Intravenous tranexamic acid has been shown to reduce bleeding and the need for transfusions in total hip arthroplasty, although it has a theoretical risk of producing thromboembolic phenomena. Recently some papers have been published using the topical application of tranexamic acid, but the ideal administration protocol has not yet been clearly defined. The aim of this paper was to demonstrate that our protocol of topical tranexamic acid is effective and safe.
MethodsProspective data collection from a case series of 80 primary hip arthroplasties, in which the following topical tranexamic acid protocol is used: 1.5 g diluted to a total volume of 60 ml were administered, applying 20 ml in the acetabular bed, 20 ml in the femoral canal and 20 ml through the Redon drain, keeping it closed for 20 min.
ResultsEighty patients were operated. Preoperative haemoglobin 14.26 g/dL; preoperative haematocrit 42.39%. An average loss of 2.74 g/dL of haemoglobin and 8% of haematocrit was obtained. Eleven percent of the patients required transfusion, of whom 67% had known previous anaemia; only 3 patients without prior anaemia required transfusion (4%). There were no thromboembolic complications in our series.
ConclusionThe use of topical tranexamic acid was safe and effective in primary total hip arthroplasty, reducing the need for blood transfusion compared to that described in the literature in untreated patients.
El ácido tranexámico intravenoso ha demostrado que disminuye el sangrado y la necesidad de transfusiones en la artroplastia total de cadera, aunque tiene un riesgo teórico de producir fenómenos tromboembólicos. Recientemente se han publicado algunos trabajos con la aplicación del ácido tranexámico tópico, sin haberse definido todavía cuál es el protocolo de administración ideal. El objetivo de este trabajo fue demostrar que nuestro protocolo de administración tópica de ácido tranexámico es eficaz y seguro.
Materiales y métodosRecogida de datos de una serie prospectiva de 80 artroplastias primarias de cadera, en las que se utilizó un protocolo de ácido tranexámico tópico: se prepararon 1,5 gramos diluidos en un volumen total de 60 ml, administrando 20 ml tras fresar el cotilo, 20 ml en el canal femoral y 20 ml a través del redón, manteniéndolo cerrado durante 20 minutos.
ResultadosSe intervinieron 80 pacientes. Hemoglobina preoperatoria 14,26 g/dL; hematocrito preoperatorio 42,39%. Se observó una pérdida media de 2,74 g/dL de hemoglobina y del 8% de hematocrito. Precisaron transfusión el 11% de los pacientes, de los cuales el 67% tenían una anemia previa conocida; solo 3 pacientes sin anemia previa precisaron transfusión (4%). No hubo ninguna complicación tromboembólica en nuestra serie.
ConclusionesLa utilización de ácido tranexámico tópico fue eficaz y segura en la artroplastia total primaria de cadera, reduciendo la necesidad de transfusión sanguínea respecto a lo descrito en la literatura en pacientes no tratados.
Osteoarthritis of the hip is one of the most common diseases in orthopaedic surgery and total hip arthroplasty (THA) is one of the most frequent procedures performed in orthopaedic surgery and has also proved to be effective, because it considerably improves the patients’ quality of life.1 One of the most common problems of THA is bleeding, which leads to up to 22%–35% of transfusions, depending on the series.2–4 The intravenous use of tranexamic acid (TXA) has been shown to reduce bleeding and the need for transfusions, although it has a theoretical risk of producing thromboembolic phenomena. Many studies suggest that these phenomena do not significantly increase with the administration of TXA in major orthopaedic surgery.5,6 TXA has proven to be cost-effective.7 Numerous TXA oral, intravenous or combined protocols have been described. Its difficulty lies in administration, since it must be administered before or after intervention at specific times according to the protocol5,8–10; this impedes correct application of it in all the centres and the reduction of its reproducibility of results. Recently, several papers have been published using the topical TXA application, without having yet defined what the ideal administration protocol would be,2,9,11–14 despite several protocols existing. Since it would only be administered intraoperatively by the surgical team, topical protocols should be more easily reproducible.
In 2017 in our department we proposed the possibility of systematically using TXA in THA. There are many administration protocols for TXA in THA, the majority with good results,2,11 and after analysing the references we chose to use the discreetly modified topical TXA protocol described by Tavares Sánchez Monge et al.2
The aim of this study was to demonstrate that our topical administration protocol of TXA in primary THA was effective and safe.
Material and methodsData collection was from a prospective series of 80 primary cementless THA by anterolateral approach performed between December 2017 and January 2019. In all interventions, a topical TXA protocol similar to that described by Tavares Sánchez Monge et al.2: was used where 5 g of TXA was prepared, diluted up to a total volume of 60 ml (3 capsules of Amchafibrin®, each of them with 500 mg of TXA and with a volume of 5 ml diluted in 45 ml of physiological saline solution). The fluid was administered at 3 different times: 20 ml after milling the cup and before implanting the component, 20 ml in the femoral canal after milling and before implanting the final stem; in both cases we waited for approximately 3 min and then the fluid was aspired and the component implanted. The final 20 ml were administered through the Redon drain after suture, keeping it closed for 20 min. The drain was removed 20−24 h after surgery. With regards to the protocol described by Tavares Sánchez Monge et al.,2 only 2 differences were introduced: the Redon drain was closed for 20 min instead of 60 and it was removed after 24 h instead of after 48 h.
Demographic data were collected as was the ASA classification of preoperative anaesthetic risk; the implanted prosthesis and the material of its components; duration of surgery (time from skin incision up to the administration of TXA through the Redon drain); preoperative haemoglobin and haematocrit levels and postoperative control levels (performed 20−24 h after surgery); the need for transfusion and the length of hospital stay.
All hip arthroplasties were performed by the same surgical team (3 surgeons who alternated in their functions as principal surgeon and assistant surgeon). They used 2 types of prostheses: an innovative system for hip arthroplasty (Exactech®, Gainesville, Florida, U.S.A.) and Equateur® cup with Integrale® (Amplitude®, Valence, France) stem. Informed consent was obtained from each patient.
Statistical analysisData were included in a data base created by the Excel 2013 programme by Microsoft® (Microsoft Corporation, Redmond, Washington, U.S.A.). Statistical analysis was performed with the help of the IBM SPSS programme version 24 (IBM Corporation, New York, U.S.A.). This consisted of a descriptive analysis of variables, calculating the distribution of frequencies for qualitative variables and arithmetic mean and standard error of mean for the quantitative variables. To determine the differences between the values of each variable in each group, an analysis of the variances (ANOVA) was made for one route with a post hoc test of multiple Bonferroni comparisons, in the quantitative variables, and using the Chi square test for qualitative variables. Differences were considered to be statistically significant for p values of <.05.
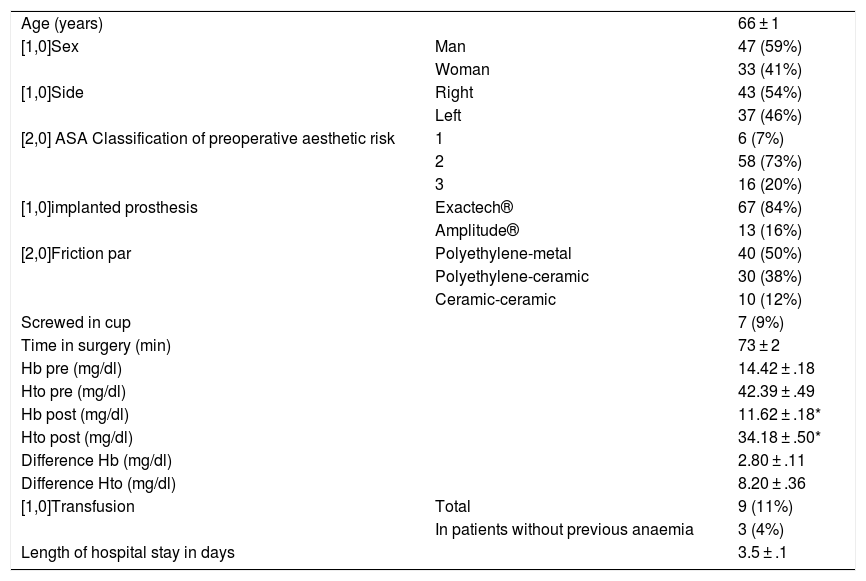
ResultsEighty patients were operated on, 47 men and 33 women (Table 1). Cementless THA was performed using a modified anterolateral approach with the patient in lateral decubitus position and the described topical TXA protocol was applied. No statistically significant differences were observed for any of the parameters studied between the 3 surgeons who acted as principal surgeon during the operations. In 7 patients (9%) it was necessary to screw in the cup because the surgeon considered the grip was not sufficient (Table 1). The need to screw in the cup, despite considerably prolonging (p < .001) time in surgery, did not significantly change the haemoglobin, haematocrit or transfusion rates.
Total primary hip arthroplasties (n = 80) with topical tranexamic acid protocol.
| Age (years) | 66 ± 1 | |
| [1,0]Sex | Man | 47 (59%) |
| Woman | 33 (41%) | |
| [1,0]Side | Right | 43 (54%) |
| Left | 37 (46%) | |
| [2,0] ASA Classification of preoperative aesthetic risk | 1 | 6 (7%) |
| 2 | 58 (73%) | |
| 3 | 16 (20%) | |
| [1,0]implanted prosthesis | Exactech® | 67 (84%) |
| Amplitude® | 13 (16%) | |
| [2,0]Friction par | Polyethylene-metal | 40 (50%) |
| Polyethylene-ceramic | 30 (38%) | |
| Ceramic-ceramic | 10 (12%) | |
| Screwed in cup | 7 (9%) | |
| Time in surgery (min) | 73 ± 2 | |
| Hb pre (mg/dl) | 14.42 ± .18 | |
| Hto pre (mg/dl) | 42.39 ± .49 | |
| Hb post (mg/dl) | 11.62 ± .18* | |
| Hto post (mg/dl) | 34.18 ± .50* | |
| Difference Hb (mg/dl) | 2.80 ± .11 | |
| Difference Hto (mg/dl) | 8.20 ± .36 | |
| [1,0]Transfusion | Total | 9 (11%) |
| In patients without previous anaemia | 3 (4%) | |
| Length of hospital stay in days | 3.5 ± .1 |
The levels are expressed as mean ± standard error of the mean.
Hb: haemoglobin; Hto: haematocrit; post: postoperative; pre: preoperative.
Postoperative haemoglobin and haematocrit levels were not significantly lower (p < .001) than the corresponding preoperative levels (Table 1). Patients who received transfusions presented with lower haemoglobin and haematocrit levels (<.001), both pre and postoperatively, than patients who did not require transfusion, but no significant differences were observed in the loss of haemoglobins or haematocrits in those patients who received transfusions compared to those who did not. If the patients were divided into 4 age quartiles, it was observed that the older patients (Q4) presented with significantly lower levels of haemoglobin and haematocrit preoperatively (p < .01) and haemoglobin (p < .05) and haematocrit (p < .01) levels postoperatively compared to the younger ones (Q1), and they also presented with a significant difference in the need for transfusion, with the rate of transfusion being higher in older patients (Q4) compared with younger ones (Q1) (p < .05). If we study by gender, the haemoglobin and haematocrit levels were lower in women (p < .001) and despite the fact there was no significant difference in the loss of haemoglobins or haematocrits compared with men, the need for transfusion was significantly higher in women (p < .001). Patients who received transfusions had a significantly longer hospital stay than those who did not require transfusion (p < .001).
Nine (11%) patients required transfusion and 6 of them had previous known anaemia (haemoglobin <12,3 g/dl) (Table 1); of the 74 patients who did not have anaemia before surgery, 3 (4%) received transfusions, one suffered an intraoperative periimplant fracture. There were no thromboembolic complications in this series.
DiscussionThe mean age of our series was 66 years, very similar to that of other primary THA series.2,9,11,13,15 The percentage of males/females was between 40% and 60%, with men or women predominating depending on the series.2,9,11 In our study, males predominated (59%). Time in surgery was highly variable (40−103 min) depending on the authors,9,11,13,15 with our data at a 73 min intermediate level. We obtained a hospital stay under 3 and a half days, which was similar to the series by Luo et al.,9,13 which is below other published series2,11; this could be linked to the postoperative Redon drain, since on removal after 24 h or on not using it,9,13 patients began early rehabilitation and were able to receive hospital discharge in less time.
In the results of this series we were able to be demonstrated that as haemoglobin and haematrocrit losses were similar, the factor determining greater risk of transfusion were low haemoglobin and haematocrit levels. Advanced age and being female were related to lower preoperative haemoglobin and haematocrit levels and have shown to be predisposing factors in the need for transfusion.
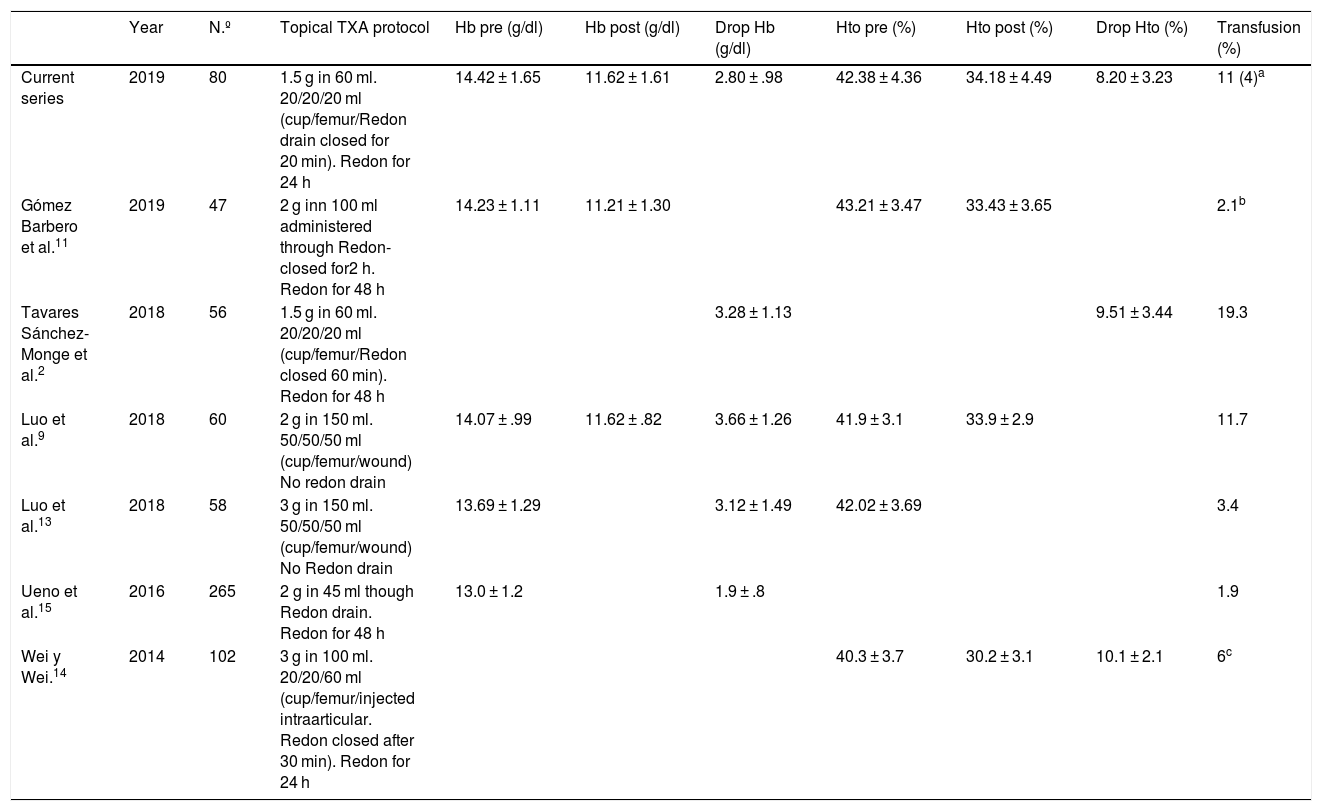
The results of using this topical TXA protocol are comparable to those published by other authors (Table 2). Our haemoglobin and haematocrit levels were slightly lower than those described by Tavares Sánchez-Monge et al.2 and the need for transfusion was lower than that described in the series where the protocol was presented.2 Compared with other topical TXA protocols (Table 2),9,11,13–15 these results are at least equally positive, and we may therefore say that the use of topical TXA is effective and safe. If we compare the haematocrit and haemoglobin losses and the percentage of patients who received transfusions with the controls groups of other authors (groups in which no intravenous or topical TXA was used),2,8,14 we observe that with our topical TXA protocol we obtained lower losses of haematocrit and haemoglobin, and fewer patients received transfusions. Furthermore, if we compare with recently published series where an intravenous TXA protocol was used,8,11,14 the results of our topical TXA are no worse regarding haemoglobin and haematocrit loss figures and the need for transfusion. Hospital stay time was considerably higher for patients who received transfusions. For all of the above, we may expect a reduction in the number of transfusions from systematic use of topical TXA which will short mean hospital stay time in patients treated using primary THA.
Table comparison between different topical tranexamic acid administration in THA.
| Year | N.º | Topical TXA protocol | Hb pre (g/dl) | Hb post (g/dl) | Drop Hb (g/dl) | Hto pre (%) | Hto post (%) | Drop Hto (%) | Transfusion (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current series | 2019 | 80 | 1.5 g in 60 ml. 20/20/20 ml (cup/femur/Redon drain closed for 20 min). Redon for 24 h | 14.42 ± 1.65 | 11.62 ± 1.61 | 2.80 ± .98 | 42.38 ± 4.36 | 34.18 ± 4.49 | 8.20 ± 3.23 | 11 (4)a |
| Gómez Barbero et al.11 | 2019 | 47 | 2 g inn 100 ml administered through Redon-closed for2 h. Redon for 48 h | 14.23 ± 1.11 | 11.21 ± 1.30 | 43.21 ± 3.47 | 33.43 ± 3.65 | 2.1b | ||
| Tavares Sánchez-Monge et al.2 | 2018 | 56 | 1.5 g in 60 ml. 20/20/20 ml (cup/femur/Redon closed 60 min). Redon for 48 h | 3.28 ± 1.13 | 9.51 ± 3.44 | 19.3 | ||||
| Luo et al.9 | 2018 | 60 | 2 g in 150 ml. 50/50/50 ml (cup/femur/wound) No redon drain | 14.07 ± .99 | 11.62 ± .82 | 3.66 ± 1.26 | 41.9 ± 3.1 | 33.9 ± 2.9 | 11.7 | |
| Luo et al.13 | 2018 | 58 | 3 g in 150 ml. 50/50/50 ml (cup/femur/wound) No Redon drain | 13.69 ± 1.29 | 3.12 ± 1.49 | 42.02 ± 3.69 | 3.4 | |||
| Ueno et al.15 | 2016 | 265 | 2 g in 45 ml though Redon drain. Redon for 48 h | 13.0 ± 1.2 | 1.9 ± .8 | 1.9 | ||||
| Wei y Wei.14 | 2014 | 102 | 3 g in 100 ml. 20/20/60 ml (cup/femur/injected intraarticular. Redon closed after 30 min). Redon for 24 h | 40.3 ± 3.7 | 30.2 ± 3.1 | 10.1 ± 2.1 | 6c |
The levels are expressed as a mean ± standard deviation.
THA: total hip arthroplasty; Hb: haemoglobin; Hto: haematocrit; Number of patients; post: postoperative; pre: preoperative; TXA: tranexamic acid.
There were no thromboembolic complications in our series, similarly to other series where TXA was administered in topical form.2,9,11,13,15 In general, adverse events of a drug are related to its plasmatic concentration and administering topical TXA (with which the plasmatic concentration is approximately 10 times lower with respect to intravenous administration),16 the theoretical risk of thromboembolic complication is lowered even further than with intravenous TXA administration.5,6
To conclude, the use of this topical TXA protocol is safe and effective in primary THA, reducing the need for blood transfusions with respect to the reference for the series where there is no use of any TXA protocol. It would be interesting to conduct further studies to compare the different topical TXA protocols to determine which of them would be most effective.
Level of evidenceLevel of evidence iv.
FinancingNone.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Miranda I, Collado-Sánchez A, Peregrín-Nevado I, Díaz-Martínez JV, Sánchez-Alepuz E, Miranda FJ. Utilización del ácido tranexámico tópico en la artroplastia total primaria de cadera. Eficacia y seguridad. Nuestra experiencia. Rev Esp Cir Ortop Traumatol. 2020;64:114–119.