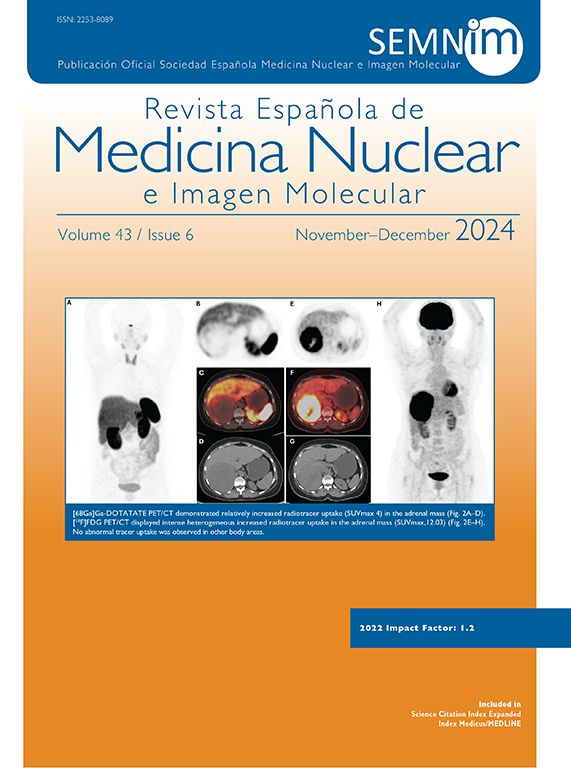
The Revista Española de Medicina Nuclear e Imagen Molecular (Spanish Journal of Nuclear Medicine and Molecular Imaging), was founded in 1982, and is the official journal of the Spanish Society of Nuclear Medicine and Molecular Imaging, which has more than 700 members.
The Journal, which publishes 6 regular issues per year, has the promotion of research and continuing education in all fields of Nuclear Medicine as its main aim. For this, its principal sections are Originals, Clinical Notes, Images of Interest, and Special Collaboration articles. The works may be submitted in Spanish or English and are subjected to a peer review process.
In 2009, it became the leading Spanish journal in the field of Medical Imaging on having an Impact Factor , awarded by the Journal Citation Reports.