To compare the efficiency and safety of platelet-rich plasma (PRP) injection versus hyaluronic acid (HA) in patients with hip osteoarthritis (OA) not responding to symptomatic treatment, and to correlate cellular composition of PRP to clinical outcomes.
Material and methodsThis is a phase III clinical trial, double-blinded, controlled and randomised into two treatment groups (PRP and HA). Patients received one hip ultrasound-guided injection. Follow up was 12 months. Pain was assessed using VAS score, HHS and WOMAC were used as functional scores, analgesia, adverse events, cellular components in peripheral blood and in PRP were recorded. Clinical response was assessed using OARSI criteria.
ResultsSeventy-four patients were included. Both groups improved in VAS, WOMAC and HHS score and reduced the amount of analgesia (p < .05). Significant differences were seen at 1 year post-treatment in HHS score (PRP 70.9 [3.7-58], HA 60.2[43-74.2] p < .05).
Platelet concentration was different between responders and non-responders (at 1 month, non-responders 449[438-578] x103 platelets/μl versus responders 565 [481-666] x103 platelets/μl, p < .044). There was a correlation between leukocytes concentration and clinical scores (VAS at six months, r = .748, p < .013, WOMAC at 6 months r = .748, p < .013). Patients with early stage hip OA showed higher response rate to PRP compared with late stage (11.51 OR, 95%CI 2.34-50.65, p < .03).
ConclusionsPlatelet-rich plasma injection improved hip function, reduced pain and the use of analgesia. It is important to bear in mind the cellular composition in order to achieve a better clinical response.
Comparar la eficacia y seguridad de la infiltración de plasma rico en plaquetas preparado por técnica abierta respecto a ácido hialurónico en pacientes con coxartrosis refractaria a tratamiento conservador. Así como correlacionar el impacto clínico entre las diferentes concentraciones celulares.
Materiales y métodosEnsayo Clínico Fase III, doble-ciego, controlado, en el que se aleatorizaron a los pacientes que cumplen los criterios de inclusión y no exclusión en dos grupos de tratamiento (PRP o AH) con una única infiltración de cadera ecoguiada. El seguimiento fue de 12 meses, registrando escala de dolor (EVA) y escalas funcionales (HHS y WOMAC), analgesia consumida, respondedores (criterios OARSI) y efectos adversos. Se analizaron, en el grupo experimental, las concentraciones celulares en sangre periférica y en el PRP infiltrado.
ResultadosSe incluyeron un total de 74 pacientes. Ambos grupos de tratamiento presentaron mejoría en las escalas EVA, WOMAC, HHS y reducción del consumo de analgesia en el tiempo (p < 0.05). Únicamente encontramos diferencias significativas entre grupos al año de tratamiento en los valores de HHS (Grupo PRP 70.9 [3.7-58] grupo AH 60.2[43-74.2] p < 0.05).
Encontramos correlación entre la concentración de plaquetas en pacientes respondedores (1 mes postratamiento; no respondedores 449[438-578] x103 plaquetas/μl, respondedores 565 [481-666] x103 plaquetas/μl, p < 0.044). Se correlaciona la concentración de leucocitos con las escalas clínico-funcionales (EVA 6 meses, r = 0.748, p < 0.013, subescala rigidez WOMAC 6 meses, r = 0.748, p < 0.013). Los pacientes con estadios de coxartrosis iniciales (KL 1 y 2) tienen mayor probabilidad de responder al tratamiento con plasma rico en plaquetas (11.51 OR, IC 95% 2.34-50.65, p < 0.03).
ConclusionesLa infiltración única de PRP es eficaz en términos de mejoría funcional, reducción del dolor y disminución del consumo de analgesia en coxartrosis. Los sistemas de preparación abiertos, son un procedimiento seguro para la obtención de PRP. Se deben indicar las infiltraciones de cadera en estadios evolutivos iniciales. Se debe tener en cuenta la composición celular para garantizar una repuesta clínica positiva.
Osteoarthritis is characterised by a cartilage impairment process, with proliferative reaction of the subchondral bone and inflammation of the synovial membrane of the hip. Osteoarthritis (OA) is among the 10 main causes of disability worldwise.1 Its management of consists of a single definitive treatment: hip arthroplasty. Conservative therapeutic options include pharmacological treatments, physiotherapy and intraarticular injections. Intraarticular injections (IA) are another tool for daily clinical practice. Their use is extensive but many doubts remain unresolved regarding their efficacy and usage protolcol.2,3
The use of blood products has recently acquired great impact, including that of platelet-rich plasma (PRP).4 PRP is a concentrate of platelets obtained from autologous blood centrifugation. Its biological composition with high concentrations of growth factors and its tissue repair and regeneration action has become the focus of the scientific community.5 Indiscriminate administration regardless of the pathology together with its level of effect and patient characteristics have led to doubts regarding its efficacy. Its action has been demonstrated in vitro in studies but no quality clinical studies exist proving its efficacy and establishing a treatment preparation and administration protocol.6–8
As a result, one of the primary objectives of this study was to establish a PRP procedural protocol, with quantification and correlation of the cellular composition and with clinical findings and evidence of treatment efficacy.
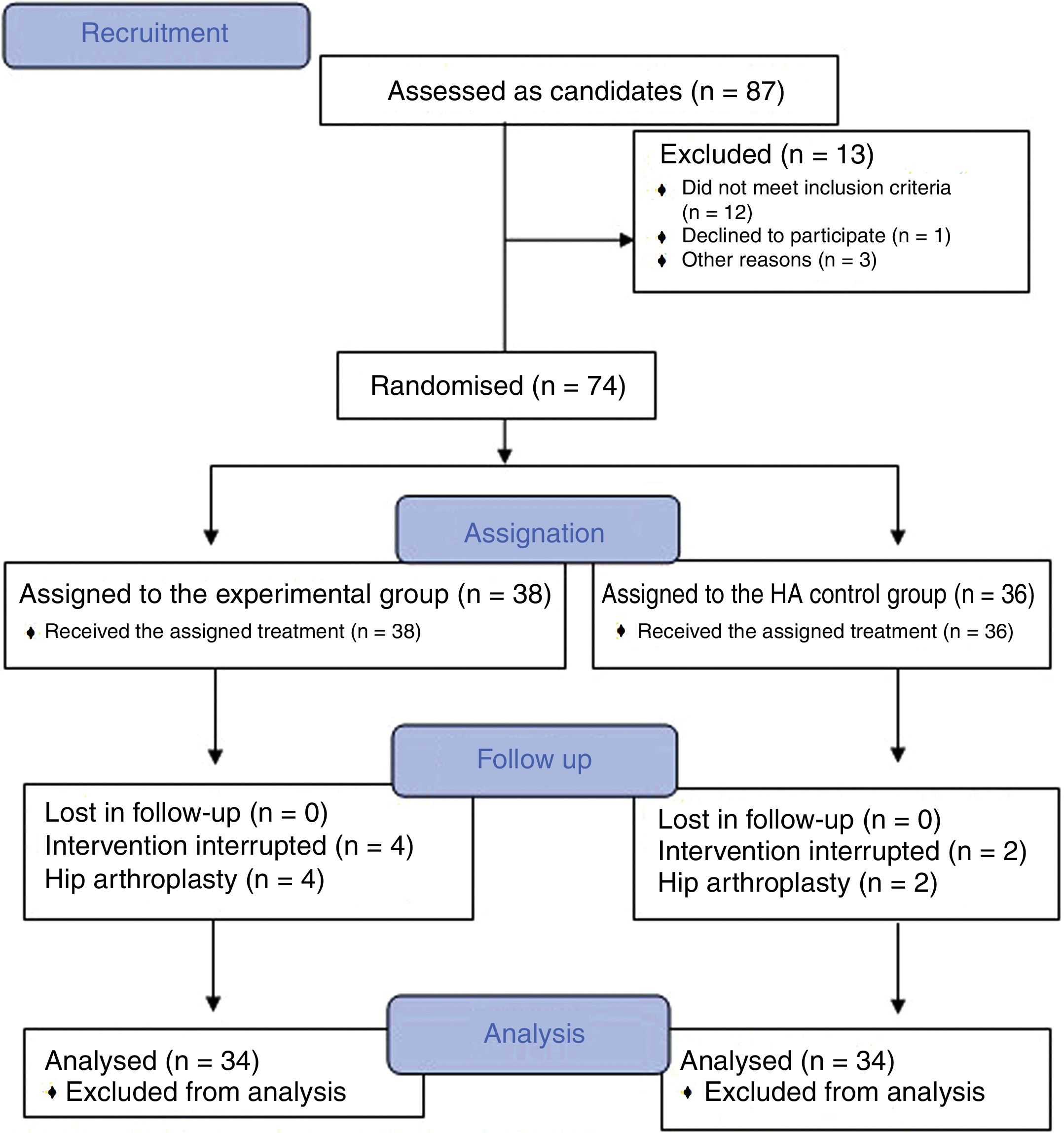
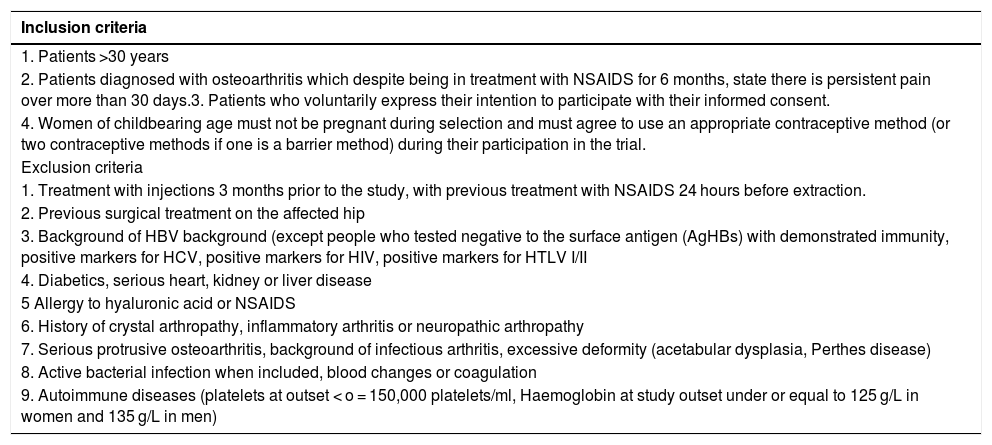
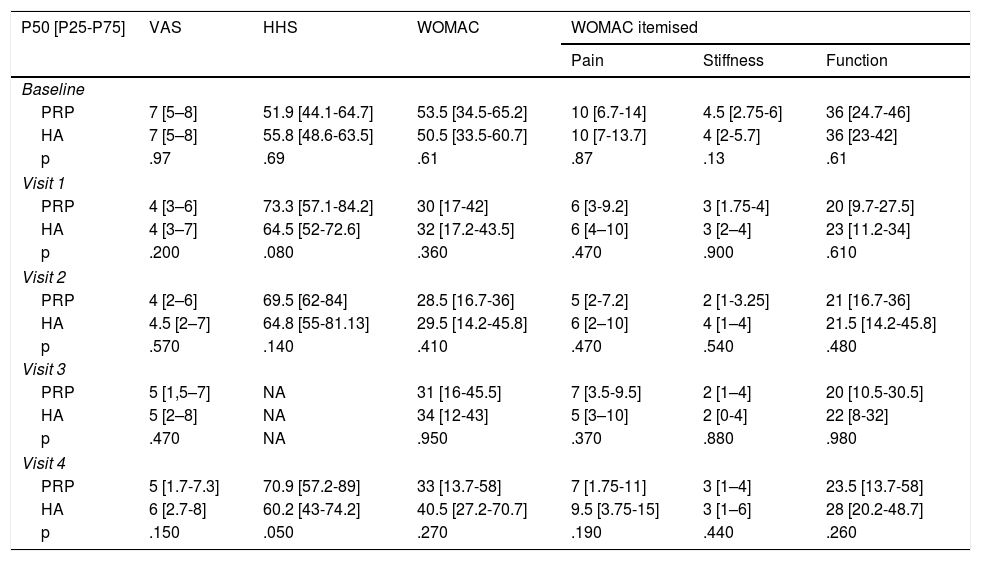
Material and methodsA randomized, double-blind, controlled trial, phase III, was conducted which included a total of 74 patients diagnosed with hip osteoarthritis for whom prolonged analgesic treatment with NSAIDS had failed and who were attended in the Orthopaedic Surgery clinical Unit of the Hospital Virgen Macarena (Seville). They met all inclusion criteria and did not present with any of the exclusion criteria (Table 1). They all voluntarily signed their informed consent to participate. The study was conducted from 2016 to 2018.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| 1. Patients >30 years |
| 2. Patients diagnosed with osteoarthritis which despite being in treatment with NSAIDS for 6 months, state there is persistent pain over more than 30 days.3. Patients who voluntarily express their intention to participate with their informed consent. |
| 4. Women of childbearing age must not be pregnant during selection and must agree to use an appropriate contraceptive method (or two contraceptive methods if one is a barrier method) during their participation in the trial. |
| Exclusion criteria |
| 1. Treatment with injections 3 months prior to the study, with previous treatment with NSAIDS 24 hours before extraction. |
| 2. Previous surgical treatment on the affected hip |
| 3. Background of HBV background (except people who tested negative to the surface antigen (AgHBs) with demonstrated immunity, positive markers for HCV, positive markers for HIV, positive markers for HTLV I/II |
| 4. Diabetics, serious heart, kidney or liver disease |
| 5 Allergy to hyaluronic acid or NSAIDS |
| 6. History of crystal arthropathy, inflammatory arthritis or neuropathic arthropathy |
| 7. Serious protrusive osteoarthritis, background of infectious arthritis, excessive deformity (acetabular dysplasia, Perthes disease) |
| 8. Active bacterial infection when included, blood changes or coagulation |
| 9. Autoimmune diseases (platelets at outset < o = 150,000 platelets/ml, Haemoglobin at study outset under or equal to 125 g/L in women and 135 g/L in men) |
With the aim of being able to reject the null hypothesis with a statistical power of at least 80%, a significance level of 5% and a confidence level of (1-α) 95%, it was necessary to include 33 patients in each group. In addition to this, bearing in mind an estimated 10% percent withdrawal rate, it was believed necessary to recruit a total of 37 patients to each group.
Study variablesSociodemographic variables were collected from the patients (age, sex, weight, height and body mass index, cause of osteoarthritis, radiographic evaluation of the osteoarthritis grade), medical history and results from tests (peripheral blood haemogram and PRP haemogram). The following were recorded: Harris Hip Score, VAS score, WOMAC score, responder-non responder (OARSI criteria, Fig. 1),9 analgesic consumption (type, frequency and daily dose defined at each visit). Cultures of the final PRP specimen were made. In its recommendation guidelines for the development of clinical trials in osteoarthritis the OARSI establishes that being a “responder” or “non-responder” to a treatment is an added indicator to functionality, overall evaluation and pain for assessment efficacy.9 Responders are considered to be those patients who present with an improvement ≥ 50% in pain or function or absolute change > 20 points or who meet with 2/3 of the following criteria: pain, function or overall patient assessment ≥ 20% or absolute change of >10 points.
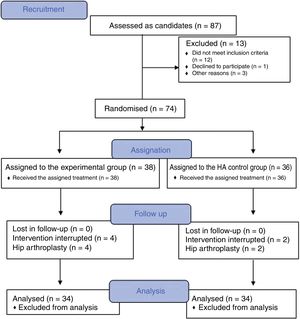
PRP obtainment and preparation protocolThe method of obtaining PRP manually through an open technique was completed complying with the necessary quality requirements in keeping with report V1/23052013 of the Spanish Agency for Medication and Healthcare Products (AEMPS for its initials in Spanish)10 and the ministerial document of Technical Requirements and Minimum Conditions for Haemodonation and for the Transfusion Centres and Services.11
The nursing staff obtained a blood sample (75 ml) from the antecubital fossa. A peripheral blood haemogram was obtained from this sample and to the remaining 70 ml was added 9 ml of sodium citrate. This was aliquoted into 7 sterile test tubes. The PRP preparation protocol was based in a simple centrifugation system, with plasmatic layer pipettes and activation with calcium chloride. One hundred grams was centrifuged for 10 minutes (Allegra X22 Centrifuge®). In a flow hood and complying with all sepsis recommendations, a pipette was used to select the upper plasma-rich fraction (2 ml of the supernatant). From the 14 ml of platelet-rich solution obtained, 5 ml was sent for blood count analysis, 2 ml to a culture and 1 ml was frozen at -80 °c. The remaining 6 ml were injection into the patient.
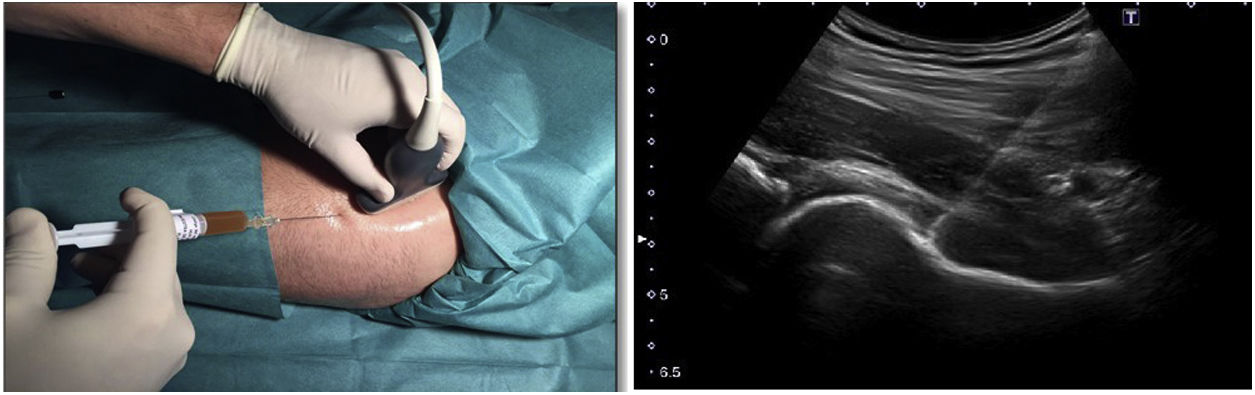
Hip infiltration protocolUltrasound-guided injection of the hip was performed in both groups, using an anterolateral approach (Fig. 2). The control group was injected with 60 mg/6 ml of hyaluronic acid through a prefilled syringe (Hialano G-F, Synvisc-One ®) and the intervention group was injected with 6 ml of plasma rich in intraarticular platelets (PRP group).
Statistical AnalysisStatistical analysis was performed with the SPSS version 15.0 (IBM, SPSS, Chicago, Illinois, United States) software. The categorical variables were shown as frequencies and/or percentages and the quantitative ones as medians/interquartile ranges or mean ± SD. To compare the quantitative variables the Student t-test was used or the Man-Whitney U test depending on its adjustment or non adjustment to normality. In the case of categorical variables the Chi square test or exact Fisher tests was used. A p value of .05 was considered statistically significant for all calculations. An analysis of the qualitative variable of efficacy was performed, considering the variable responder (yes or no), WOMAC, HHS and VAS, among the two treatment groups in each visit. For tendency analysis the Wilks Lambda test was used.
To analyse the correlation between the concentration of platelets and leukocytes with the clinical response of the patient, the bivariate Pearson correlation test or its non parametric alternative (Spaarman Rho test) was used. This evaluation was performed by measuring the WOMAC, HHS, VAS and responder-non responder scales in all visits in the experimental treatment group (PRP).
To assess the clinical factors which impact the clinical response to treatment the effect of the variables was analysed using the logistic regression multivariate study.
Ethical aspectsThis study was developed following approval by the ethics committee, following the recommendations of the Declaration of Helsinki of 1964. Signed informed consent was obtained from all the patients included in the study.
Data protection obtained in the clinical trialThe contents of the data collection notebooks and the confidentiality of data of each patient has been respected at all times. The appropriate procedures were followed to ensure compliance with that stipulated by Organic Law 15/99 of 13th December on Protection of Personal Data.
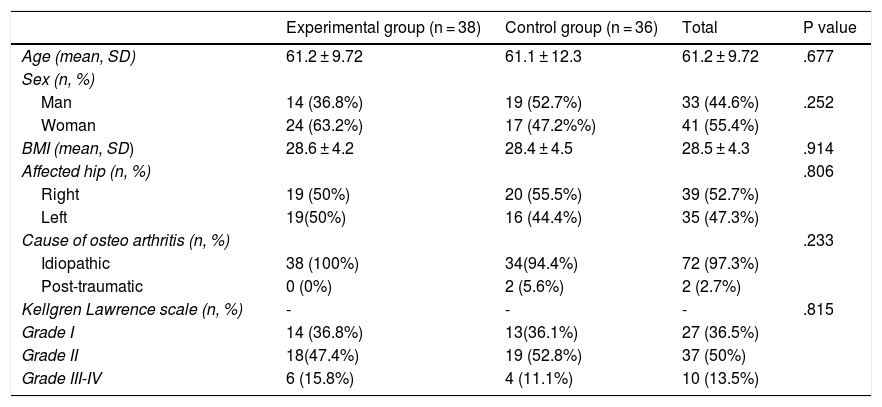
ResultsThe study included a total of 74 patients, divided into two groups: the experimental group (PRP, 38 patients) and the control group (HA, 36 patients). Table 2 contains the baseline characteristics of both groups.
Descriptive analysis of the study population
| Experimental group (n = 38) | Control group (n = 36) | Total | P value | |
|---|---|---|---|---|
| Age (mean, SD) | 61.2 ± 9.72 | 61.1 ± 12.3 | 61.2 ± 9.72 | .677 |
| Sex (n, %) | ||||
| Man | 14 (36.8%) | 19 (52.7%) | 33 (44.6%) | .252 |
| Woman | 24 (63.2%) | 17 (47.2%%) | 41 (55.4%) | |
| BMI (mean, SD) | 28.6 ± 4.2 | 28.4 ± 4.5 | 28.5 ± 4.3 | .914 |
| Affected hip (n, %) | .806 | |||
| Right | 19 (50%) | 20 (55.5%) | 39 (52.7%) | |
| Left | 19(50%) | 16 (44.4%) | 35 (47.3%) | |
| Cause of osteo arthritis (n, %) | .233 | |||
| Idiopathic | 38 (100%) | 34(94.4%) | 72 (97.3%) | |
| Post-traumatic | 0 (0%) | 2 (5.6%) | 2 (2.7%) | |
| Kellgren Lawrence scale (n, %) | - | - | - | .815 |
| Grade I | 14 (36.8%) | 13(36.1%) | 27 (36.5%) | |
| Grade II | 18(47.4%) | 19 (52.8%) | 37 (50%) | |
| Grade III-IV | 6 (15.8%) | 4 (11.1%) | 10 (13.5%) | |
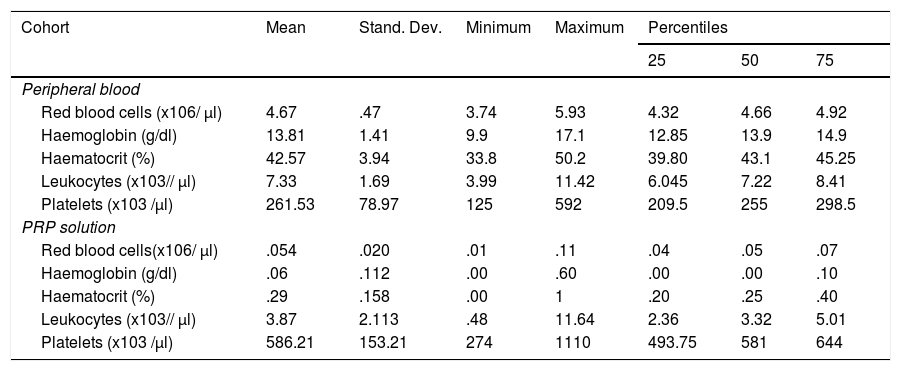
Cellular concentration in peripheral blood and PRP solution in the experimental group
| Cohort | Mean | Stand. Dev. | Minimum | Maximum | Percentiles | ||
|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | |||||
| Peripheral blood | |||||||
| Red blood cells (x106/ μl) | 4.67 | .47 | 3.74 | 5.93 | 4.32 | 4.66 | 4.92 |
| Haemoglobin (g/dl) | 13.81 | 1.41 | 9.9 | 17.1 | 12.85 | 13.9 | 14.9 |
| Haematocrit (%) | 42.57 | 3.94 | 33.8 | 50.2 | 39.80 | 43.1 | 45.25 |
| Leukocytes (x103// μl) | 7.33 | 1.69 | 3.99 | 11.42 | 6.045 | 7.22 | 8.41 |
| Platelets (x103 /μl) | 261.53 | 78.97 | 125 | 592 | 209.5 | 255 | 298.5 |
| PRP solution | |||||||
| Red blood cells(x106/ μl) | .054 | .020 | .01 | .11 | .04 | .05 | .07 |
| Haemoglobin (g/dl) | .06 | .112 | .00 | .60 | .00 | .00 | .10 |
| Haematocrit (%) | .29 | .158 | .00 | 1 | .20 | .25 | .40 |
| Leukocytes (x103// μl) | 3.87 | 2.113 | .48 | 11.64 | 2.36 | 3.32 | 5.01 |
| Platelets (x103 /μl) | 586.21 | 153.21 | 274 | 1110 | 493.75 | 581 | 644 |
Of the patients included, 9 (12.1%) were on the waiting list for surgery, 3 of whom (33.3%) belonged to the experimental group and who finally decided not to be operated on due to significant improvement.
To assess efficacy, internationally validated scales were used for both pain and functionality. Also, instead of controlling analgesic medication it was left open to the patient to assess consumption using the DDD described in the section on methodology. In our study, both treatment groups presented with improvement in scales and in analgesic consumption.
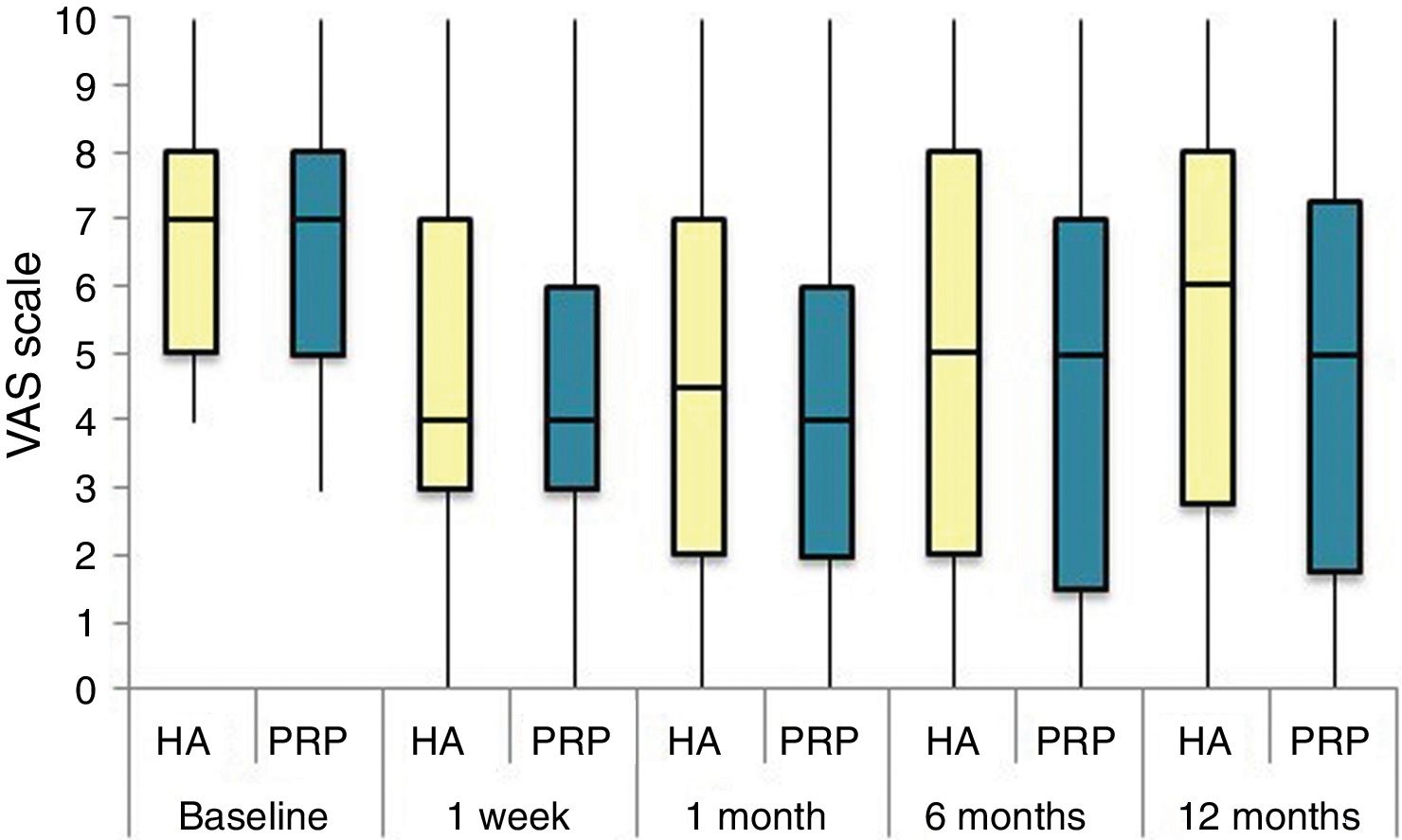
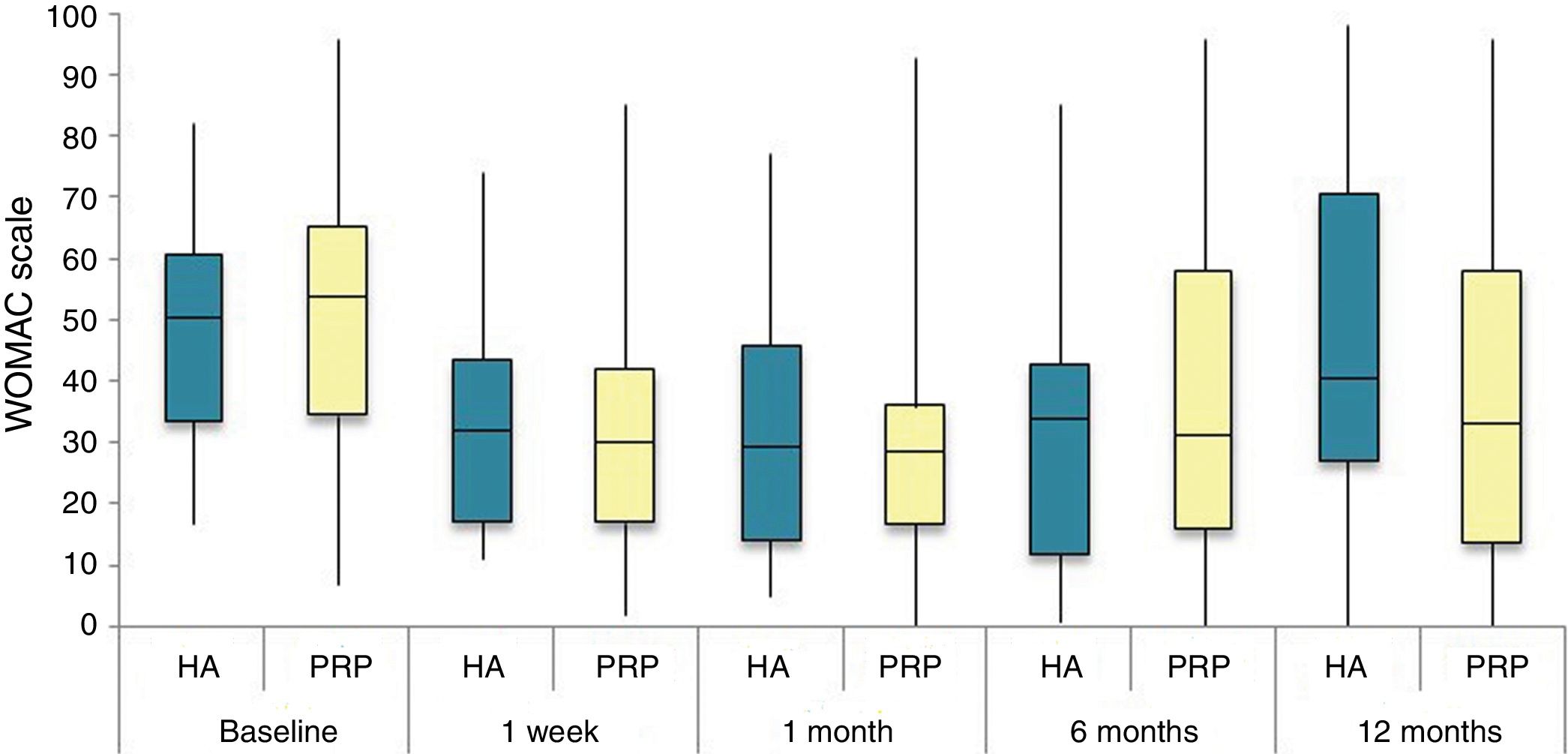
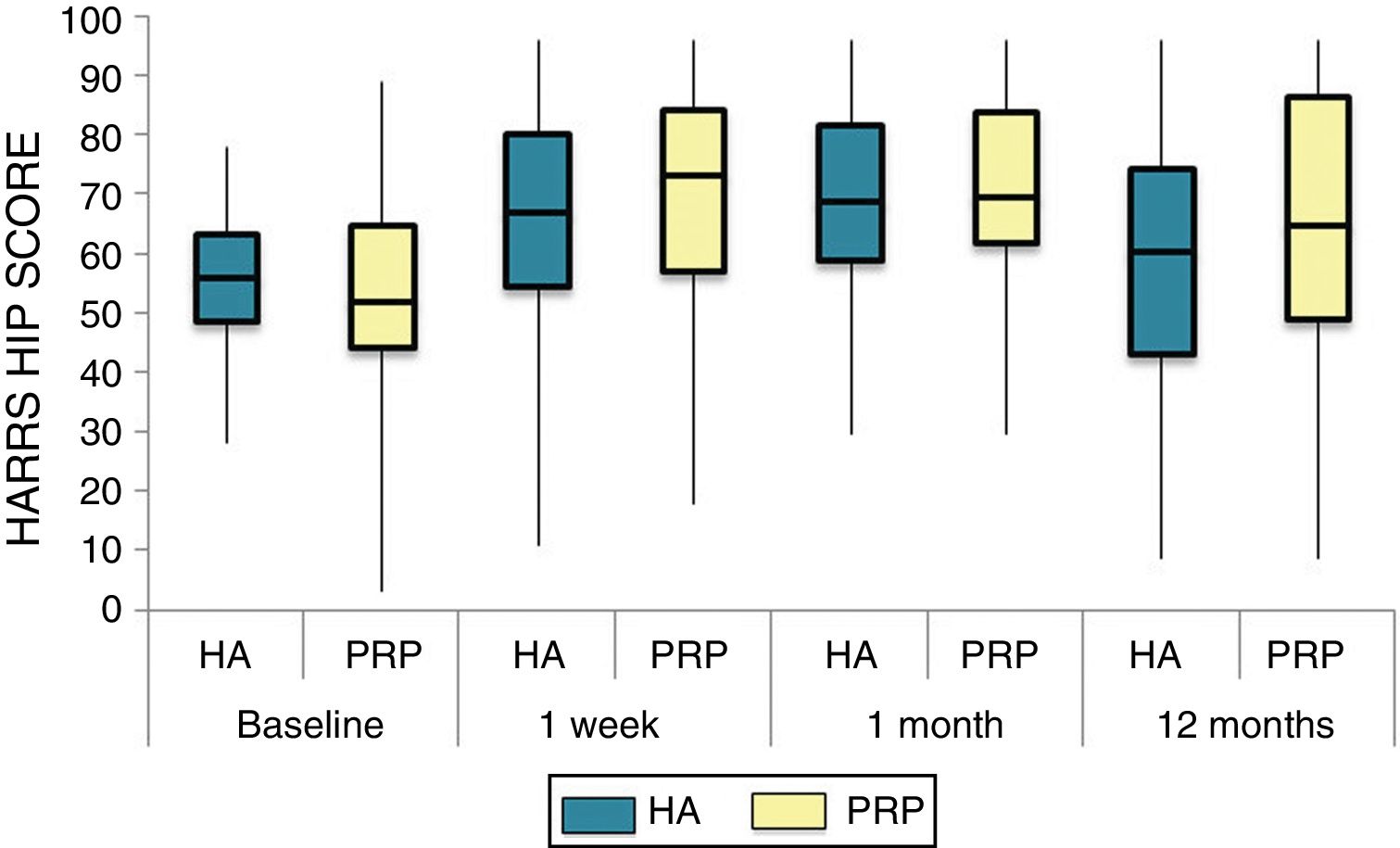
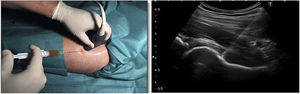
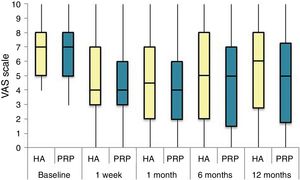
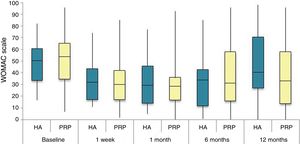
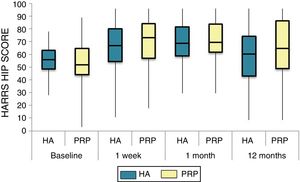
The differences in the scales at the end of the period (12 months after single injection) were significantly better compared with the baseline figures of the two groups: WOMAC, HHS, VAS (Figs. 3–5) (visit 12 months-baseline visit λ: .856 [VAS] p< .01, .426 [HHS] p< .01; .951 [WOMAC] p< .01). Without comparing the experimental and control group only statistically significant differences were found in the HHS scale 12 months after treatment (Table 4, PRP group 70.9 [3,7-58] HA group 60.2[43–74,2] p< .05). No adverse effects were recorded.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC 0-100 points) for each treatment group during 12 month follow-up. Diagram of box-and-whisker plots. The horizontal line represents the median, the limits of the box represent the quartiles and the error bars represent the 95% CI. HA: hyaluronic acid; PRP: platelet-rich plasma.
Harris Hip Score (HHS; 0-100 points) for each treatment group during 12 month follow-up. Diagram of box-and-whisker plots. The horizontal line represents the median, the limits of the box represent the quartiles and the error bars represent the 95% CI. HA: hyaluronic acid; PRP: platelet-rich plasma.
Results of clinical and functional scales
| P50 [P25-P75] | VAS | HHS | WOMAC | WOMAC itemised | ||
|---|---|---|---|---|---|---|
| Pain | Stiffness | Function | ||||
| Baseline | ||||||
| PRP | 7 [5–8] | 51.9 [44.1-64.7] | 53.5 [34.5-65.2] | 10 [6.7-14] | 4.5 [2.75-6] | 36 [24.7-46] |
| HA | 7 [5–8] | 55.8 [48.6-63.5] | 50.5 [33.5-60.7] | 10 [7-13.7] | 4 [2-5.7] | 36 [23-42] |
| p | .97 | .69 | .61 | .87 | .13 | .61 |
| Visit 1 | ||||||
| PRP | 4 [3–6] | 73.3 [57.1-84.2] | 30 [17-42] | 6 [3-9.2] | 3 [1.75-4] | 20 [9.7-27.5] |
| HA | 4 [3–7] | 64.5 [52-72.6] | 32 [17.2-43.5] | 6 [4–10] | 3 [2–4] | 23 [11.2-34] |
| p | .200 | .080 | .360 | .470 | .900 | .610 |
| Visit 2 | ||||||
| PRP | 4 [2–6] | 69.5 [62-84] | 28.5 [16.7-36] | 5 [2-7.2] | 2 [1-3.25] | 21 [16.7-36] |
| HA | 4.5 [2–7] | 64.8 [55-81.13] | 29.5 [14.2-45.8] | 6 [2–10] | 4 [1–4] | 21.5 [14.2-45.8] |
| p | .570 | .140 | .410 | .470 | .540 | .480 |
| Visit 3 | ||||||
| PRP | 5 [1,5–7] | NA | 31 [16-45.5] | 7 [3.5-9.5] | 2 [1–4] | 20 [10.5-30.5] |
| HA | 5 [2–8] | NA | 34 [12-43] | 5 [3–10] | 2 [0-4] | 22 [8-32] |
| p | .470 | NA | .950 | .370 | .880 | .980 |
| Visit 4 | ||||||
| PRP | 5 [1.7-7.3] | 70.9 [57.2-89] | 33 [13.7-58] | 7 [1.75-11] | 3 [1–4] | 23.5 [13.7-58] |
| HA | 6 [2.7-8] | 60.2 [43-74.2] | 40.5 [27.2-70.7] | 9.5 [3.75-15] | 3 [1–6] | 28 [20.2-48.7] |
| p | .150 | .050 | .270 | .190 | .440 | .260 |
Regarding consumption of analgesics, meured as the defined daily dose, there was a drop in the overall analgesia prescription by 73.3% (initial visit compared with final visit, control group of the 62.9% in the experimental group of 84.2%, p >.05) without any findings of significant differences.
Following the recommendations by the OARSI9 we analyzed the patients establishing whether they were “responders” or “non responders” at each visit. In all visits we observed a higher percentage of responder patients in the experimental group finding the maximum level of responders in both groups one month after treatment with no statistically significant differences (PRP vs. HA responders: 1st visit after one week: 76.3% vs. 61.6%; visit after one month: 81.6% vs. 69.4%; visit after 6 months: 73.7% vs. 58.3%; visit after 12 months: 64.7% vs. 44.1%, p >.05).
The cellular composition test results of the PRP solutions are contained in Table 3. According to the PAW classification we obtained a P2XB solution, depending on DEPA; DCA being the mean concentration obtained with our preparation protocol of 586,216 ± 153,208 × 103 platelets/μl.
The platelet concentration index in the PRP treatment group was 2.22 with a performance of 44.57%. Mean concentration of leukocytes in the cohort study was 3.87 ± 2.11 × 103 leukocytes/ μl.
On studying the responder and non-responder patient characteristics we found that the responder patient had higher platelet concentrations with statistically significant differences (one month after treatment; non responders 449[438-578] ×103 platelets/μl, responders 565 [481-666] ×103 platelets/μl, p< .044).
On studying the correlation between analytical values and clinical-functional scales we found there was a negative correlation (r = -.359, p< .029) between the levels of platelets and the VAS in the visit after 6 months, although the correlation was low. However, we did find a high positive correlation between the leukocyte concentrations and the WOMAC stiffness subscale in non responder patients in that same visit (r = .748, p< .013).
In the multivariate analysis using binomial logistic regression we found that the grade I-II Kellgren-Lawrence scale was independently related to the clinical response in the visit after one month (11.51 OR, 95% CI 2.34-50.65, p< .03).
DiscussionThe use of PRP preparations are booming in such heterogeneous disciplines as aesthetic medicine, sports medicine and dentistry. However, despite their extended use evidence on their composition and clinical efficacy is highly limited. In the literature we found a high variability regarding the methodology of preparation, dosing and indication.4,5 Globally, however, a trend in favour of PRP solutions compared with other treatments has been observed.5–7
It seems logical to think that formula conditions (time and speed of centrifugation, use of platelet activators, methods of freezing and unfreezing, open/close techniques) may alter the composition of the final formula and there are indeed many studies which warn of the enormous variability the final medication may provide.7,12–14 This, together with the lack of any standardisation regarding its dosage (number of injections, time between doses, volume to be injected) and usage indication meant that for this study we wanted to assess a new formula and correlate it with clinical results in patients with osteoarthritis. The clinical results described to date vary depending on the study. Thus, Dallari et al.15 report differences on the WOMAC scale at two and six months after injection with PRP and its comparator (hyaluronic acid) whilst Battaglia et al.15 and Doria et al.16 both report an absence of differences. Up until now a single meta-analyses in osteoarthritis has been published by Ye et al.17 in 2018 where a total of 4 trials were included and the author concluded that PRP injections showed superiority on the VAS scale at the first visit after treatment (2 months). In our study we found there were significant differences in the HHS scale one year after treatment and an improvement on the WOMAC and VAS but without any significant differences.
Our study did not aim to evaluate the cost-benefit of the use of PPR compared with hyaluronic acid or other types of injection. However, it is true that after our experience, making PRP in the flow chamber which is available in any hospital represents a currently unused resource and would lower costs and have a considerable financial impact on our public health system.
The index of responders described in the studies on osteoarthritis vary between 40% at 6 months (Sánchez et al.18) and 21% at 12 months (Dallari et al.14). In our study larger variations have been observed at the same assessment times (74% at 6 months and 65% at one year) and particularly higher (82%) one month after treatment. This has all been accompanied by a drop in the global prescription of analgesics in both groups and a reduction in surgical procedures programmed. This is of great relevance to health which has not been assessed in PRP studies to date.
In the multivariate analysis of our study we found that the Kellgren–Lawrence grades I-II were correlated independently with being a responder after month 1 (11.51 OR, 95% CI 2.34-50.65, p< .03). These data are in keeping with those published by Dallari et al.14, which positively correlate with the Kellgren-Lawrence scale and VAS values (r = .392, p< .04). In other indications such as osteoarthritis of the knee these results were corroborated as in the study by Filardo et al.19 where a favourable trend to treatment with PRP was found in those patients with radiological compromise lower or equal to 2 on the Kellgren-Lawrence scale.
The single PRP injection prepared by open technique at 100 g for 10 minutes activated by calcium chloride appears to be a safe and effective practice for patients with osteoarthritis, particularly patients at incipient radiological stages (grade 1-2 KL).
The comparison of different PRP preparation systems has been extensively described in the literature, quantifying cellular performance and in some case the concentration of certain growth factors and cytokines.13,14,19 However, there is no defined correlation between its optimum concentration and clinical efficacy.17–20 Sundman et al.21 state that the effectiveness of PRP is due to attributing the metabolic equilibrium between the anabolic effect of growth factors and the catabolic effect of cytokines added to an appropriate ratio of platelet/leukocyte concentration to obtain tissue regeneration. In our study we observed that the patient responders present a higher platelet concentration to the non responders (non responder group 480.14 ± 97.60 × 103 platelets/μl, responders 585.74 ± 148.62 × 103 platelets/μl p< .04).We also found there was a negative correlation between the values of the VAS scale and platelet concentration (visit 3, r = −.36, p< .03) and a high positive and significant correlation between the results of the stiffness subscale of the WOMAC and the concentration of leukocytes (r = .75, p < .01).
In view of these results, we would defend the concept of personalised medicine as proposed by Doria et al.16 Knowing the composition in key elements such as the ratio of platelets/leukocytes, cytokines and growth factors of the final PRP solution according to patient profile (syndrome and specific tissue)enables us to select more efficiently and target the patient who would benefit significantly from these autologous medicaments.
Further studies like this one are needed to specify the PRP components and the correlation with clinical response to guide the orthopaedic surgeon in the ideal formula for the patient characteristics and those of their pathology. Despite the limitation of the sample size and the need to validate this protocol with a higher number of patients, we found there was a maximum correlation of favourable clinical results on administrating solutions with a minimum concentration of 565 [481-666] ×103 platelets/μl and poor in leukocytes (>4.9 leukocytes ×103/μl) and it could therefore be considered a preliminary protocol for patients in the initial stages of osteoarthritis.
ConclusionPRP solutions are as effective and safe as those of hyaluronic acid for the treatment of hip osteoarthritis in its initial stages. This usage may lead to a reduction in the consumption of analgesia and in surgical treatments. Cellular concentrations present in the PRP solutions correlate with the clinical and functional result. The clinician must be aware of and take into consideration the final product characteristics obtained in order to guarantee the best result.
Level of evidenceLevel of evidence I.
FinancingThis study was financed by the Spanish Orthopaedic Surgery Society (SECOT Foundation Research Grant 2014).
Conflict of interestsThe authors have no conflict of interests to declare.
We wish to thank the Department of Analytical Chemistry, Faculty of Sciences of the Biomedical Research Institute (IBS) of the University of Granada for its collaboration in the analysis of growth factors.
Please cite this article as: Villanova-López MM, Núñez-Núñez M, Fernández-Prieto D, González-López C, García-Donaire J, Pérez-Pérez A, et al. Ensayo clínico fase III para evaluar la eficacia y seguridad del uso de plasma rico en plaquetas frente a ácido hialurónico en coxartrosis Rev Esp Cir Ortop Traumatol. 2020;64:134–142.