | |
| N. of previous operations | |||
| 0 | 9 | (50) | |
| 1 | 7 | (38.89) | |
| 2 | 1 | (5.56) | |
| 3 | 1 | (5.56) | |
| N of grafts | |||
| 1 | 18 | (88.89) | |
| 2 | 2 | (11.11) | |
| Total area of graft cm² | 3.75 ± 1.02 | (1.8−6.0) | |
| Age of graft (days) | 20.5 | (15−28) | |
Variables are expressed as mean ± SD (range) or n. (%).
It is important that there are no mirror lesions, no disturbances in the axis, and no ligamentous lesions, and if so, the abnormality must be corrected beforehand.
There were no postoperative complications or infections.
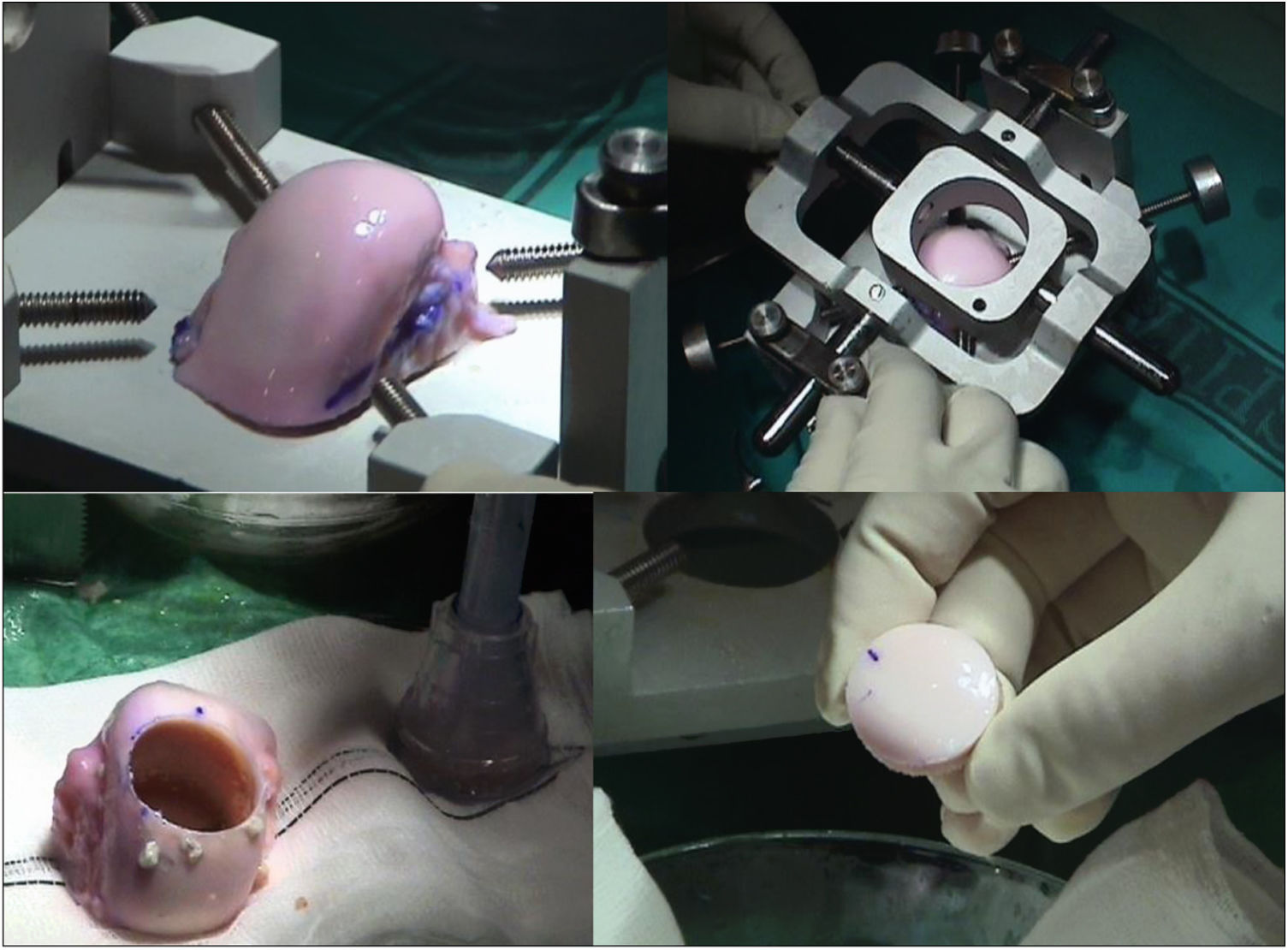
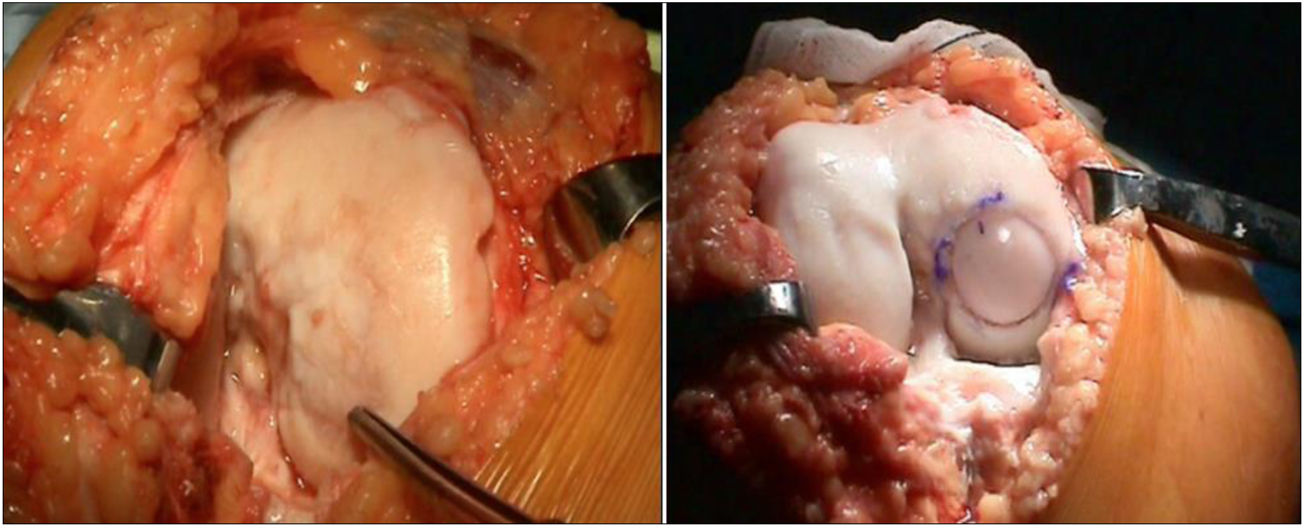
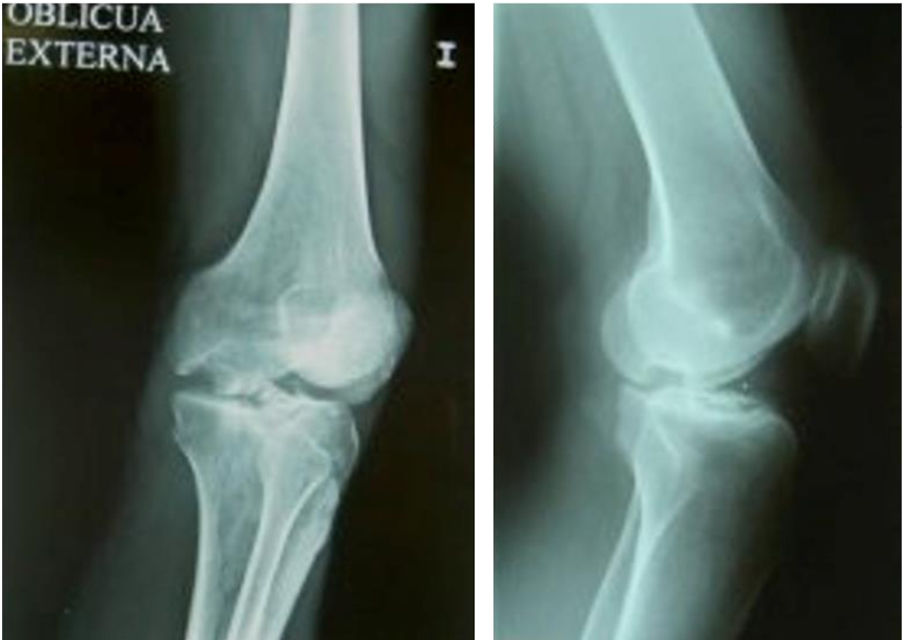
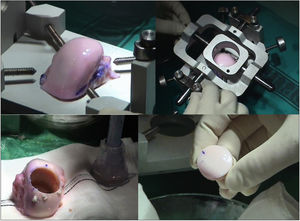
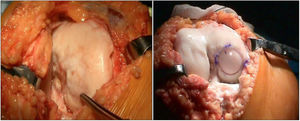
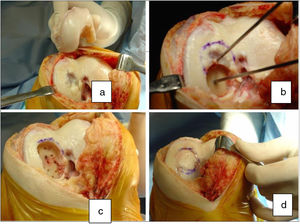
The surgical technique used comprised an internal or external knee arthrotomy, followed by preparation of the damaged area using the Maxioats® (Artrhex®) technique23 (Figs. 1 and 2).
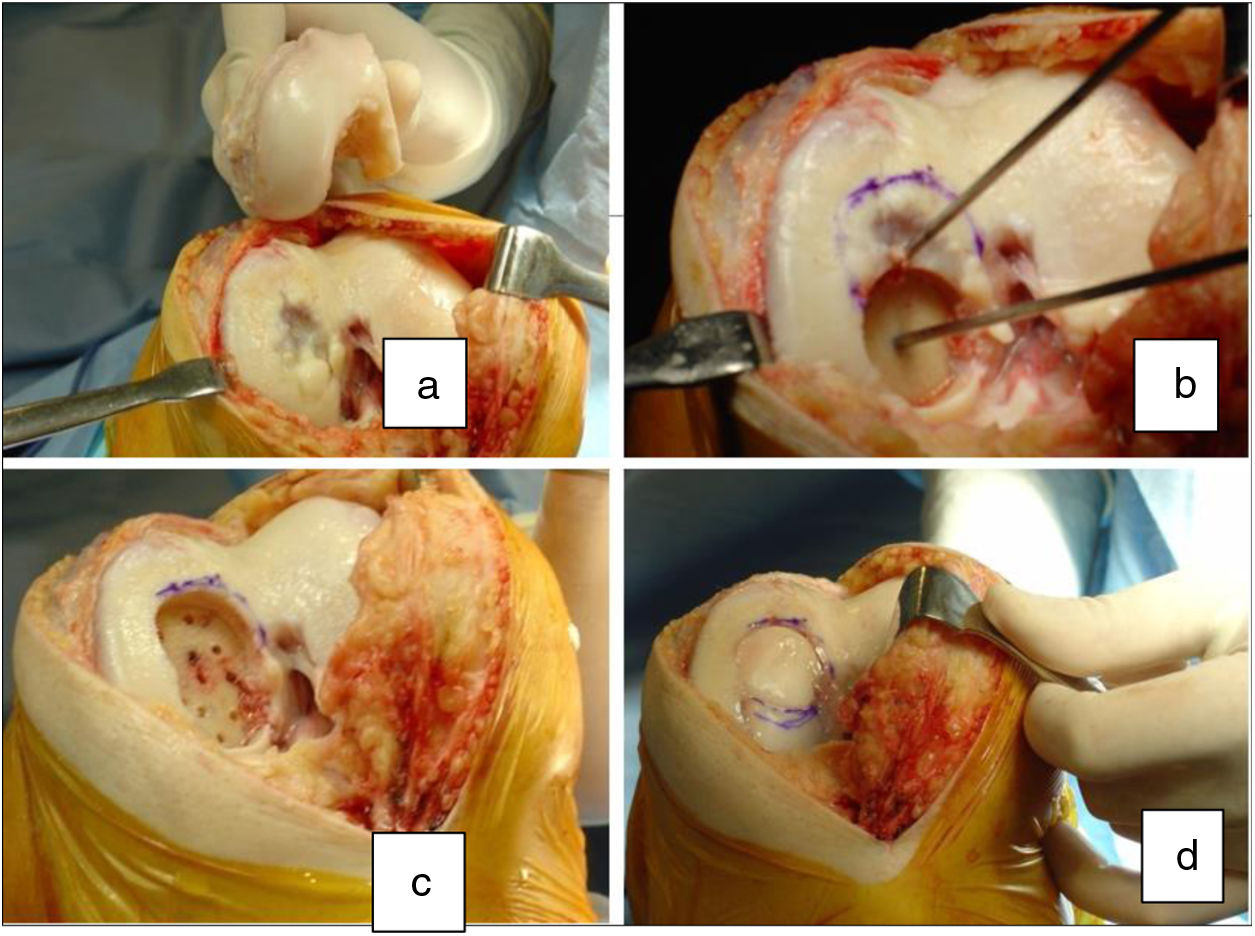
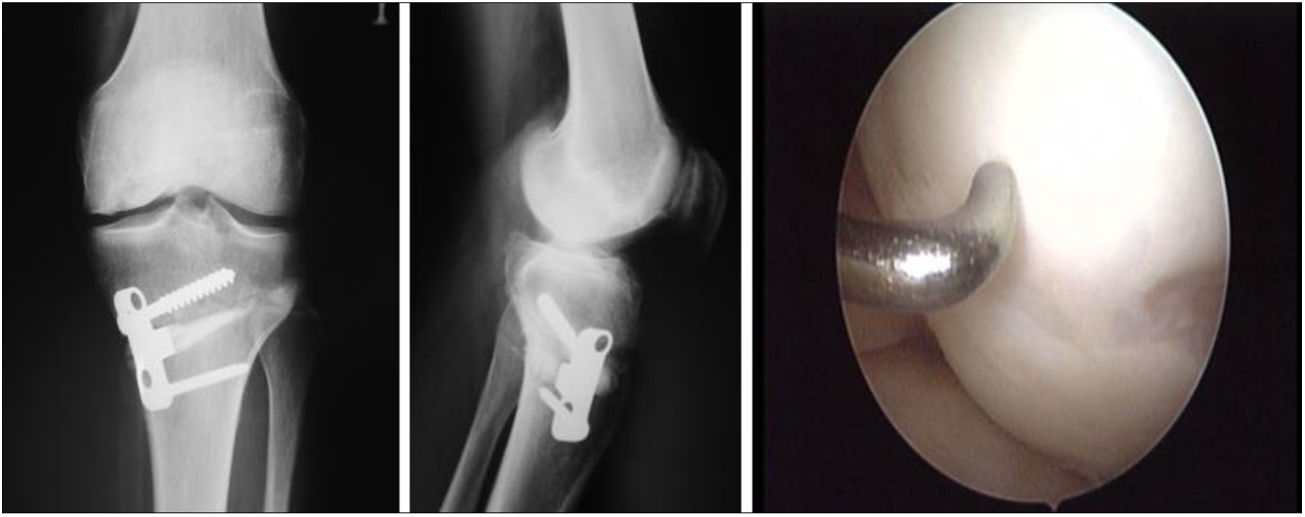
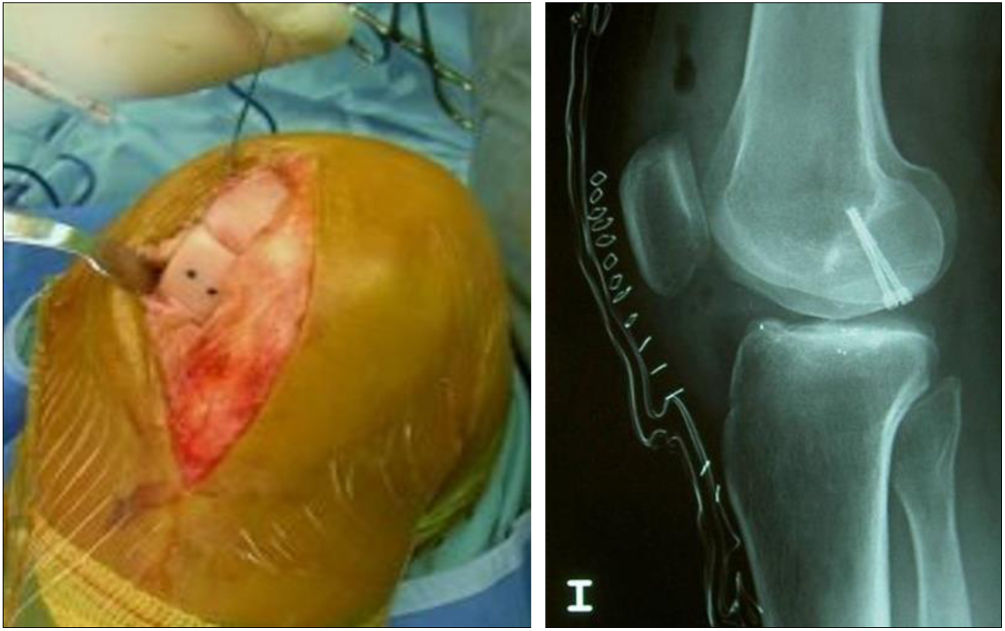
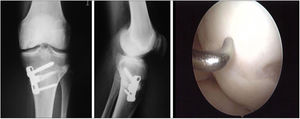
Two patients required special milling of the graft, as the Maxioats® technique did not cover the defect; one received a double graft and, at one year, arthroscopic control of the graft (Figs. 3 and 4); in the other case, the graft was milled by hand, given the defect, and fixed with 2 Herbert screws (Figs. 5 and 6).
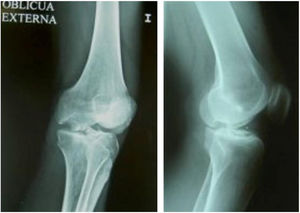
Same patient as Fig. 3, at one year, valgus osteotomy and arthroscopic revision.
Same patient as Fig. 5. Result of manually milled graft fixed with Herbert screws.
In one case the surgical technique was performed completely arthroscopically.
A diagnostic imaging follow-up protocol was created. This protocol is for preoperative and postoperative assessment of the lesion, analysing in the latter the 3 stages of the healing process of the transplanted grafts: proliferative, transitional and maturation. The method comprised first MRI scans in the month prior to surgery, and at 3, 6 and 12 months after surgery.
MRI was sometimes used with gadolinium-DTPA contrast agents, which can describe biomechanical and biochemical changes associated with chondral matrix degeneration. In addition, cartilage-specific slices demonstrate the close relationship between chondral defects, clinical symptoms, and the likelihood of symptom progression.24
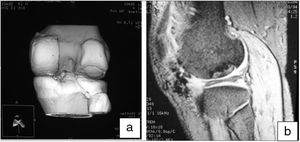
We also used helical CT to monitor bone integration in the host/donor area, checking whether or not there were bone bridges at 3 and 6 months.
There are different clinical evaluation methods, in our case we chose the following protocols for clinical follow-up: International Knee Documentation Committee (IKDC) (knee-specific),25 Knee injury and Osteoarthritis Outcome Score (KOOS) and satisfaction protocol. The assessed quality of cartilage repair was monitored using the International Cartilage Repair Society Score (ICRS), in the cases where we performed a second arthroscopy, in 8 patients we applied the Oswestry Arthroscopy Score (OAS).26
The radiological assessments were performed by MRI and helical CT.
We used SPSS® version 11.5 (SPSS, Inc, Chicago, IL, USA) for the statistical analysis. The results were considered statistically significant with a P value <.05.
ResultsWe performed the first intervention of the series of 40 cases in 2003 and the last in 2019. We included in this study those with a minimum follow-up of 10 years; the oldest patients in the series have been followed up for 17 years.
The patients underwent clinical and radiological follow-up protocols. The IKDC protocol, and more specifically the subjective knee assessment form,25 shows clear improvement, with a preoperative mean of 30 and postoperative mean of 62, out of a maximum of 79 (N: 20); P < .0001. The 5 components of the KOOS protocol: pain: 66–85; specific symptoms: 72–82; activities of daily living: 74–91; function in sport and recreation: 37–70; knee-related quality of life: 25−60. In terms of degree of satisfaction, 89% were satisfied or very satisfied. Regarding the grade of cartilage repair (ICRS), the chondral lesion modification, 19 patients progressed from grade IV to grade I and one is currently grade IV. These results were obtained by MRI,24 with specific cartilage slices, including with gadolinium-DTPA contrast agents.
In the cases we examined a second time by arthroscopy, we observed the survival of the implanted graft, integration of the edges, and the macroscopic appearance of the cartilage surface, using the OAS protocol, which is complementary to the ICRS results (Table 2).
Result over 20 knees with allografta.
| Protocols | Preoperative | Results | P value* |
|---|---|---|---|
| IKDC | 30.7 ± 9 | 65.2 ± 13.8 | <.0001 |
| KOOS | <.0001 | ||
| Symptoms | 62.5 ± 18.0 | 82.5 ± 15.5 | |
| Pain | 66.5 ± .18.6 | 85.3 ± 16.3 | |
| Activities of daily living | 74.5 ± 18.9 | 91.1 ± 14.7 | |
| Sport/leisure | 37.8 ± .22.6 | 70.2 ± 26.4 | |
| Quality of life | 25.4 ± 17.6 | 60.0 ± 24.3 | |
| ICRS | Grade IV: 20 patients | Grade I: 18 patients | |
| OAS | Grade II: 2 patients | ||
| Satisfaction | |||
| Very satisfied | 67.7 | ||
| Satisfied | 21.3 | ||
| Somewhat satisfied | 5.5 | ||
| Somewhat dissatisfied | 3.0 | ||
| Dissatisfied | 2.4 |
ICRS: International Cartilage Research Society; IKDC: International Knee Documentation Committee; KOOS: Knee injury and Osteoarthritis Outcome Score; OAS: Oswestry Arthroscopy Score.
It should be noted that all the patients were discharged after 6 months except for one, the gunshot injury, who had to be reoperated as the graft had partially failed, and another allograft had to be performed on the resulting defect, which is currently a grade I; the patient with the patella transplant, underwent arthrolysis and she progressed favourably.
Two other patients underwent osteotomy, one at one year and the other at 6 years, as the internal impingement had progressed; the latter, whom we had lost to follow-up, made the poorest progress and is awaiting arthroplasty.
Plain anteroposterior and lateral studies of the joint were used for postoperative radiographic measurement, to check the correct implantation of the graft in all cases, with no loosening or subsequent mobilisation observed, except in one case where the graft had collapsed slightly due to partial integration.
Helical CT was used in follow-up to check the integration of the recipient area with the graft. In all cases it was observed that at 3 months there were sufficient bone bridges to consider the graft integrated. In one case bone integration bridges were observed at one and a half months. This is a very useful method to directly assess the bone integration of the graft in the recipient bed.
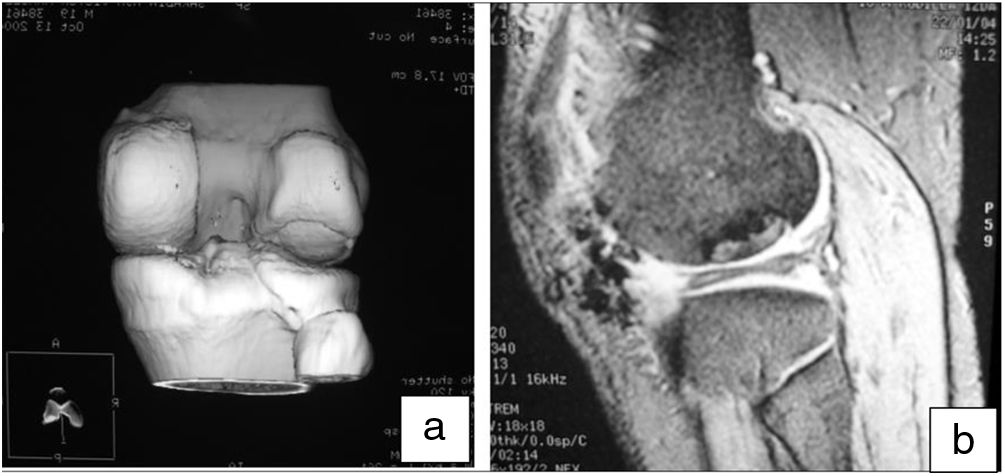
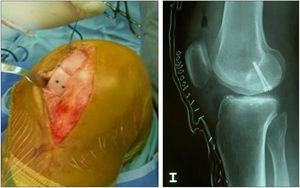
Moreover, MRI was useful to check the gradual decrease in bone oedema in the graft-receiving area interface, which decreased progressively in successive controls (3–6 months) in all cases (Fig. 7).
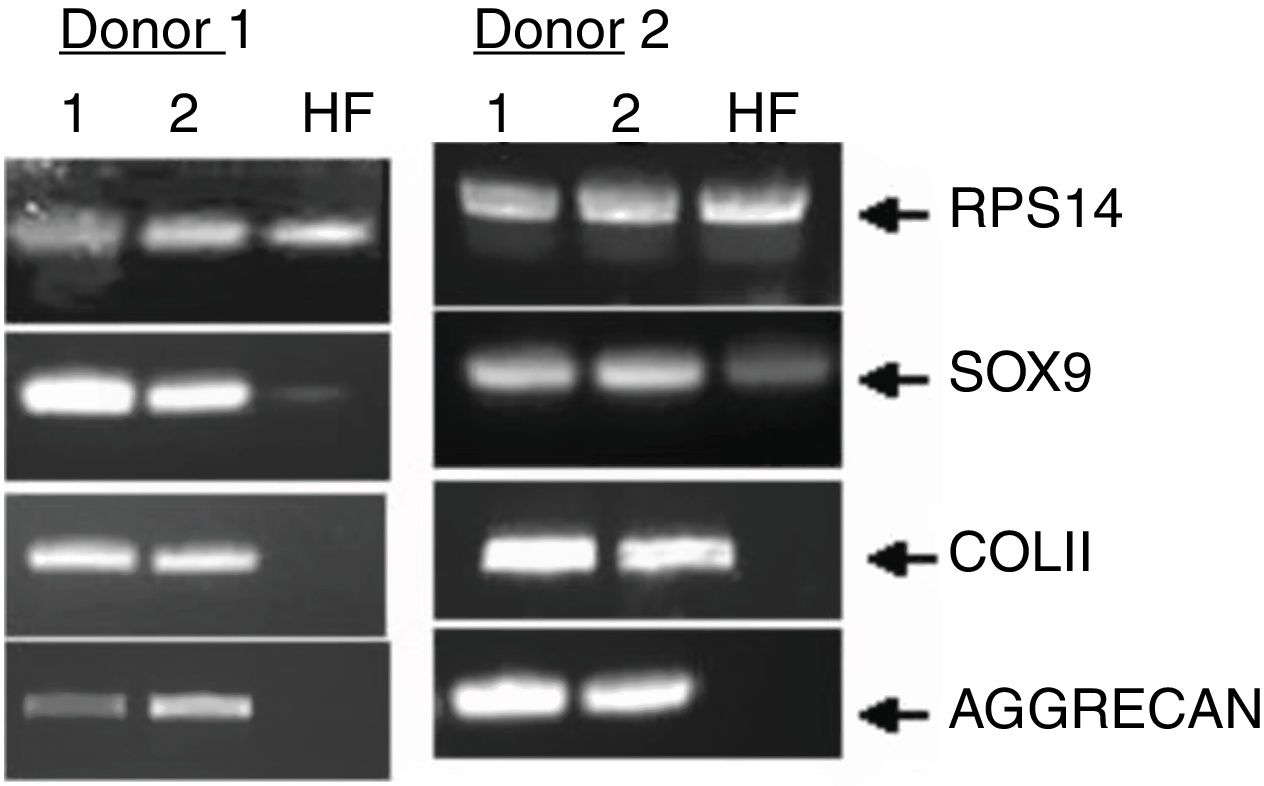
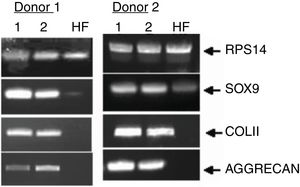
Finally, we monitored the graft by means of biopsy in 3 of the cases that underwent a control arthroscopy, the result being mature hyaline cartilage. In all cases we carried out a pre-implant viability study using tetrazolium salts, confirming the viability of the graft in all cases. Apart from this and as additional control, in one case we added a pre-implantation genetic study of the preserved cartilage, the result being the expression of specific chondrocyte genes (Fig. 8).
DiscussionThe presence of viable chondrocytes in this type of procedure is essential since chondrocytes are essential for maintenance of the extracellular matrix and to prevent degeneration of the graft over time.
Programmed cell death (apoptosis) is one of the factors that can compromise the effectiveness of these processes. This occurs during storage and preparation as well as during implantation. Recent studies indicate that the rate of apoptosis is responsible for the death of chondrocytes associated with transplantation.27
It is therefore important to establish methods of assessing graft quality, which in this case depends on the viability of the chondrocytes. Although it is important to establish viability, there is no quantitative method to determine cell viability before the graft is used for transplantation. We use tetrazolium salts for this purpose, a method that has already been tested.20,21
Since osteochondral transplants began in the 1970s, osteochondral lesions, osteonecrosis, and osteochondritis dissecans have increasingly been treated using osteochondral transplants.2–6
OC transplants are techniques on the increase, with very good results, accounting for more than 80% of published cases, including post-traumatic lesions.2,3,5,7
The indications for transplantation have grown and now extend to bipolar lesions,28 to massive joint grafts29 and as a means of rescue when first-choice treatments fail.30
The alternative to autograft is to transplant with allografts, either fresh, implanted immediately after harvesting,5,12 or after storage.13
First the size and location of the lesion must be considered, and whether there are any abnormalities in the mechanical axes, ligamentous insufficiencies or meniscal lesions that need to be repaired beforehand.
The clinical outcome and survival of the allograft depends on the presence of viable chondrocytes that allow remodelling of the extracellular matrix of the cartilage after transplantation. In this case, there has been discussion as to the best way to preserve the graft prior to transplantation: in lactated Ringer's solution at 4°7 or in culture media at 37 °C.
We chose storage in cell culture medium in an incubator at 37 °C.16,17
In our case and as we have been able to demonstrate, cartilage survival in perfect conditions is in a storage medium such as DMEM and in an incubator at 37 °C, for at least 28 days.20
In the transplants we performed, the age of the graft from extraction to implantation was a minimum of 15 days and a maximum of 28 days.
More recent publications highlight that storage at 37 °C is superior to 4 °C in experimental medium in animals,16,31 concluding that storage of OA at 37 °C especially protects the superficial layer of cartilage, which is the most sensitive and vulnerable, increasing chondrocyte viability rates over time.
Our study is a retrospective case series, there was no control group available compared to other possible treatment modalities, although this method is often considered a last resort option, studies rarely compare this technique with others that restore cartilage.
ConclusionsAllogeneic osteochondral transplantation, preserved fresh at 37 °C, is a safe and effective method of treating symptomatic osteochondral lesions of the knee in young patients.
This technique has the advantage of transplanting viable and mature hyaline cartilage, which is mechanically stable, based on optimal preservation in a culture medium at 37 °C.
At a minimum follow-up of 10 years the osteochondral transplant is well integrated, as demonstrated in imaging controls and good control of pain and joint function has been achieved.
Level of evidenceLevel of evidence IV.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Gómez Cimiano FJ, Garcés Zarzalejo C, Estellés M. de León LR, Gómez de la Lastra L, Galindo Rubin C. Trasplante osteocondral de rodilla, mediante aloinjertos, conservados en fresco a 37 °C. Determinación de la viabilidad del cartílago humano, indicaciones, técnica y evidencia. Seguimiento mínimo, 10 años. Rev Esp Cir Ortop Traumatol. 2021;65:340–348.