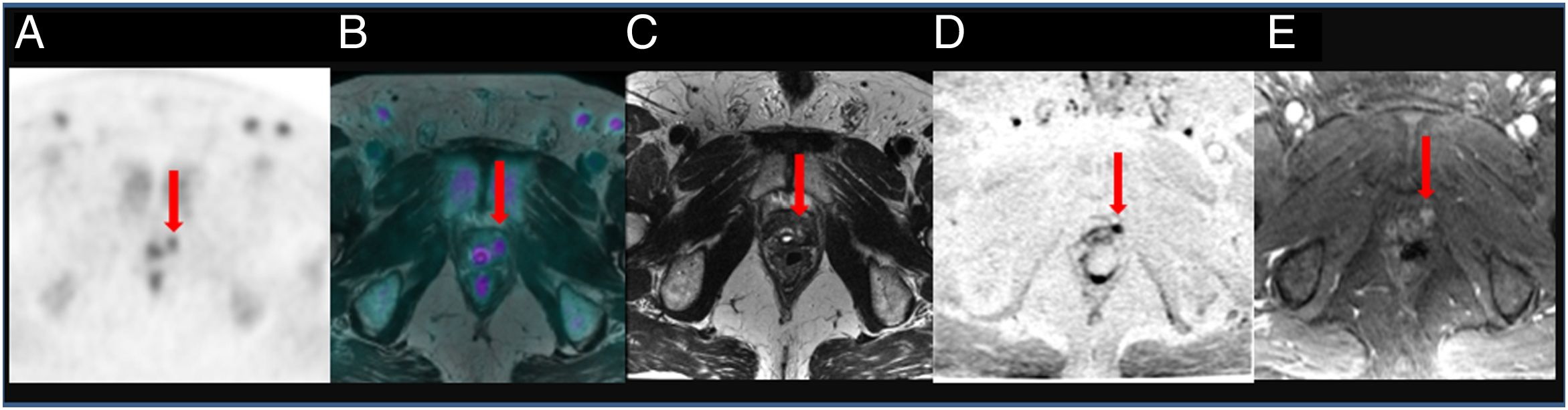
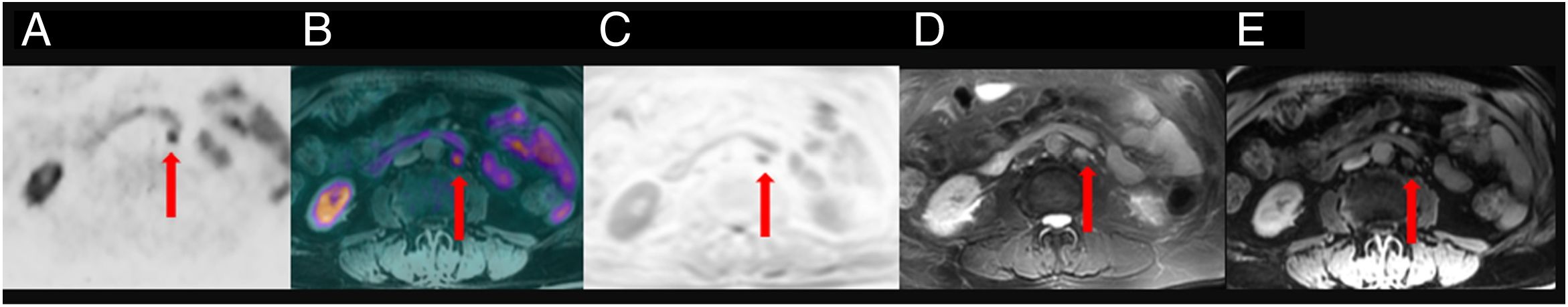
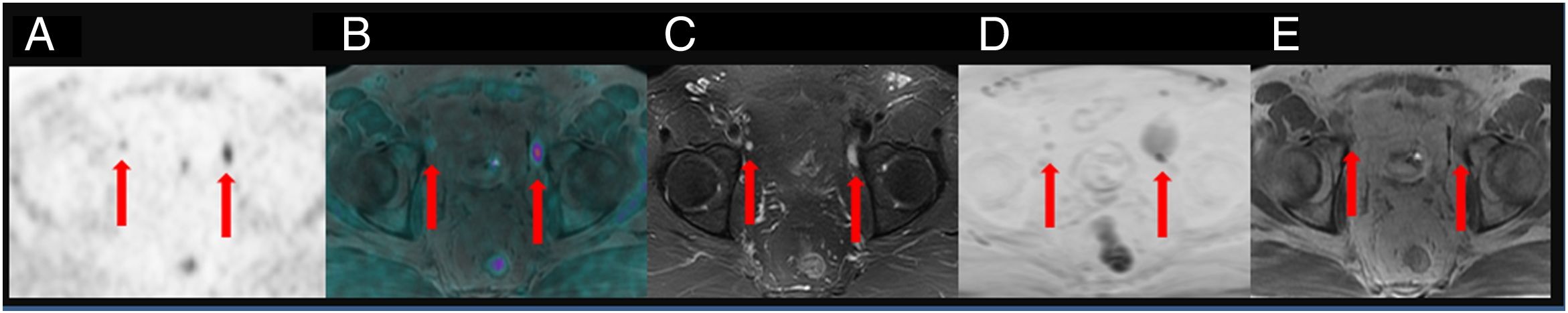
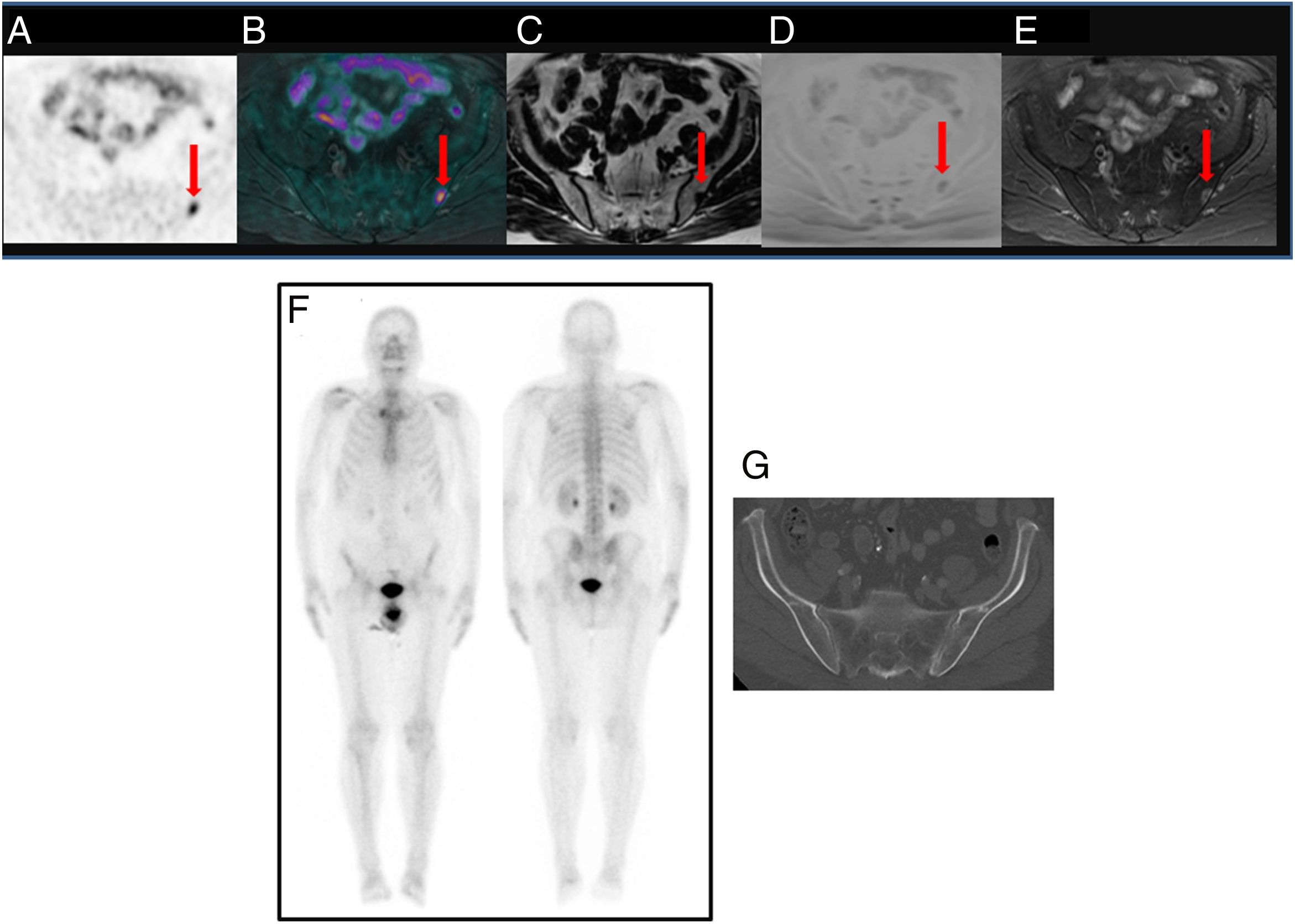
To assess the detection rate of 18F-Choline PET/MRI and subsequent changes in therapy approach for patients with prostate cancer treated by prostatectomy and with rising levels of PSA <1ng/ml.
MethodsProspective study with our first 36 patients with prostatectomy for prostate cancer and rising levels of PSA, who were referred for an 18F-Choline PET/MRI study.
A dual-phase study was acquired after intravenous administration of 185±10% MBq of 18F-Choline: (1) early imaging (immediately after tracer administration) of prostate area (emission PET/Multiparametric MRI). (2) Whole-body imaging 1h after tracer injection (emission PET/MRI: T1, T2, STIR, diffusion).
The therapy approach for patients was decided upon the Oncology Committee consensus based on 18F-Choline PET/MRI findings.
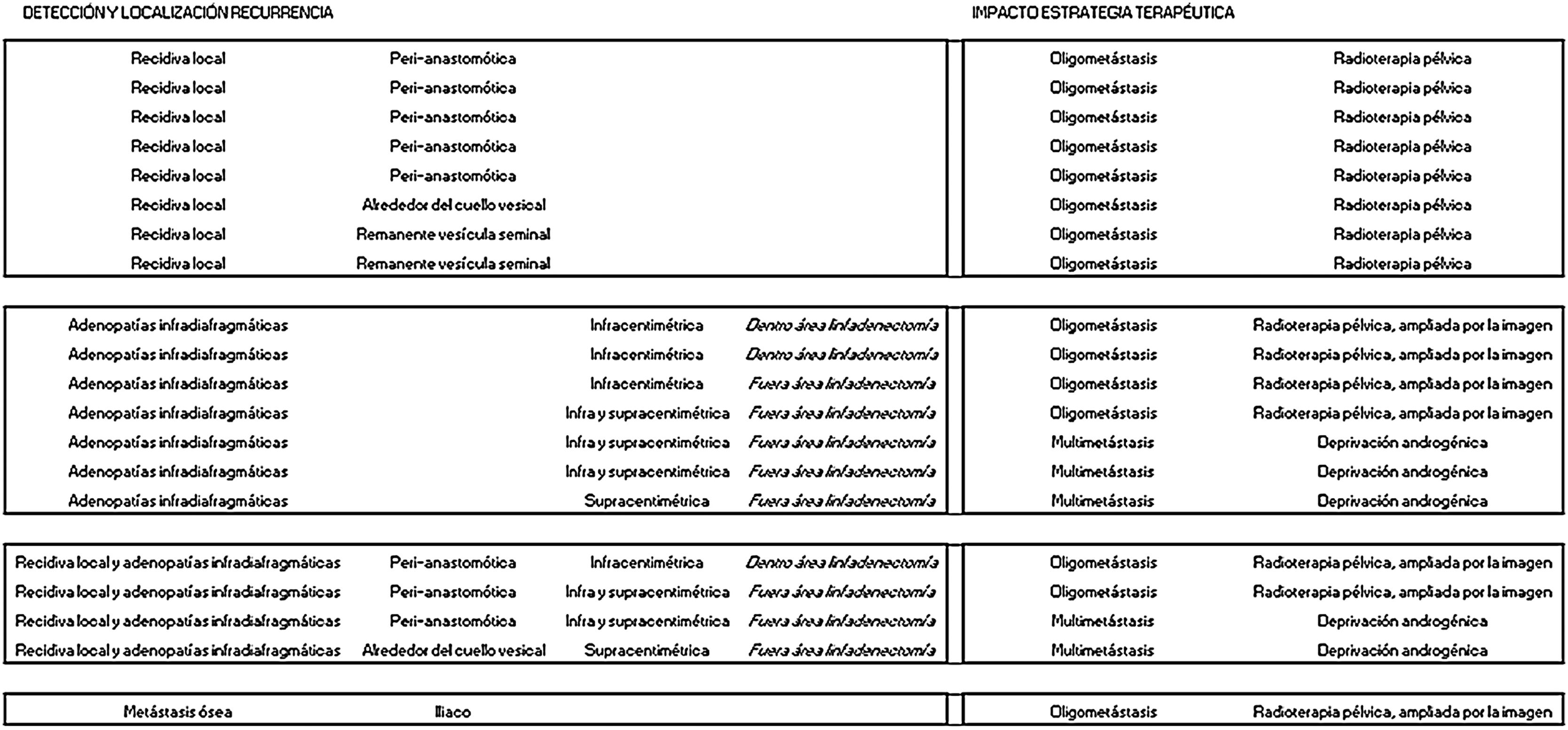
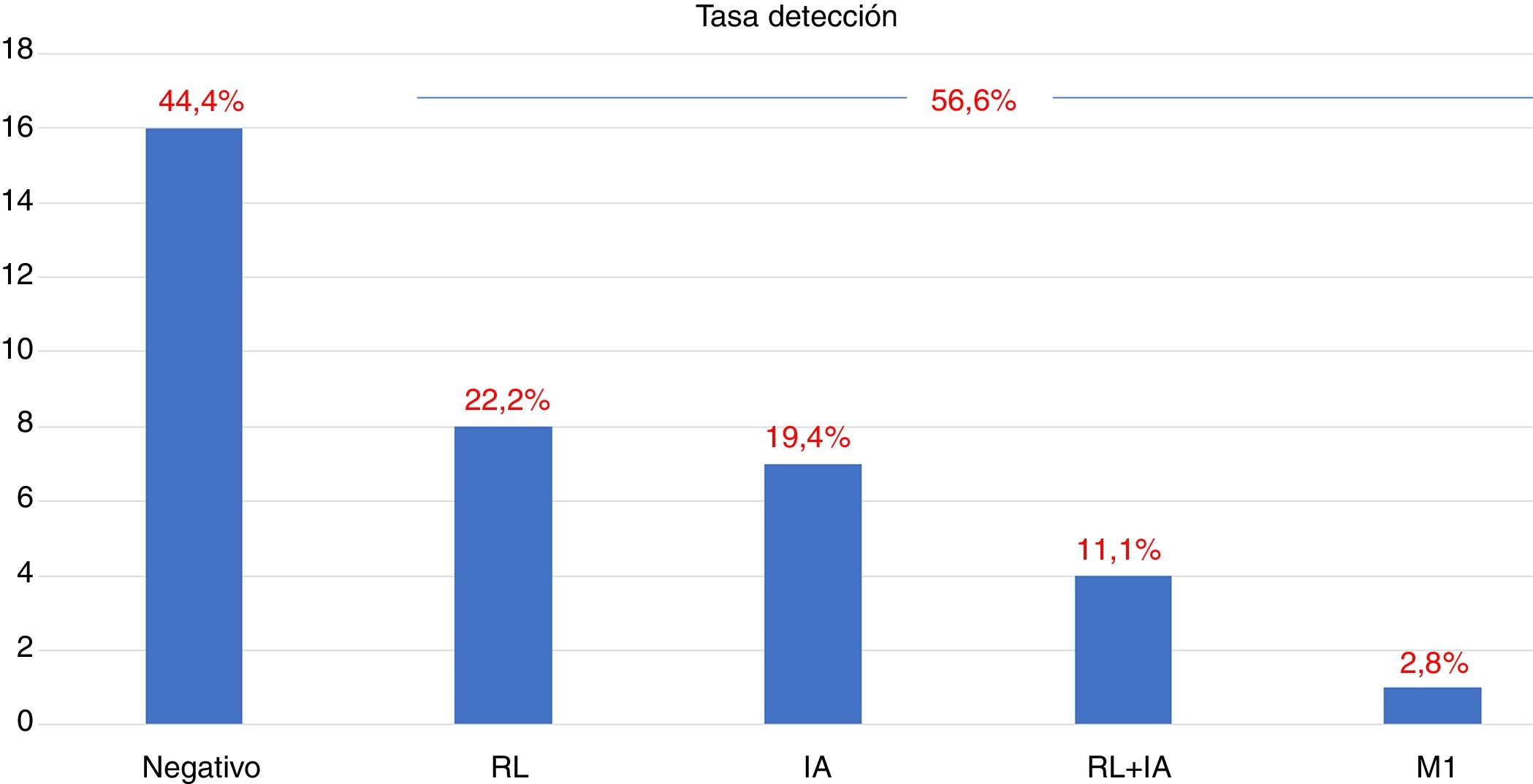
ResultsTwenty out of 36 patients (55.6%) were positive for the 18F-Choline PET/MRI study: 8 (22.2%) within the prostatectomy bed, 7 (19.4%) with infradiaphragmatic lymph nodes, 4 (11.1%) with local recurrence and infradiaphragmatic lymph nodes, and 1 (2.8%) with bone metastasis.
Sixteen out of the 36 patients (44.4%) were negative for the 18F-Choline PET/MRI study.
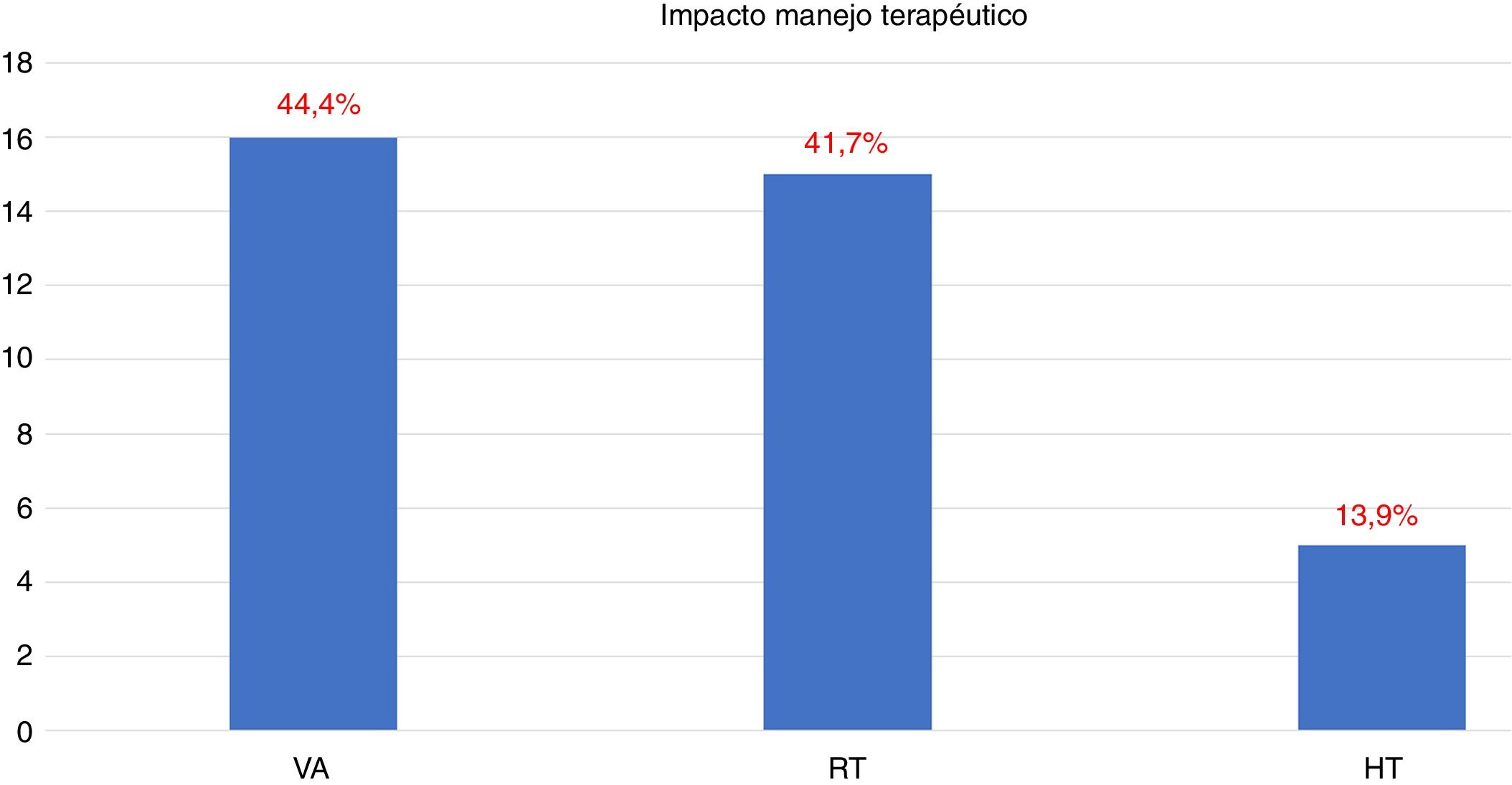
18F-Choline PET/MRI findings had an impact on the therapy approach to follow: 15 patients (41.6%) showed oligometastatic disease which was treated by imaging-guided radiotherapy, 5 (13.9%) with multiple metastatic disease were treated by androgen deprivation therapy, 16 (44.4%) negative were under active surveillance.
ConclusionHybrid 18F-Choline PET/MRI procedure showed a high detection rate for recurrence in prostate cancer patients treated with prostatectomy and rising PSA levels <1ng/ml, and 18F-Choline PET/MRI findings resulted in a better tailored therapy approach delivered to our patients.
Evaluar la tasa de detección de la PET/RM con 18F-colina y los cambios en el manejo terapéutico de los pacientes con cáncer de próstata tratados con prostatectomía que presentan elevación del PSA <1 ng/ml.
MétodoEstudio prospectivo de los 36 primeros pacientes con cáncer de próstata tratados con prostatectomía, con elevación de PSA<1 ng/ml, a los que hemos realizado una PET/RM con 18F-colina.
Tras la administración de 185±10% MBq de 18F-colina se ha adquirido un estudio en dos fases: 1) precoz prostática (inmediatamente tras la administración del trazador): emisión PET/RM multiparamétrica. 2) Estudio una hora postinyección de cuerpo completo: emisión PET/RM: T1, T2, STIR, difusión.
El comité oncológico ha decidido la estrategia terapéutica de los pacientes según los hallazgos de la PET/RM con 18F-colina.
ResultadosDe los 36 pacientes, 20 (55,6%) han mostrado positividad del estudio PET/RM con 18F-colina: en 8 (22,2%) lecho de prostatectomía, en 7 (19,4%) adenopatías infradiafragmáticas, en 4 (11,1%) recidiva local y adenopatías infradiafragmáticas, en 1 (2,8%) una metástasis ósea.
De los 36 pacientes, en 16 (44,4%) el estudio PET/RM con 18F-colina ha sido negativo.
Los hallazgos de la PET/RM con 18F-colina han condicionado la estrategia terapéutica: en 15 pacientes (41,6%) enfermedad oligometastásica tratada con radioterapia guiada por la imagen, en 5 (13,9%) enfermedad multimetastásica tratada con privación androgénica, en 16 (44,4%) negativo en vigilancia activa.
ConclusiónLa técnica híbrida PET/RM con 18F-colina ha demostrado una elevada tasa de detección de la recidiva en los pacientes tratados con prostatectomía que presentan PSA <1 ng/ml, permitiendo una estrategia terapéutica personalizada según los hallazgos de la exploración.
Article
Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)