Low back pain is a common cause of lost playing time in young athletes, and spondylolysis is its most common identifiable cause. Despite technological advances in radiology, which can lead to an early diagnosis with better prognosis, progression to spondylolisthesis is sometimes asymptomatic and may not be detected until late stages. There are wide variations, suggesting lack of consensus as regards the objective of treatment, which consists of clinical, radiological, biomechanical or functional improvement. There is also a lack of agreement regarding the ideal conservative treatment, surgical indications and need of slip reduction, and most of the established recommendations are not evidence based. We present a review of literature, which summarizes the current knowledge of spondylolysis and spondylolisthesis in children and adolescents.
El dolor lumbar es causa frecuente de cese de actividades deportivas en atletas jóvenes, y la espondilolisis es su causa identificable más común. Aunque los avances en las técnicas radiológicas permiten su diagnóstico en fases precoces, en algunos casos la progresión a espondilolistesis es asintomática y no se detecta hasta fases avanzadas. No hay consenso en el objetivo del tratamiento, que consiste en la resolución clínica, radiológica, biomecánica o funcional, según autores. También hay falta de acuerdo en el tratamiento conservador ideal, en las indicaciones quirúrgicas y en la necesidad de reducción de la espondilolistesis, y muchas recomendaciones establecidas no están avaladas por la evidencia. Presentamos una revisión de la bibliografía que resume el conocimiento actual de la espondilolisis y espondilolistesis en niños y adolescentes.
The term spondylolysis (from the Greek spondylo=vertebra, lisis=separation) refers to a unilateral or bilateral defect of the pars interarticularis, an isthmus of the posterior arch of the vertebral body between the superior and inferior joint facets. Spondylolisthesis (olisthesis=sliding) describes the ventral migration of a vertebral segment over another, usually preceded by spondylolysis, and identified as a clinical entity by obstetrician Herbiniaux in 1782.1 Both have been frequently described as causes of lumbar and radicular pain in all age groups.
EpidemiologyThe incidence in the general population varies depending on the reports and is difficult to determine, as it is normally only investigated in patients who suffer symptoms. In a classic dissection study, Roche and Rowe2 found a 4.2% overall prevalence of spondylolysis. More recently, random groups of computed tomography (CT) scans obtained in adults due to reasons unrelated to lumbar pain (aortic, abdominal and urological pathology) have been studied and a defect of the pars has been observed in 9–11% of cases, nearly doubling the figure detected through simple radiographs and with no significant association to the presence of lumbar pain.3,4 There is considerable ethnic variability, with a distribution of frequency from highest to lowest among Eskimos (around 40%), Caucasians (5–12%) and African–Americans (1–3%).5 In general, a higher incidence has been reported among males, but this statement is controversial. It seems true that the progression to spondylolisthesis, which takes place in 25% of cases,6 is more likely among females.3–5 Both genders require surgical treatment with similar frequencies.7
Lumbar pain is a frequent cause of temporary interruption of sports activities among professional athletes, and spondylolysis is the most common identifiable cause.8 Soler and Calderón9 carried out a study among 3152 professional athletes in Spain and registered a prevalence of spondylolysis of 8.02% (not much higher than among the general population with a similar age), with no significant differences observed between genders but with great variability among disciplines. From higher to lower frequency, it was identified in throwing sports, artistic gymnastics, rowing, weightlifting, combat sports, swimming (breaststroke and butterfly stroke), volleyball, rhythmic gymnastics and synchronized swimming. In athletes with lumbar pain, the prevalence of spondylolisthesis was higher among adolescents (47%) than adults (5%).8–10
There is a strong association between defects of the pars and the presence of spina bifida occulta, which is only found in 5% of the general population but in up to one-third of patients with isthmic spondylolisthesis. It is advisable to investigate the coexistence of spinal dysrhaphism prior to a possible posterior approach, in order to prevent accidentally damaging the elements of the dural sac and to assess if repair of the pars is feasible.11
PathogenesisThe exact etiology of the condition is not known, although it is likely that elongation of the pars has a multifactorial origin due to predisposing factors (hereditary, vertebral dysplasia–spina bifida, elongation of the facets, anomalies of the soft tissues or the physis, and sacropelvic morphology) and environmental factors (straight posture, gait and repeated load of the lumbosacral spine).
Although there is a genetic predisposition towards spondylolysis, it is not a congenital pathology and there have been no cases described among newborns. Neither are there any patients in age groups which have not learned to walk. This indicates that the increase in load generated by bipedal posture plays a significant role in the development of the condition.12 The incidence increases after the start of walking until 18 years, and then remains stable until adulthood. Its development at the end of the period of spinal growth is not frequent.2,6
In children and adolescents, the posterior arch is not completely ossified and the intervertebral disk is very elastic, making the pars more susceptible to failure due to fatigue caused by tension and shearing forces, especially among those who practice sports involving repeated hyperextension of the thorax.
Unlike degenerative spondylolisthesis, which is 5–6 times more frequent in L4–L5, children and adolescents are more likely to suffer in L5–S1 (71–95%), followed by L4–L5 (5–15%), L3–L2 (less than 5%) and L2–L1 (less than 1%).5,7
The prevalence in the lumbosacral joint among young patients is explained because the sacrum is relatively immobile, whereas the lumbar spine is the segment with greatest mobility. The anterior elements (the disk and the vertebral body) resist compression, whilst the posterior elements (bone) represent the support of the shearing forces. Under normal conditions, stability of the joint is achieved through a series of static stabilizers (orientation of the interfacet joint surface of L5–S1, integrity of the discs and the ligamentous complex), and dynamic stabilizers (neuromuscular system). Generally, these elements are able to maintain the alignment of the affected vertebra.
The pars interarticularis of L5, particularly its isthmic and lateral portions, represents the weakest bone link between these elements, and that is where the maximum mechanical stress is found, as it must resist both axial and shearing forces.
Spondylolysis has a biomechanical and anatomical explanation. On the one hand, the load on the posterior bone arch increases considerably from L1 to L5 during extension. These shearing forces are greater in patients with high pelvic incidence and lumbar lordosis (parameters which will be defined in the radiographic analysis of pelvic morphology), which explains why high-grade spondylolisthesis is observed more frequently among these patients.
Another hypothesis postulates that an adequate separation between the adjacent joint facets enables a superposition of the posterior elements during hyperextension. A recent anatomical study demonstrated that individuals who did not have sufficient interfacet distance in a craniocaudal direction were more likely to develop a spondylolytic defect by entrapment of the pars of L5 between the inferior facets of L4 and the superior facets of S1 during repeated lumbar extension.13
Labelle et al.14 related pelvic morphology and posture to the direction of the forces acting on the pars, and established that patients with a high pelvic incidence and sacral inclination suffered a shear phenomenon with an increase of the tension forces acting on the pars, whereas in patients with less pelvic incidence and sacral inclination, the lesion was produced by axial compression and tear of the pars, usually known as “nutcracker phenomenon”.
ClassificationIn 1976, Wiltse et al.15 described 5 types of spondylolisthesis according to their dysplastic, isthmic, degenerative, posttraumatic and pathological etiology. In 1997, Marchetti and Bartolozzi16 developed a new classification which distinguished acquired from developmental spondylolisthesis. The latter groups together both dysplastic and isthmic forms, and is subdivided into high and low grade depending on the presence and severity of a series of dysplastic joint changes (lumbosacral kyphosis, trapezoidal shape of L5, dome-shaped surface of the sacrum, sacral dysplasia and kyphosis, dysplasia of the posterior elements and small transverse apophysis).
These classifications are useful to identify the etiopathology, but not to establish a therapeutic algorithm. In 2008, Mac-Thiong et al.17 proposed a new classification with 6 subtypes, currently accepted by the Spinal Deformity Study Group (SDSG), which excludes the grade of dysplasia and is based on 3 radiographic characteristics: (1) grade of displacement (high or low), (2) pelvic incidence (low, normal or high), and (3) sagittal, regional (spinopelvic) or global imbalance.
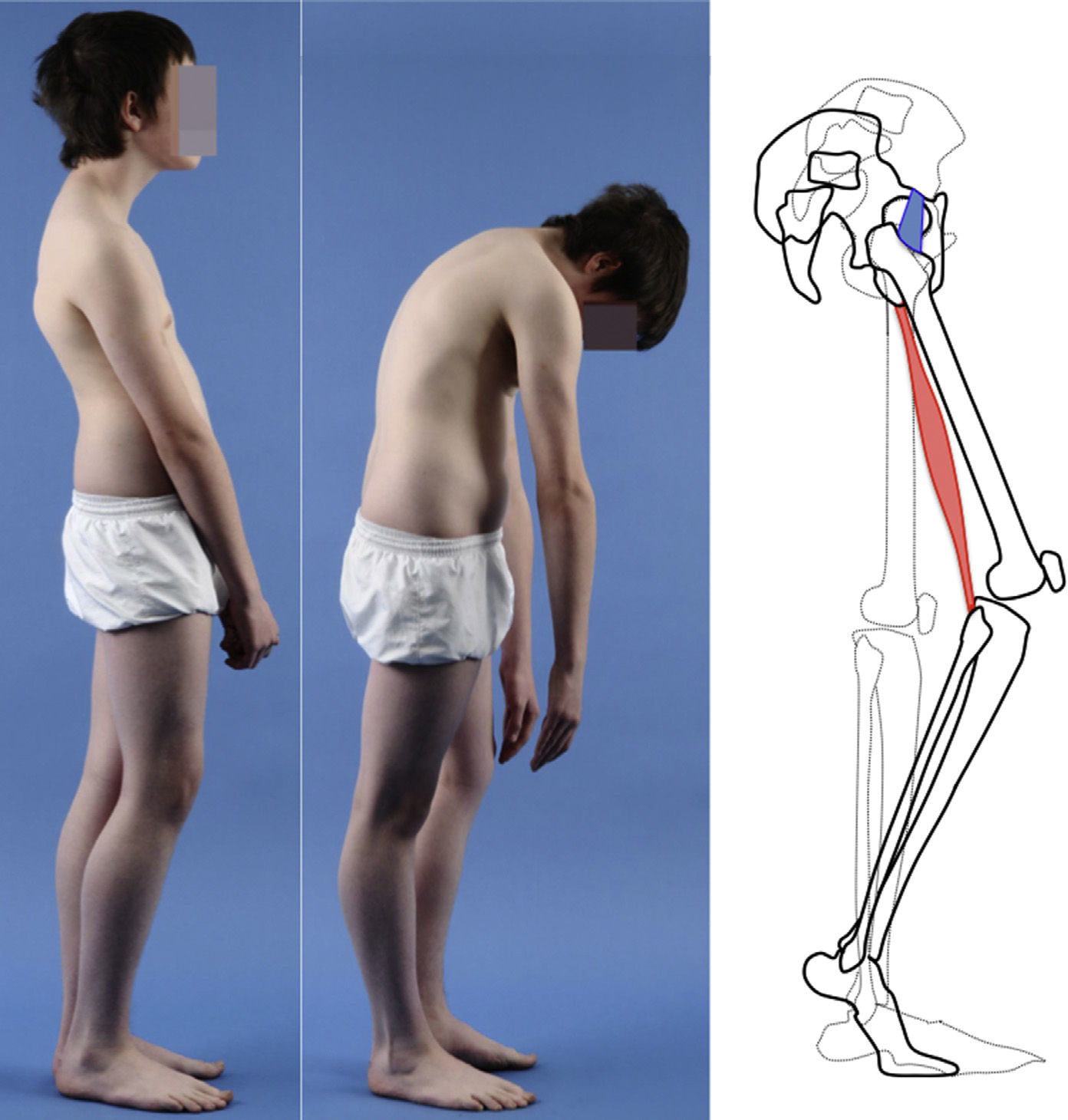
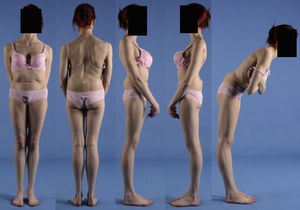
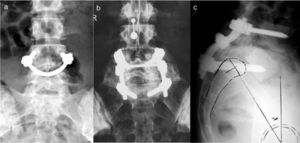
Clinical presentationThe majority of low-grade spondylolysis and spondylolisthesis cases are asymptomatic. Patients who attend consultation often do so in late infancy and early adolescence, when the sliding progresses and the first symptoms appear. Patients present central lumbar pain with mechanical characteristics, worsened by sports activities and prolonged standing, hamstring contracture with limitation of forward bending of the thorax and increase of the popliteal angle, present in 80% of symptomatic patients18 (Figs. 1 and 2).
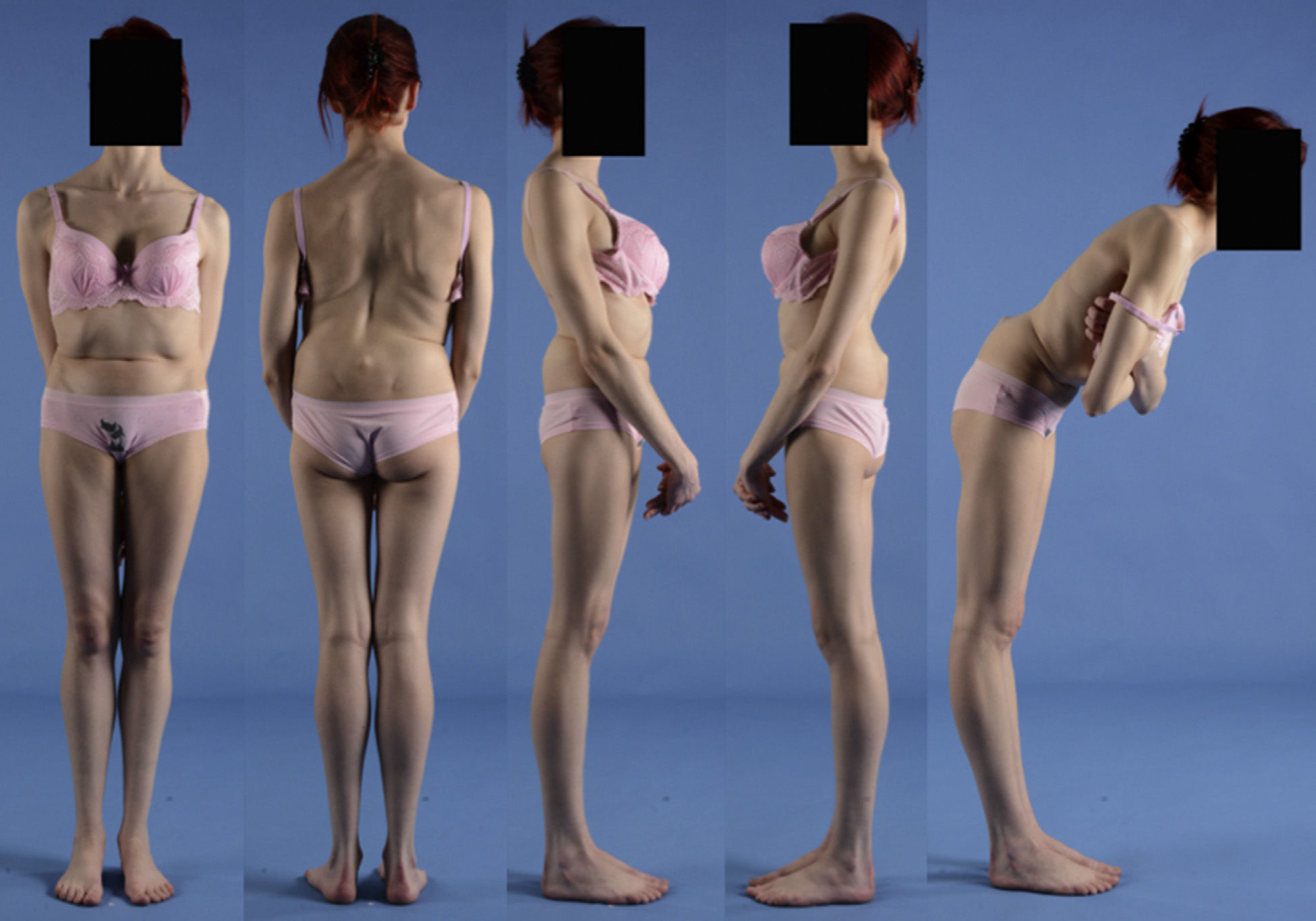
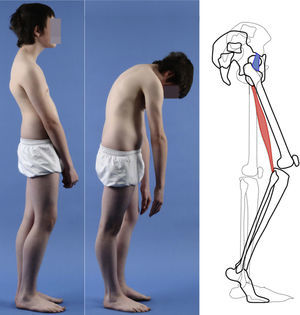
A 17-year-old female with high-grade spondylolisthesis. It is possible to observe the typical abdominal fold, the prominence of posterior elements of the listhetic vertebra and the verticalization of the sacrum. Right thoracic curve with coronal imbalance and limitation of thoracic flexion.
Pain during lumbar hyperextension is a common finding in the clinical exploration of high-grade spondylolisthesis cases. The “stork test” or “flamingo test” (hyperextension whilst standing on one leg) reproduces pain in the affected side. It is also common to detect pain irradiating to the lower limbs, which may follow a radicular path or not.
The Phalen-Dickson sign is present in up to 53% of high-grade spondylolisthesis cases,19 although it can also be observed in any grade of sliding, and describes a posture with an increased support base and crouch gait (see video in additional material [Appendix B Annex 1]). In order to compensate the lumbosacral kyphosis produced by the listhesis (displacing the center of gravity forward), patients retrovert the pelvis verticalizing the sacrum and bend the knees to displace the center of gravity backwards. Pelvic retroversion entails an extension of the hips. Maintaining this abnormal posture causes the hamstring contracture which, in the most severe cases, leads patients to walk on tiptoe (Fig. 1).
Irritation of the L5 root is rare in low-grade isthmic spondylolisthesis, and is produced by entrapment of the root within the fibrous tissue that forms around the pars defect. In high-grade spondylolisthesis, displacement of the L5 vertebra produces a radiographic foraminal stenosis in 57–74% of cases, but this is only symptomatic in 26% of adolescents.19 The severity of symptoms is usually correlated with the grade of vertebral displacement.20,21 Unlike in adults, central canal stenosis is rare in adolescents.
The presence of an abdominal fold (Fig. 2) is common in cases of severe listhesis. Palpation of the spinous processes can reveal an imbalance, with prominence of the process of the vertebra underlying the defect.
Almost half of the patients present lumbar or thoracolumbar scoliosis, possibly antalgic, with an increased incidence among those with a higher grade of dysplasia and displacement (Fig. 2, see video in the additional material [Appendix B Annex 1]). Unless early treatment is applied, the scoliotic curve can become structural.22
Natural historyThe long-term prognosis of low-grade isthmic spondylolisthesis is usually benign, and most patients improve with conservative measures.23 The risk of progression of spondylolisthesis is maximal during the period of rapid growth. The mean progression of listhesis is also greater during adolescence (7%), somewhat less between the second and third decades of life (4%) and minimal in adult age (2%).12 An independent risk factor for lumbar pain is sliding over 25%,24 but there are some asymptomatic cases of high-grade listhesis which could be explained by a spontaneous fusion.25
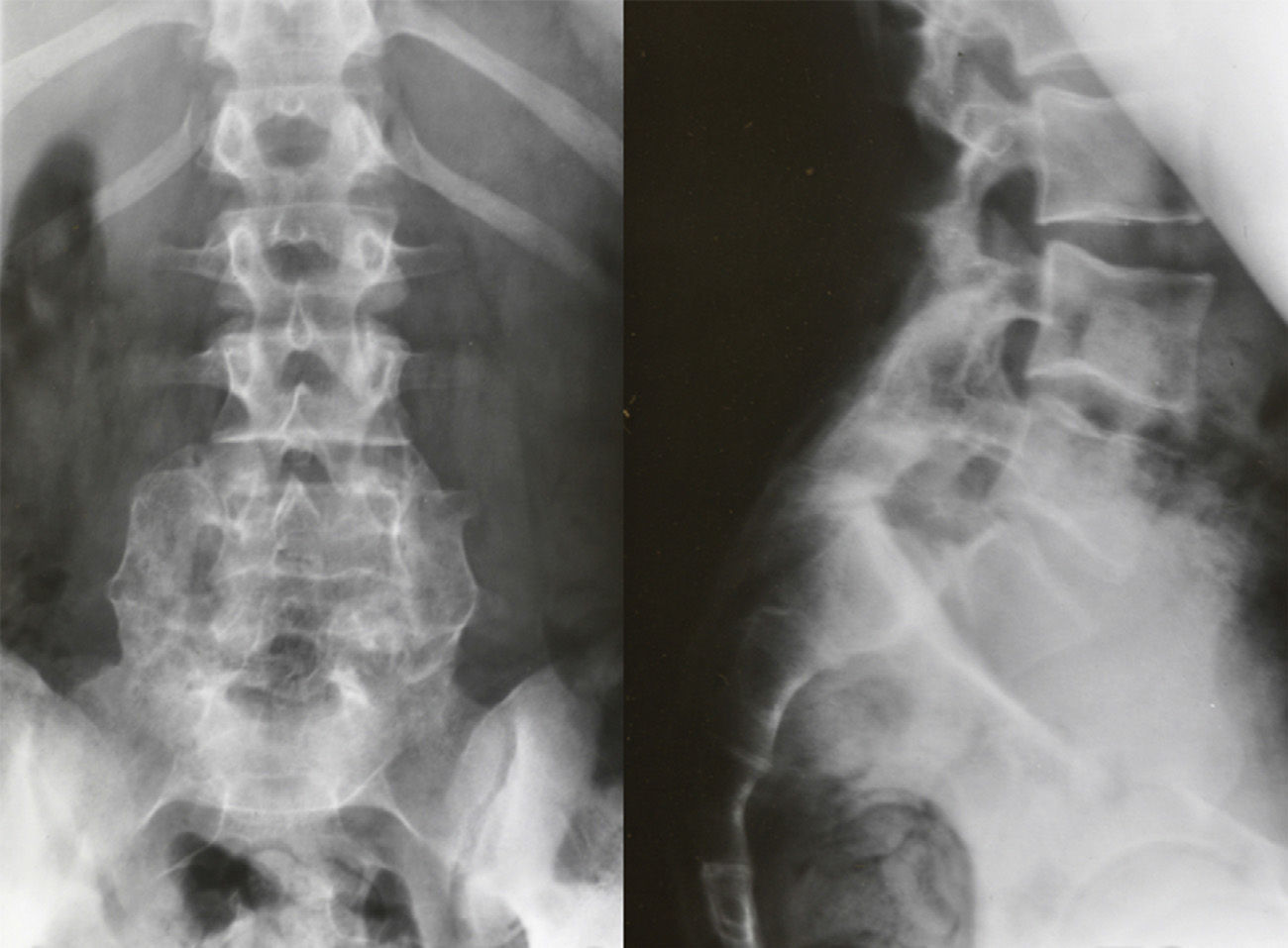
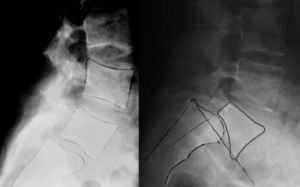
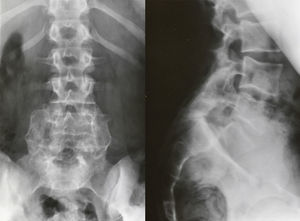
Isthmic spondylolisthesis usually associates a tear of the pars, whereas in the dysplastic type elongation of the pars takes place without bone discontinuity, the upper plate of the sacrum acquires a dome-shaped morphology and the vertebral body of L5 is trapezoidal (Fig. 3). The risk of progression is higher in dysplastic spondylolisthesis.
There is no consensus on which factors favor progression, but Fredrickson et al. monitored a group of patients over 45 years and demonstrated that the percentage of sliding, age of onset, lumbar index and sliding angle at the time of presentation had no predictive value for final sliding.6,12
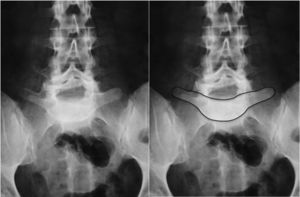
Image-based diagnosisFerguson-type simple anteroposterior radiographic projections (patient in supine position with angulation of the beam 30–35°, parallel to the L5–S1 discal space) of the lumbosacral joint, as well as lateral whilst standing on both legs, are the initial tests of choice. In some cases they can even be diagnostic, although they have a high rate of false negatives, especially in cases of unilateral spondylolysis. Obtaining oblique projections to detect the typical “Scottie dog” image of the collar or neck fracture in unilateral lesions of the pars has not demonstrated an increase in sensitivity and specificity, despite entailing a considerable increase in cost and exposure to radiation.26 In cases of high-grade spondylolisthesis it is possible to observe the “inverted Napoleon hat sign” (Fig. 4) in the anteroposterior projection, formed by the superposition of the dislocated body of L5 and the sacrum.27
A magnetic resonance imaging (MRI) scan is justified to rule out other causes of pain (neoplastic processes, infections, hernias and degenerative discal changes). In addition, it can establish the condition of the intervertebral disk in cases in which pars repair is being considered. The use of 3mm axial sections through the pedicle and the intervertebral space, with parasagittal projections at the level of the pars, can also be useful in the staging of lesions. In acute lesions, an increase in signal intensity in T2-enhanced sequences of the pedicles adjacent to the affected pars indicates edema of the bone marrow, which suggests a potential for consolidation.6 It is also useful to rule out canal and foraminal stenosis.
Computed tomography (CT) offers the best visualization of the bone morphology and allows a differential diagnosis with other lesions. The Tokushima group classified spondylolytic lesions by stages according to CT images into: (1) early, with focal bone absorption or a lineal defect, (2) progressive, with a wide defect and fragmentation, and (3) terminal, in cases with sclerosis, corresponding to pseudoarthrosis. This classification prognosticates the capacity for consolidation according to the evolutionary stage in which the treatment is initiated, although it has not been validated.28,29
Single photon emission tomography (SPECT) has been described as the most sensitive imaging technique. It enables stress reactions and subacute pars lesions to be diagnosed before the fracture becomes visible in simple radiographs, and the image may persist for 1 year.30 However, this is a costly and scarcely accessible technique in our medium, which also has the disadvantage of high exposure to radiation.
Bone scintigraphy (BS) has high sensitivity for spondylolytic lesions, which, according to the level of uptake of the radionuclide, can be distinguished into “hot” (osteoblastic activity, with potential for consolidation) or “cold” (established pseudoarthrosis). Nevertheless, specificity is very low, as other causes of lumbar pain, like osteoid osteoma,30 may also generate “hot” areas.
Lesions are considered acute when there is hyperuptake of radionuclides in BS, hyperintensity of the pedicles in T2-enhanced sequences of the MRI, or lineal defects in the CT (early stage), despite being undetectable in simple radiography and, therefore, having a good potential for consolidation.28–30
Radiographic measurements for the classification of spondylolisthesis are summarized into:
- 1.
Local analysis: lumbosacral.
A series of measurements in lateral simple radiographs, which must be carried out whilst standing on both legs, since some movements are spontaneously reduced in the supine position,27 to measure the severity of displacement and monitor its progression31:
- •
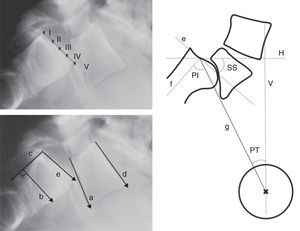
Level of sliding (anterior translation): measured through several methods (Taillard, Boxall, Wright and Bell, Meyerding). The most commonly used is the Meyerding classification, according to which the absence of displacement is classified as grade 0, a displacement of 0–25% as grade I, 25–50% as grade II, 50–75% as grade III, 75–100% as grade IV and over 100% as grade V or spondyloptosis. Low-grade spondylolisthesis is that of grade II or less, whereas high-grade refers to cases of grade III or above (Fig. 5).
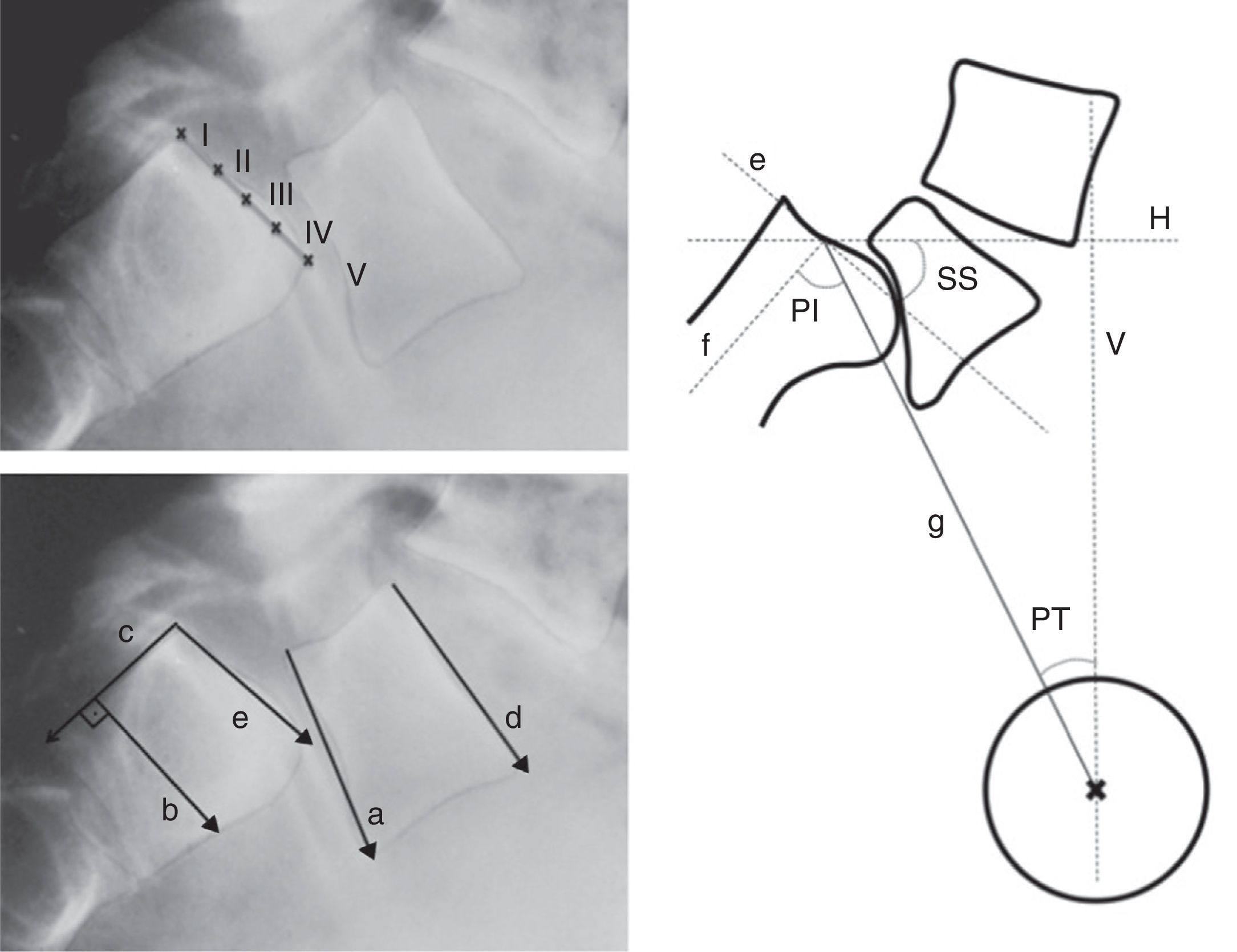
Figure 5.Radiographic measurements. Above, level of Meyerding sliding. Below, reference lines to measure the lumbosacral angle according to Boxall (a, b), Dubousset (c, d) and Wiltse (a, e). On the right, pelvic incidence (PI), pelvic rotation or tilt (PT) and sacral slope (SS). (a) Inferior plate of L5; (b) perpendicular line to c; (c) posterior edge of the sacrum; (d) superior plate of L5; (e) superior plate of the sacrum; (f) perpendicular to e; (g) line joining the center of e with the center of the femoral head (or half the distance between the center of both femoral heads); H: horizontal; V: vertical.
(0.14MB). - •
Lumbosacral angle or slip angle (LSA): describes the sagittal angulation between L5 and S1, or grade of lumbosacral kyphosis. According to the definitions of different authors, it is formed by27:
- 1.
A line running parallel to the inferior plate of L5 and another running perpendicular to the posterior edge of the sacrum (Boxall).
- 2.
Lines running parallel to the superior plate of L5 and the posterior edge of the sacrum (Dubousset).
- 3.
Lines running parallel to the inferior plate of L5 and the superior of the sacrum (Wiltse).
- 1.
The Dubousset lumbosacral angle is the most easily reproducible. It enables pre- and postoperative assessment as it does not require reference points in the area of arthrodesis (Fig. 5).
- •
Lumbar lordosis (LL): this should be measured from the superior plate of L5 to the superior plate of the limit vertebra of the lordotic curve, which is not necessarily L1, as lordosis is usually more extensive in high grades of listhesis.
- •
- 2.
Regional analysis: sacropelvic.
The radiographic parameters describing the morphology and spatial orientation of the pelvis in the sagittal plane are:
- •
Pelvic incidence (PI): angle between the perpendicular to the midpoint of the superior plate of the sacrum and the line running from this point to the center of the femoral head.
- •
Pelvic rotation or tilt (PT): angle formed by the intersection between the vertical and the line connecting the midpoint of the superior plate of the sacrum with the axis of both femoral heads. This is the best indicator of pelvic anteversion or retroversion.
- •
Sacral inclination or slope (SS): angle formed by the surface of the superior plate of S1 and the horizontal.
SS and PT are postural parameters (dynamic), and vary according to the version of the pelvis on the axis of the hips, whereas PI is an anatomical value (static), which is constant for each individual. These 3 measurements are related to each other through the formula PI=SS+PT (Fig. 5).
Anterior rotation, anteversion, flexion and anterior inclination of the pelvis increase SS and reduce PT. Posterior rotation, retroversion, extension and posterior inclination of the pelvis entail a reduction of SS and an increase of PT.
PI correlates to lumbar lordosis and is higher than average among the majority of patients with high-grade spondylolisthesis (69–70°), although a clear cause/effect relationship has not been demonstrated, since not all patients with spondylolisthesis present an elevated PI nor do all patients with elevated PI develop spondylolisthesis.14,32,33 Hresko et al.34 retrospectively compared the measurements obtained among a series of patients with high-grade spondylolisthesis against a group of healthy patients, all of them with a PI >70°. Patients with abnormally elevated retroversion were considered imbalanced (elevated PT and reduced SS), and surgical reduction was recommended in these cases.
- •
- 3.
Analysis of overall balance.
- •
Sagittal C7 plumb line: vertical line passing through the center of the C7 body. There is an overall balance when this passes through or behind the femoral heads.14
- •
Sagittal vertical axis: the horizontal distance between the sagittal C7 plumb line and the posterosuperior edge of the S1 body. In healthy individuals, this should be ±2.5cm.
- •
Anterior L5 slip and lumbosacral kyphosis tend to displace the center of gravity forwards, but even in high-grade spondylolisthesis it is rare for an overall sagittal imbalance to take place, which is preserved through a series of compensation mechanisms: (1) lumbar hyperlordosis, (2) pelvic retroversion with extension of the hips and (3) knee flexion.
TreatmentAlthough the majority of spondylolisthesis cases in children and adolescents are low-grade cases with scarce symptoms and low risk of progression they must be diagnosed and receive early treatment, if necessary. The treatment depends on the age and potential growth of each patient, the presence and severity of symptoms, the evolutionary phase and the grade of displacement.
The management of this pathology and assessment of its results have been the object of considerable controversy in the multiple articles published since its recognition as an entity until the present day. The assessment of the effectiveness of the treatment has been based on the resolution of the symptoms, the recovery of overall sagittal balance, radiographic consolidation of the defect and/or return to normal activity, according to each author.
Conservative treatmentThe initial management of symptomatic spondylolysis and spondylolisthesis in children and adolescents must be conservative.
There is no consensus on the best conservative treatment, but most authors agree on the following recommendations:
- •
Restriction of activities involving the transmission of extension and torsion forces through the pars.
- •
Antilordotic lumbosacral orthesis to unload the posterior vertebral elements and so reduce the forces crossing the pars. This has been reported to reduce lumbar pain in up to 80% of cases,29 but no orthesis has been demonstrated to effectively reduce distal movement to L4–L5 and, to date, no statistically significant differences have been found in the clinical and radiographic results between groups treated with and without orthesis. Therefore, the improvement could be due to a restriction of activity and a benign natural course of the pathology.35
- •
Thorax and pelvis stabilization exercise regime after the orthesis with the objective of reducing extension forces in the lumbar spine and reducing spasm, improving flexibility of the extensor musculature of the hips, hamstrings, lumbar and abdominal regions. As in the previous case, it has not been demonstrated that the improvement is attributable to the benefits of rehabilitation therapy rather than the restriction of other types of sports activities.35
- •
Follow-up every 6–12 months until skeletal maturity (Risser IV–V), which in girls takes place approximately 2 years after menarche, to detect a possible progression of sliding.23
A recent metaanalysis involving observational studies suggested that the conservative treatment of low-grade spondylolisthesis and spondylolysis achieved excellent or good functional results in 84% of patients, according to the criteria of Steiner and Micheli, although fusion of the pars was only achieved in 28% of cases.35 This indicates a lack of clinical-radiographic correlation, although there could be a publication bias, since a greater tendency towards publishing positive clinical results and negative radiographic results of conservative treatment has been observed.
Unilateral lesions have shown significantly higher consolidation rates than bilateral cases (71% versus 18%).
The success of the consolidation depends on the level of displacement (significantly worse when it exceeds 5%)36 and the evolutionary phase of the defect at the time of starting the treatment (fusion rates of 68%, 28% and 0% in the early, progressive and terminal phases of Tokushima, respectively).35
Surgical treatmentIndication of surgery follows persistence of symptoms despite conservative treatment, which vary according to the age of patients. Low lumbar pain is the main symptom, and usually develops during the peak of rapid growth.30,37 It is possible that skeletal growth or the changes in activity during puberty contribute to the development of the symptoms, since the incidence of lumbar pain also increases during adolescence in the general population.37
Surgical treatment is rarely necessary in preadolescent children, as they do not normally suffer pain. In these patients, surgery is considered in case of hamstring contracture or postural scoliosis with a long evolution, potentially structural in the long term.22
Low-grade spondylolisthesisA superiority of pars repair and non-instrumented fusion of a single level when the intervertebral disk is healthy has not been demonstrated in low-grade spondylolisthesis and spondylolysis.
Pars repairThe objective of direct repair with reconstruction and graft of the pars defect is to reestablish the anatomy and stability of the segment, maintaining its mobility, and to prevent a subsequent slip. Some authors8,38 consider the improvement of symptoms following diagnostic infiltration of local anesthetic in the pars defect as a predictive factor of good results of the surgical repair, but this is not very useful in children.
The ideal conditions to carry out a repair are38:
- •
Age under 20 years.
- •
Separation under 2mm.
- •
Preferably L4.
- •
Absence of dysplasia of the posterior elements.
- •
Absence of discopathy.
- •
Minimal listhesis.
Any technique employed will require the debriding of the pseudoarthrosis and graft with autogenous bone from the iliac crest, followed by stabilization of the floating distal fragment. Occasionally, it may be necessary to remove the instrumentation once fusion has been achieved because of the irritation caused by the implant, especially in athletes. The results are good or excellent in 70–100% of cases.
Since the first surgical repair technique with instrumentation, described by Buck in 1970, various fixation methods have been developed, with good results regarding consolidation rates and quality of life. The most commonly used techniques are:
- 1.
Morscher technique, with a special hook which is easy to place but has pseudoarthrosis rates of up to 35%.39
- 2.
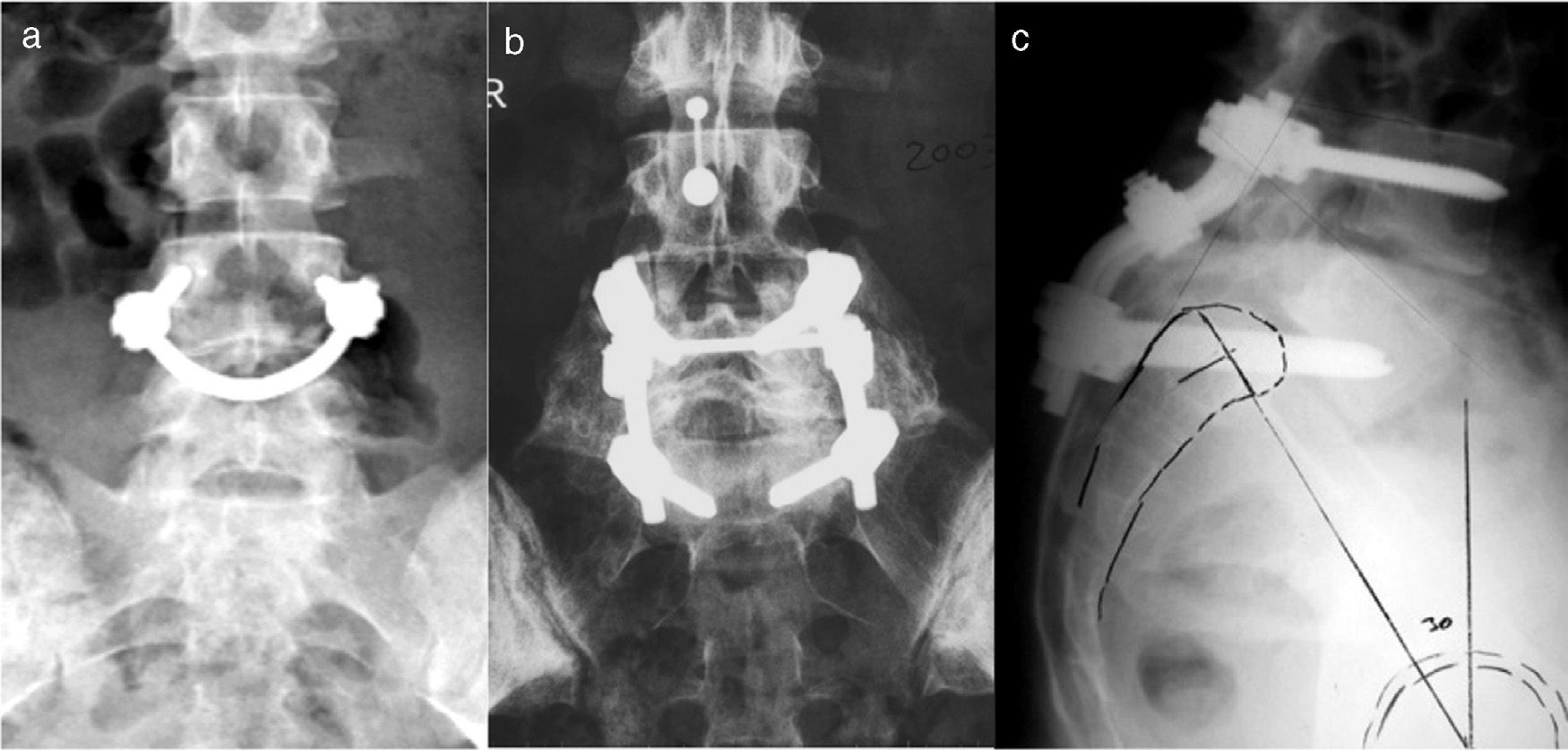
“Smiling face”, pedicular screws connected by a bar which grips the spinous process. Increasing the number of fixation points increases the strength of the system and achieves high rates of consolidation40 (Fig. 6a).
- 3.
Direct fixation of the pars with a screw or DPSF, which achieves the highest rates of fusion, However, this is a very technically demanding procedure.41
This is still the basis of surgical treatment in children and adolescents with L5 spondylolysis or low-grade lumbosacral spondylolisthesis in the presence of symptoms, and achieves long-lasting and satisfactory results. Although the rate of pseudoarthrosis is high (nearly one third of patients), in the long term there have been reports of clinical improvement in over 80% of cases after the intervention.37,42 This clinical and radiographic discrepancy has several possible explanations: (1) the pseudoarthrosis causes a relative stability of the segment, (2) denervation of the isthmus, which is the source of pain, takes place during the intervention, and (3) the natural history of the pathology tends towards a disappearance of the symptoms.37
The technique most favored by the authors is the paramedian bilateral approach of Wiltse through a cutaneous incision by the midline, and achieving fusion of both transverse apophyses of L5 and the sacral ala, providing an autologous iliac crest graft (Fig. 7). Postoperative support with a brace for 3 months has been recommended, but the need for this measure is controversial.43 Hamstring contracture improves within 12–18 months after the intervention in the majority of patients.
High-grade spondylolisthesisThe objective of surgical treatment of high-grade spondylolisthesis is to alleviate pain, resolve the neurological dysfunction and achieve a solid arthrodesis, minimizing the number of fused segments. The pain and functional limitation have been attributed to sagittal imbalance,14 but this is controversial.
Several surgical techniques have been described for the treatment of high-grade spondylolisthesis, but there are still discrepancies between in situ fusion and reduction, because there are very few evidence-based studies comparing both treatments and it has not been demonstrated that reduction provides better clinical results.44–47
In situ posterolateral fusionThis is still the most commonly accepted technique for the treatment of high-grade spondylolisthesis, and it has reported an improvement of symptoms in the majority of patients.48 It has not been demonstrated that a level of sliding over 50% entails a poor postoperative result,43 but it has been associated to persistence of lumbosacral kyphosis and a lack of evident fusion in simple radiographs.43,49,50
At present, in situ fusion is usually indicated in skeletally immature patients with an adequate sagittal balance. Critics of this technique usually refer to the high rate of pseudoarthrosis, progression of sliding due to remodeling in flexion of the fusion mass and late neurological involvement.19 In order to minimize these problems, instrumented and non-instrumented circumferential fusion techniques have been proposed (Fig. 6b). Support techniques of the anterior spine entail a higher risk of lesion of the large vessels and the lumbar sympathetic chain, a cause of retrograde ejaculation among males.
Initial decompression is not recommended among patients without evident radiculopathy.43
ReductionAt present, there are no comparative studies with a level of evidence 1 or 2 which prove superior clinical results of reduction versus in situ fusion in high-grade spondylolisthesis. Among the authors who advocate reduction, another point of controversy is whether the potential benefit is due to the translational (level of siding) or angular (lumbosacral kyphosis) correction.
The theoretical advantage of reduction is that it achieves an alignment of the fusion mass which increases compression forces, decreases the risk of progression, recovers sagittal balance, corrects the posture and improves self-perception.43,51,52
The arguments against the technique are a higher neurological risk and morbidity associated with a more complex surgical procedure. The most common lesion is L5 radiculopathy. Different mechanisms for the lesion have been proposed: direct trauma during debridement, radicular impingement on the iliolumbar ligaments, increase of the tension or posterior displacement by dragging of the disk during reduction, which narrows the foramen.53
Petraco et al.54 proved that stretching of the root during the process of translational reduction is not lineal; instead, 71% of the elongation takes place when the last 50% of the deformity is reduced. They also proved that the recovery of lordosis relaxed the root. For this reason, many surgeons advocate a partial reduction. Avoiding complete extension of the knees in the immediate postoperative period is recommended in order to avoid late neuroapraxias.53 Other risks which have been described are kyphotic imbalance above the fused level in anatomical monosegmental reductions,55 higher surgical morbidity due to the increase in surgical time, greater transfusion requirements caused by the increase in blood loss, and fractures caused by sacral insufficiency and angulation of the sacrum.34,54,56
In recent decades, numerous surgical options have been proposed for the reduction and fixation of high-grade spondylolisthesis, which vary in terms of approach route, type of reduction (translational or angular), grade of correction (partial or total), decompression, use of instrumentation and arthrodesis (anterior, circumferential or posterolateral). The 2 most commonly accepted techniques are currently:
- a.
Translational reduction with instrumented circumferential arthrodesis. Intersomatic fusion is carried out with fusion cages, if the space allows it, or with iliac crest autologous graft. As an alternative to reduce the risk of lesion by traction of the root of L5 during the reduction of the L5 vertebral body on S1, some authors have proposed shortening the segment through an osteotomy of the sacral dome.46
- b.
Angular reduction of the lumbosacral kyphosis through lumbosacral transfixation with screws, with excellent rates of union (Fig. 6c).
Transfixation with angular correction requires less dural manipulation, does not produce traction on the roots and has demonstrated a similar biomechanical stability of the 3 spinal segments to that achieved by translational reduction. A study comparing both techniques could not find any differences in the level of correction of lumbosacral kyphosis.51,57
SpondyloptosisDislocation of the L5 body in front of S1. In 50% of spondyloptosis cases from L5 to S1 there is a spontaneous fusion.58
Surgical indication is established in cases with severe lumbar pain and neurological symptoms (L5–S1 radiculopathy or neurogenic claudication).58
Surgical options are isolated decompression in symptomatic patients in which there is a certainty of spontaneous fusion, lumbosacral transfixation and translational reduction techniques.52
In order to avoid neurological lesion, surgical techniques have been developed involving slow reduction with an external fixator59 or segment shortening techniques. Gaines et al. described vertebrectomy of L5 with good cosmetic results but with a 77% rate of neurological lesion.60 Shortening through resection of the sacral dome has a possibly lower incidence of neurological lesion.46
- •
Spondylolisthesis in children and adolescents is a pathology with a benign curse, and only a small percentage of patients develop a symptomatic progression.
- •
Limiting the participation of children and adolescents with low-grade spondylolisthesis and spondylolysis in sports activities is not justified.
- •
There is no consensus on which are the predictive factors of progression of sliding at the time of clinical presentation.
- •
Although treatment is rarely required, it is important to take it into account in the differential diagnosis of lumbar pain in children and adolescents.
- •
The recent classification by Mac-Thiong et al. proposes a therapeutic algorithm.
- •
There is no evidence of superiority of reduction or in situ fusion in the surgical treatment of high-grade spondylolisthesis.
Level of evidence V.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsThe authors have no financial or personal relationship with any person or institution which could lead to a conflict of interests in relation to this article.
Please cite this article as: Mora-de Sambricio A, Garrido-Stratenwerth E. Espondilolisis y espondilolistesis en niños y adolescentes. Rev Esp Cir Ortop Traumatol. 2014;58:395–406.