Although Candida is a commensal of the urogenital tract, intrauterine fungal infections are extremely uncommon in clinical practice.
AimsIn the present work we evaluated whether amniotic fluid (AF) possesses direct antifungal activity against clinical isolates of Candidaalbicans and other Candida species.
MethodsA total of 23 AF samples from pregnant women with gestational age of 38–41 weeks were obtained under aseptic conditions by the aspiration of the amniotic sac during cesarean section. Different Candida species were inoculated in amniotic fluid and Sabouraud broth, used as control, and were incubated at 37°C for 48h. Quantitative cultures of test samples and controls were performed at 0, 4, 8, 12, 24, and 48h.
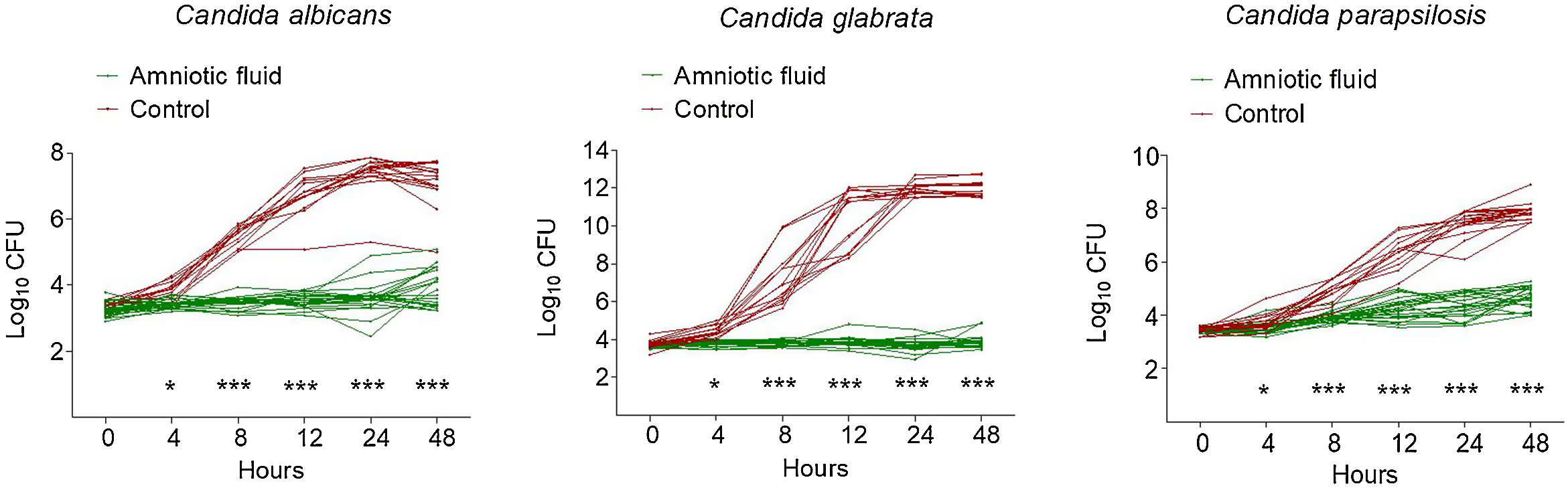
ResultsAF collected from 23 pregnant women had consistent and significant inhibitory activity against all Candida isolates tested. Nonetheless, a complete inhibition of growth by all 23 AF samples tested was observed only against Candida glabrata.
ConclusionsIt is likely that the antifungal activity of the AF against C. albicans, C. glabrata and Candida parapsilosis observed in vitro also exists in vivo, contributing to protect against intrauterine fungal infections.
Aunque Candida es un comensal del tracto urogenital, las infecciones fúngicas intrauterinas son extremadamente poco frecuentes en la práctica clínica.
ObjetivosEn el presente trabajo evaluamos si el líquido amniótico posee actividad antifúngica directa contra aislamientos clínicos de Candida albicans y otras especies del género Candida.
MétodosSe obtuvieron un total de 23 muestras de líquido amniótico de mujeres embarazadas con edad gestacional de 38 a 41 semanas en condiciones asépticas por aspiración del saco amniótico durante la cesárea. Se inocularon diferentes especies de Candida en líquido amniótico y caldo Sabouraud, que se usó como control, y se incubaron a 37°C durante 48h. Se realizaron cultivos cuantitativos de las muestras y controles a las 0, 4, 8, 12, 24 y 48h.
ResultadosEl líquido amniótico recogido de 23 mujeres embarazadas tuvo actividad inhibidora consistente y significativa contra todos los aislamientos de Candida probados. No obstante, la inhibición completa del crecimiento con las 23 muestras de líquido amniótico analizadas se observó solo frente a Candida glabrata.
ConclusionesProbablemente la actividad antifúngica del líquido amniótico observada in vitro contra C. albicans, C. glabrata y Candida parapsilosis también existe in vivo, y contribuye a la protección de las infecciones fúngicas intrauterinas.
Candida species are part of the commensal genital microbiota of approximately 20% of asymptomatic, healthy women during reproductive age.2 Colonization rates increase to about 30% in pregnant women, particularly during the third trimester, due to the high levels of circulating estrogens that promote yeast adhesion and germination.12,15 Among Candida species, Candida albicans is the most frequently isolated. However, due to the widespread use of azoles, other multidrug-resistant species, including Candida glabrata, Candida parapsilosis and Candida tropicalis, are emerging causes of systemic infections worldwide.5 Despite the high vaginal Candida colonization rates, intrauterine fetal infections due to these yeasts are rarely encountered.4 This observation is consistent with the antifungal properties of the amniotic fluid (AF) against Candida albicans.9,11 However, there is no information on AF activity against non-C. albicans Candida species.
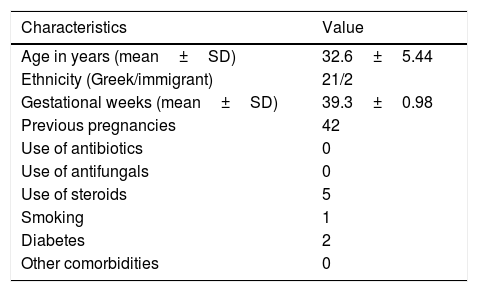
In this study we evaluated the antifungal effect of AF obtained during the 38th–41st week of gestation against C. albicans, C. glabrata, and C. parapsilosis clinical isolates. The study included 23 pregnant women followed up at the Obstetrics Department of the University Hospital of Heraklion, in Crete, Greece, from November 2015 to May 2016. The demographics and clinical details of the 23 AF donors are shown in Table 1. The requirements to be enrolled were being in a gestational age of 38–41 weeks at delivery, and giving birth by cesarean section without having taken antibiotics in the previous month. Ethical approval for the study was obtained by the IRB of the hospital (15095/30-3-2016). Written consent was signed by each study participant.
Demographics and clinical characteristics of the 23 AF donor women.
| Characteristics | Value |
|---|---|
| Age in years (mean±SD) | 32.6±5.44 |
| Ethnicity (Greek/immigrant) | 21/2 |
| Gestational weeks (mean±SD) | 39.3±0.98 |
| Previous pregnancies | 42 |
| Use of antibiotics | 0 |
| Use of antifungals | 0 |
| Use of steroids | 5 |
| Smoking | 1 |
| Diabetes | 2 |
| Other comorbidities | 0 |
Amniotic fluid samples were obtained under aseptic conditions by aspirating through the amniotic sac during cesarean section. All women had intact membranes. Samples contaminated with meconium or blood were excluded. The first 10ml of the amniotic fluid were discarded in order to avoid blood, and the remainder was collected in sterile tubes and immediately centrifuged at 3000rpm for 15min. The supernatant was frozen at −80°C until further processing.
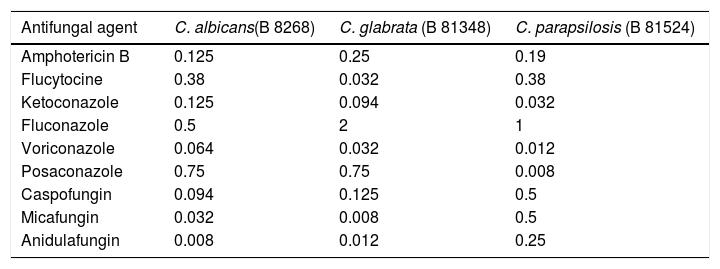
The strains used to assay the inhibitory activity of AF were recovered from patients with invasive candidiasis and identified by sequencing of the internal transcribed spacer 1 and 2 (ITS1-5.8SrRNA-ITS2). The isolates are stored in the library of the Department of Clinical Microbiology and Microbial Pathogenesis of the University Hospital of Heraklion, Greece and are available upon request. The sequences of all three isolates were submitted to Genbank, being assigned the following accession numbers: Candida albicans B 8268 (GenBank accession no. MW616800), Candida glabrata B 81348 (GenBank accession no. MW616799), and Candida parapsilosis B 81524 (GenBank accession no. MW616798). The activity of the main antifungal agents against these isolates are shown in Table 2. Yeasts grown overnight on Sabouraud dextrose agar (bioMérieux, Marcy L’ Etoile, France) were suspended in sterile normal saline. Serial dilutions of the suspensions with additional normal saline to achieve a concentration of 104yeasts/ml were made. From this final dilution, 0.2ml were added to 1.8ml of amniotic fluid, and other 0.2ml to 1.8ml of Sabouraud broth. The latter was the positive control for Candida growth. Amniotic fluids and controls were incubated at 37°C for 48h. Aliquots of 0.2ml were collected both from the amniotic fluids under testing and the controls at 0, 4, 8, 12, 24, and 48h, being serially diluted with normal saline up to 10−12. Samples of 0.1ml of each dilution were streaked on Sabouraud dextrose agar plates, which were incubated for 48h at 37°C, and colony forming units (CFUs) were counted.7 All experiments were performed in triplicate, in order to check the reproducibility of the results. Differences in CFUs between control and AF samples were compared by unpaired Student's t-test. Statistical significance was set at p<0.05. All statistical analyses were performed with Graphpad Prism, V.4 (GraphPad Software Inc, CA, USA).
Minimum inhibitory concentrations (μg/ml) of nine antifungal agents against the three clinical isolates. E-test was the method used.
| Antifungal agent | C. albicans(B 8268) | C. glabrata (B 81348) | C. parapsilosis (B 81524) |
|---|---|---|---|
| Amphotericin B | 0.125 | 0.25 | 0.19 |
| Flucytocine | 0.38 | 0.032 | 0.38 |
| Ketoconazole | 0.125 | 0.094 | 0.032 |
| Fluconazole | 0.5 | 2 | 1 |
| Voriconazole | 0.064 | 0.032 | 0.012 |
| Posaconazole | 0.75 | 0.75 | 0.008 |
| Caspofungin | 0.094 | 0.125 | 0.5 |
| Micafungin | 0.032 | 0.008 | 0.5 |
| Anidulafungin | 0.008 | 0.012 | 0.25 |
As shown in Fig. 1, all 23 AF samples possessed a significant inhibitory activity against all three clinical isolates of Candida. The antifungal activity of AF was more pronounced against C. glabrata, since all AF samples prevented this species from growing (Fig. 1). In contrast, when compared to the initial inoculum (0h), minimal growth was observed in C. albicans (with certain AF samples) and C. parapsilosis (all AF samples) at 48h (Fig. 1).
Kinetics of growth of Candida clinical isolates in the presence of amniotic fluid versus Sabouraud broth (control). Each line represents the growth curve over time of a sole Candida isolate, grown either in amniotic fluid or in Sabouraud broth (control). Each symbol represents the log10 CFU value at different time points. p<0.0001; Student's t test.
Azole-resistant non-C. albicansCandida species, including C. glabrata, are increasingly encountered as causes of urogenital infections worldwide.16 Despite the increased rates of Candida vaginal colonization during pregnancy, intrauterine infections are extremely uncommon.4,10 This is suggestive of a host effector mechanism with specific antifungal properties, including the antifungal properties of AF. Antimicrobial components of AF include cytokines and host defense proteins, including defensins, lactoferrin, lysozyme, and other antimicrobial peptides.6 In particular, beta-defensin 2 (HBD-2) has been documented as a molecule that prevents Candida proliferation in vitro.17 Previous works also attributed the antifungal activity of AF to spermine,14 and to anti-Candida antibodies.13
Several researchers studied the antibacterial activity of AF against aerobic and anaerobic bacteria.3,18 The inhibitory activity of AF against C. albicans has been shown previously.1,8,9,11,13,14 This is the first study on the activity of AF against C. albicans and other Candida species. Our results show that AF has inhibitory activity not only against C. albicans, but also against C. glabrata and C. parapsilosis. Only C. glabrata growth was completely inhibited by all 23 AF samples.
The in vitro antifungal activity of AF against C. albicans, C. glabrata and C. parapsilosis likely exists also in vivo, thus contributing to the prevention of intraamniotic fungal infections. It would be interesting to study the activity of AF against Candida auris and other emerging Candida species. In particular, it is important to understand whether the antifungal activity of AF is mediated by the restriction of important growth factors to the pathogen (e.g., nutritional immunity) or the presence of antimicrobial peptides (e.g. defensins).
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no competing interests.