Staphylococcus aureus and Candida albicans have been co-isolated from biofilm-associated diseases such as denture stomatitis, periodontitis, and burn wound infections, as well as from medical devices. However, the polymicrobial biofilm of both microorganisms has not been fully characterized.
AimsTo characterize the polymicrobial biofilm of C. albicans and S. aureus in terms of microbial density, synergy, composition, structure, and stability against antimicrobials and chemical agents.
MethodsCrystal violet assay was used to measure the biofilm formation. Scanning electron microscopy and confocal microscopy were used to analyze the structure and chemical composition of the biofilms, respectively.
ResultsSupplemented media with fetal bovine serum (FBS) decreased the biofilm formation of S. aureus and the polymicrobial biofilm. For C. albicans, depending on the culture media, the addition of glucose or FBS had a positive effect in biofilm formation. FBS decreased the adhesion to polystyrene wells for both microorganisms. Supplementing the media with glucose and FBS enhanced the growth of C. albicans and S. aureus, respectively. It seems that C. albicans contributes the most to the adhesion process and to the general structure of the biofilms on all the surfaces tested, including a catheter model. Interestingly, S. aureus showed a great adhesion capacity to the surface of C. albicans in the biofilms. Proteins and β-1,6-linked polysaccharides seem to be the most important molecules in the polymicrobial biofilm.
ConclusionsThe polymicrobial biofilm had a complex structure, with C. albicans serving as a scaffold where S. aureus adheres, preferentially to the hyphal form of the fungus. Detection of polymicrobial infections and characterization of biofilms will be necessary in the future to provide a better treatment.
Staphylococcus aureus y Candida albicans son aislados conjuntamente de infecciones asociadas a la formación de biopelículas, tales como periodontitis, estomatitis e infecciones provenientes de quemaduras, así como en dispositivos médicos. Sin embargo, la biopelícula formada por ambos microorganismos no ha sido completamente caracterizada.
ObjetivosCaracterizar la biopelícula de C. albicans y S. aureus en cuanto a densidad microbiana, sinergismo, composición, estructura y estabilidad frente a agentes químicos y antimicrobianos.
MétodosEl análisis de la formación de biopelícula se realizó mediante el ensayo de cristal violeta. Se analizó la composición química y la estructura de las biopelículas mediante microscopio confocal y microscopio electrónico de barrido, respectivamente.
ResultadosLa adición al medio de suero bovino fetal (SBF) redujo la biopelícula mono- y polimicrobiana de S. aureus. En C. albicans, con la adición de glucosa o SBF, se incrementó la formación de biopelícula. La adhesión de los microorganismos a las placas de poliestireno se redujo en presencia de SBF. La suplementación del medio con glucosa y SBF favoreció la proliferación de C. albicans y S. aureus, respectivamente. C. albicans mostró una mejor adhesión y contribuyó más a la densidad total de la biopelícula en diferentes superficies probadas, incluyendo un modelo de catéter. De manera interesante, S. aureus mostró una mejor adhesión a la superficie de C. albicans en la biopelícula. Las proteínas y los polisacáridos con enlaces β-1,6 parecen ser las moléculas más abundantes en la biopelícula.
ConclusionesLa biopelícula formada por ambas especies resultó ser más compleja, con C. albicans formando una superficie sobre la que S. aureus se adhiere, preferentemente a la forma miceliar de la levadura. El estudio de las biopelículas asociadas a infecciones polimicrobianas será necesario en el futuro con el fin de proporcionar un mejor tratamiento.
Candida albicans is the most common fungal species in the human microbiota colonizing the oral cavity, skin, and gastrointestinal and reproductive tract of healthy individuals. Changes in the local environment, host immunity, and the microbiota of each person may induce the proliferation of C. albicans and a subsequent infection that may turn from a superficial skin or mucosal infection into a candidemia,17 a bloodstream infection with high mortality rates.19,30 It has been shown that C. albicans is the third most common pathogen from intravascular catheter-associated infections and the fourth most common microorganism isolated from nosocomial bloodstream infections.6,32 Also, it is the most frequent fungal species isolated from medical devices such as catheters, prostheses, implants, pacemakers, dental materials, etc.14 It has been suggested that the ability to colonize and infect these surfaces is related to its capacity to form biofilms.7,8
In a similar way, Staphylococcus aureus is a commensal bacterium found on the skin and mucous membranes of healthy individuals. S. aureus colonizes the nasal cavity of around 30% of the human population.15,31 However, this microorganism is also considered a human pathogen and causes community-acquired and hospital-acquired infections, which in some cases are difficult to treat due to the presence of MRSA (methicillin-resistant S. aureus).27S. aureus causes a wide range of infections, from mild skin and soft-tissue infections to bacteremia, endocarditis, osteomyelitis, etc.,28 and has also the capacity to adhere to medical devices, resulting in biofilm formation and infection.16,18
Polymicrobial infections have been associated with higher mortality rates when compared with monomicrobial infections.9,20,25 In this regard, S. aureus and C. albicans have been co-isolated from biofilm-associated diseases.1 For example, it has been reported that 27% of nosocomial bloodstream infections caused by C. albicans were polymicrobial, with S. aureus as the third most common pathogen isolated along with the fungus.13 Furthermore, the lethality of S. aureus in an intraperitoneal infection model in mice was much higher in a co-infection with C. albicans.3 For this reason, the relevance of polymicrobial infections has been recognized as a major problem.2 The aim of this work was to characterize the polymicrobial biofilm of C. albicans and S. aureus on different surfaces, including a catheter model. We also analyzed the stability and composition of the biofilm using destabilizing chemical compounds and antimicrobials.
Materials and methodsGrowth conditions of S. aureus and C. albicansS. aureus ATCC 29213 was seeded on brain heart infusion (BHI) agar plates and incubated at 37°C for 24h. Then, a colony was inoculated into BHI broth and incubated at 37°C with shaking at 50rpm for 24h. In order to know the bacterial colony forming units (CFU), we generated a growth curve correlating the optical density of the cultures at 600nm with CFU on BHI agar plates. C. albicans ATCC 10231 was seeded on Sabouraud dextrose (SD) agar plates, and incubated at 37°C for 24h. Then, one colony was inoculated into SD broth at 30°C with shaking at 50 rpm for 24h. The number of yeast cells was obtained by means of the trypan blue exclusion test using a hemocytometer.
Mono- and polymicrobial biofilm formation with C. albicans and S. aureusTo standardize the optimal concentration of C. albicans and S. aureus that yield a mono- or polymicrobial biofilm in 96-well cell culture plates (Costar, NY, USA), we used S. aureus in concentrations of 1×107 CFU, 1×108 CFU, and 1×109 CFU per well; the concentrations for C. albicans were 1×106 CFU and 1×107 CFU per well. A final volume of 200μl of RPMI-1640 medium (Gibco, NY, USA) per well was used, with pH 7 adjusted with MOPS (3-(N-morpholine)propanesulfonic acid, Sigma, Saint Louis, USA). Microplate wells with a mono- or polymicrobial suspension were incubated at 37°C in a humidified chamber for 24h. Then, the excess of cells was removed and the plate was washed three times by submersion in a recipient containing tap water. In order to stain the cells, we added 125μl of a 0.4% solution of crystal violet per well, and the plate was incubated at room temperature for 15min. Afterwards, the excess of colorant was discarded, the plate was rinsed in tap water three times by submersion, and the excess of liquid was removed using paper towels. At this time, it was possible to photograph the plate for a qualitative analysis. Finally, in order to quantify the biofilm formed, we added 125μl of a 30% solution of acetic acid per well, and the content of each well was transferred after 15min of incubation at room temperature to a new microplate to assess the absorbance at 595nm in a microplate reader. The absorbance value of the negative control containing only RPMI-1640 medium was subtracted from that obtained for each well. In order to compare biofilm formation in different media, Dulbecco's modified eagle's medium (DMEM) was used as DMEM low glucose concentration (Gibco), and DMEM high glucose concentration (Gibco). RPMI-1640 containing 0.2% glucose (low) was adjusted to 2% glucose (high).
Adherence and growth kinetics of S. aureus and C. albicansAdhesion experiments testing S. aureus (1×108 CFU) or C. albicans (1×106 CFU) were carried out in 12-well plates (Costar) containing the different culture media with or without fetal bovine serum (FBS). After 90min of incubation at 37°C, the cell suspension was discarded and the adhered cells were washed twice with phosphate-buffered saline (PBS). The attached cells were detached with a cell scraper (CellTreat, Pepperell, USA) and collected in 1ml of PBS. The suspensions of cells were diluted and plated on BHI agar (S. aureus) or SD agar (C. albicans) in order to count CFU. For the growth kinetics analysis a colony of S. aureus was picked up and diluted in 1ml of RPMI-1640. One hundred microliters of the suspension were transferred to 900μl of RPMI-1640 supplemented or not with glucose and/or FBS. The resulting suspensions were incubated with shaking at 37°C. For C. albicans, a 100μl suspension containing 1×105 cells was added to 900μl of RPMI-1640 supplemented or not with glucose and/or FBS. The suspensions were incubated with shaking at 37°C. The optical density was checked at 595nm at different times.
Biofilm inhibition and destabilization using chemical agents and antimicrobialsIn order to study the inhibition of biofilm formation we used proteinase K (Sigma) at 100μg/ml, sodium (meta)periodate (Sigma) at 40mM, or DNase I (Thermo Fisher Scientific, Waltham, USA) at 50 μg/ml in the following manner: together with a suspension of S. aureus (1×107 cells) and/or C. albicans (1×106 cells) in polystyrene 96-well microplates, or on pre-formed biofilms, after a washing step with PBS to remove the excess of cells, for three hours. In a different experiment, the polymicrobial biofilm destabilization was also analyzed adding vancomycin (Sigma) at 10, 100, and 1000μg/ml, and/or amphotericin B (Thermo Fisher Scientific) at 0.25, 2.5, and 25μg/ml for 24h into the wells containing the pre-formed biofilms after a washing step with PBS. Biofilm formation and quantification was carried out as aforementioned in this section.
Analysis of biofilms by confocal laser scanning microscopyLab-Tek II system (Thermo Fisher Nunc, NY, USA) has been used for studying biofilm formation with confocal microscopy, as it consists of a slide with a removable chamber that keeps sterile conditions for cell culture.22 Two hundred microliters of S. aureus (1×107 cells) and/or C. albicans (1×106 cells) were added into the wells of Lab-Tek chamber slides. Incubation for biofilm formation was carried out in a humidified chamber for 24h at 37°C. Non-adhered cells were removed and the slides were stained with FilmTracer FM 1–43 Green Biofilm Cell Stain, Ex/Em 472/580nm (Thermo Fisher Scientific), FilmTracer SYPRO Ruby Biofilm Matrix stain, Ex/Em 450/610nm (Thermo Fisher Scientific), wheat germ agglutinin, Alexa Fluor 488 conjugate, Ex/Em 495/519nm (Thermo Fisher Scientific), or BOBO-3 Iodide, Ex/Em 570/602nm (Thermo Fisher Scientific) following the manufacturer's instructions. The removable cells were discarded, and ProLongGold Antifade Mountant (Thermo Fisher Scientific) was added to the slide. Three images per treatment in triplicate were obtained using a confocal microscope (Zeiss, LSM700) with Zen Black software, selecting one as representative. This experiment was repeated twice.
Analysis of biofilms by scanning electron microscopy (SEM)S. aureus (1×107 cells) and/or C. albicans (1×106 cells) were added to the wells of Lab-Tek humidified chamber slides, letting biofilm to be formed for 24h at 37°C. For the catheter model, a catheter section of around 1cm in length was placed into a 1.5ml tube containing 1ml of RPMI-1640 medium with S. aureus (1×107 cells) and/or C. albicans (1×106 cells). The tubes were placed in a rotating mixer at 25rpm and incubated for 24h at 37°C. Afterwards, the non-adhered cells were removed and the samples were fixed with 2.5% glutaraldehyde. The samples were then dehydrated with increasing concentration of ethanol (60%, 70%, 80%, 90%, 96%, and 100%), for 10min with each concentration. Finally, the samples were dried at 37°C/5% CO2 for 24h and coated with 10Å of gold, using Denton Vacuum Desk II. At least three images per treatment in triplicate were obtained and analyzed under JEOL JSM 5900LV scanning electron microscope (JEOL, Akishima, Tokio, Japan), selecting one image as representative. This experiment was repeated twice.
Statistical methodsGraphPad Prism 8.0 was the software used to evaluate the quantitative data in this study. Tukey's multiple comparison test (two-way ANOVA) was used to analyze the differences in biofilm formation according to the media used, as well as the growth kinetics (p≤0.05). Tukey's multiple comparison test (one-way ANOVA) was used to evaluate the adhesion of the microorganisms in different culture conditions (p≤0.05). Dunnett's multiple comparison test (one-way ANOVA) was used to analyze biofilm inhibition and destabilization using chemical reagent and antimicrobials (p≤0.05).
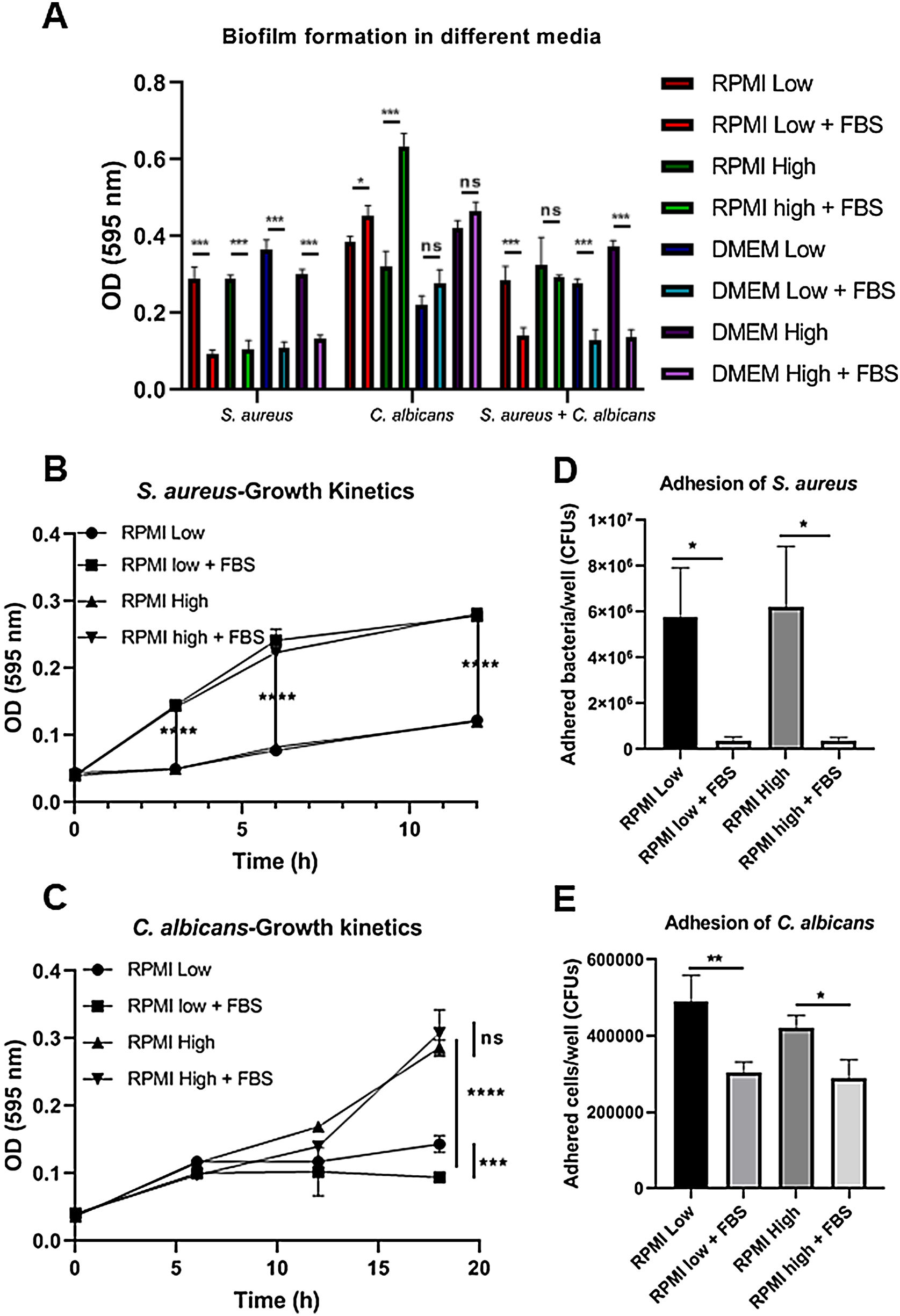
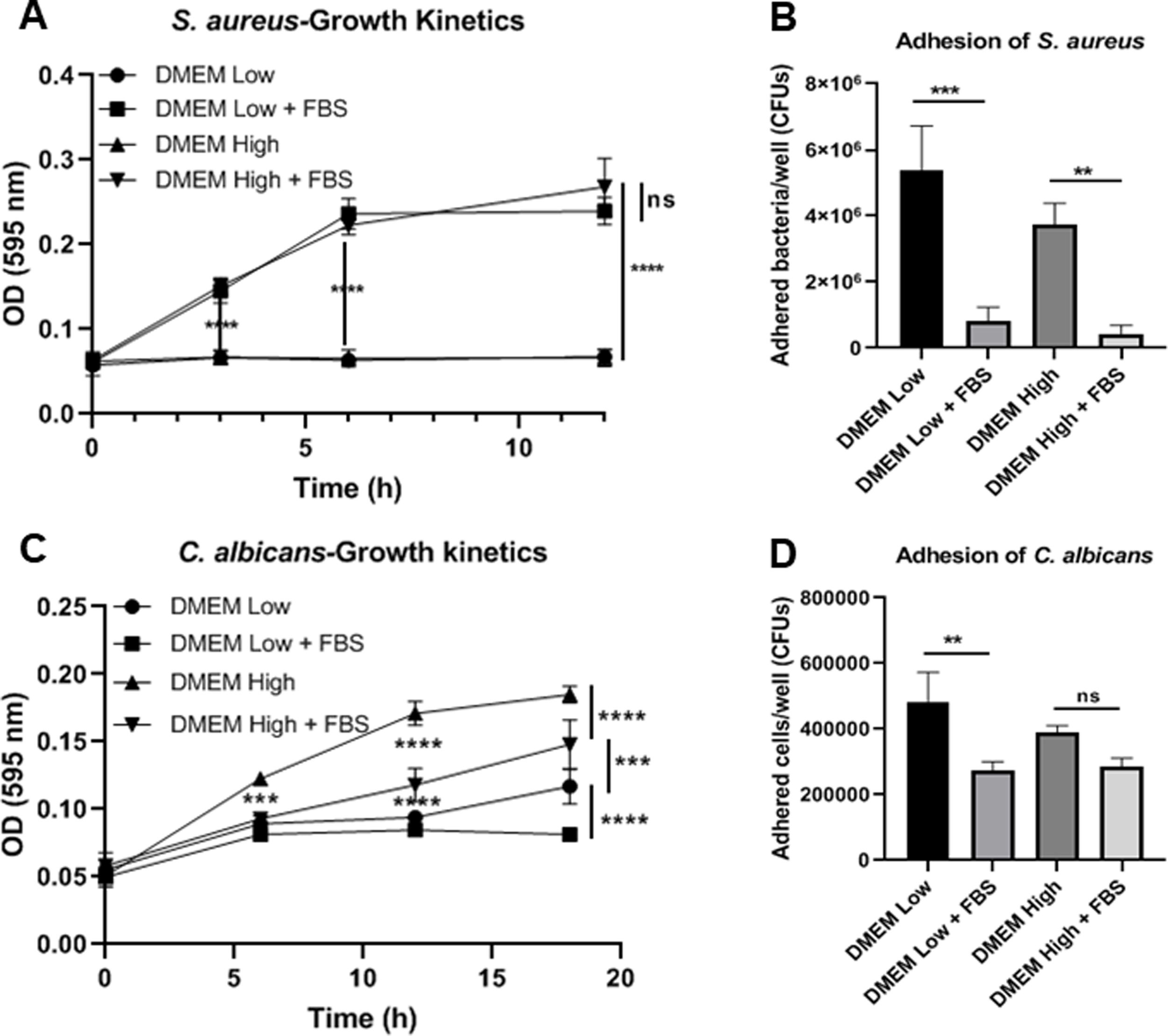
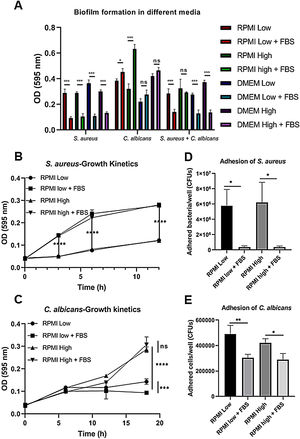
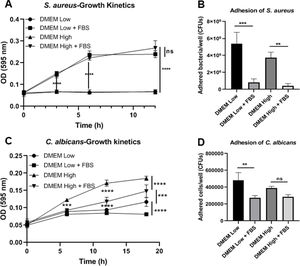
ResultsBiofilm formation by S. aureus and C. albicans in different culture mediaIn order to study the association of S. aureus and C. albicans through biofilm formation, we analyzed the microbial density necessary to establish an in vitro polymicrobial biofilm in 96-well microplates, and the biofilm formed was analyzed with the crystal violet method. A CFU value of 1×106 per well for C. albicans was better than 1×107, whereas for S. aureus concentrations from 1×107 to 1×109 bacteria per well did not result in a significant change (data not shown). Then, the incubation in different culture media supplemented or not with FBS showed that the monomicrobial biofilm density was higher in C. albicans (inoculum of 1×106 CFU per well) than in S. aureus (inoculum of 1×107 CFU per well). The addition of FBS affected both the biofilm formed by S. aureus and the polymicrobial biofilm (Fig. 1A). In the case of C. albicans, biofilm formation was not altered by FBS, but glucose addition increased the biofilm density in DMEM. In RPMI, the presence of glucose and FBS increased considerably the biofilm density.
Biofilm formation in different culture media. (A) Biofilm formation by S. aureus (1×107 bacteria per well) and/or C. albicans (1×106 cells per well) was carried out in 96-well plates using different media supplemented or not with FBS: RPMI low and high glucose concentrations, and DMEM low and high glucose concentrations. (B and C) Growth kinetics analysis for S. aureus and C. albicans in RPMI low and high glucose concentrations, supplemented or not with FBS. Two-way ANOVA with multiple comparisons (Tukey test) was used to compare among treatments (p≤0.05). (D and E) Adhesion of S. aureus or C. albicans in RPMI low and high glucose concentrations, supplemented or not with FBS, was evaluated on 12-well plates. The different media were compared by means of one-way ANOVA with multiple comparisons (Tukey test, p≤0.05). This experiment was repeated twice in triplicate.
Crystal violet assay analyzes only the overall density of the biofilm, considering neither microbial viability nor microbial growth. Using the different media supplemented or not with dextrose and FBS we found that FBS highly promoted the proliferation of S. aureus, while bacterial growth was limited in RPMI or null in DMEM without FBS (Fig. 1B, Suppl. Fig. 1A). For C. albicans, the optimal growth was observed with the addition of dextrose, while the addition of FBS increased slightly that growth at 37°C (Fig. 1C, Suppl. Fig. 1C). Analyzing the in vitro adhesion in 12-well plates, we observed that the addition of FBS affected significantly the adhesion of both microorganisms (Fig. 1D and E, Suppl. Fig. 1B and 1D).
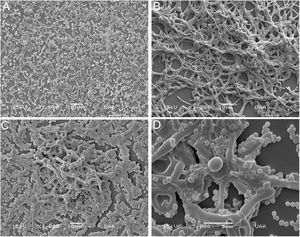
Analysis of biofilm formation on different surfaces with scanning electron microscopyConcerning biofilm formation in Nunc Lab-Tek II chamber slides, we observed that C. albicans formed a more structured biofilm if compared with that of S. aureus (Fig. 2A, B). The polymicrobial biofilm was more complex, with C. albicans forming an outwards scaffold with hyphae and pseudohyphae where S. aureus attached (Fig. 2C, D). It is worth to mention that the adhesion of the biofilms to this surface (Permanox™ plastic slides) was not as good as to the polystyrene surface, since the biofilms were easily detached in the washing step.
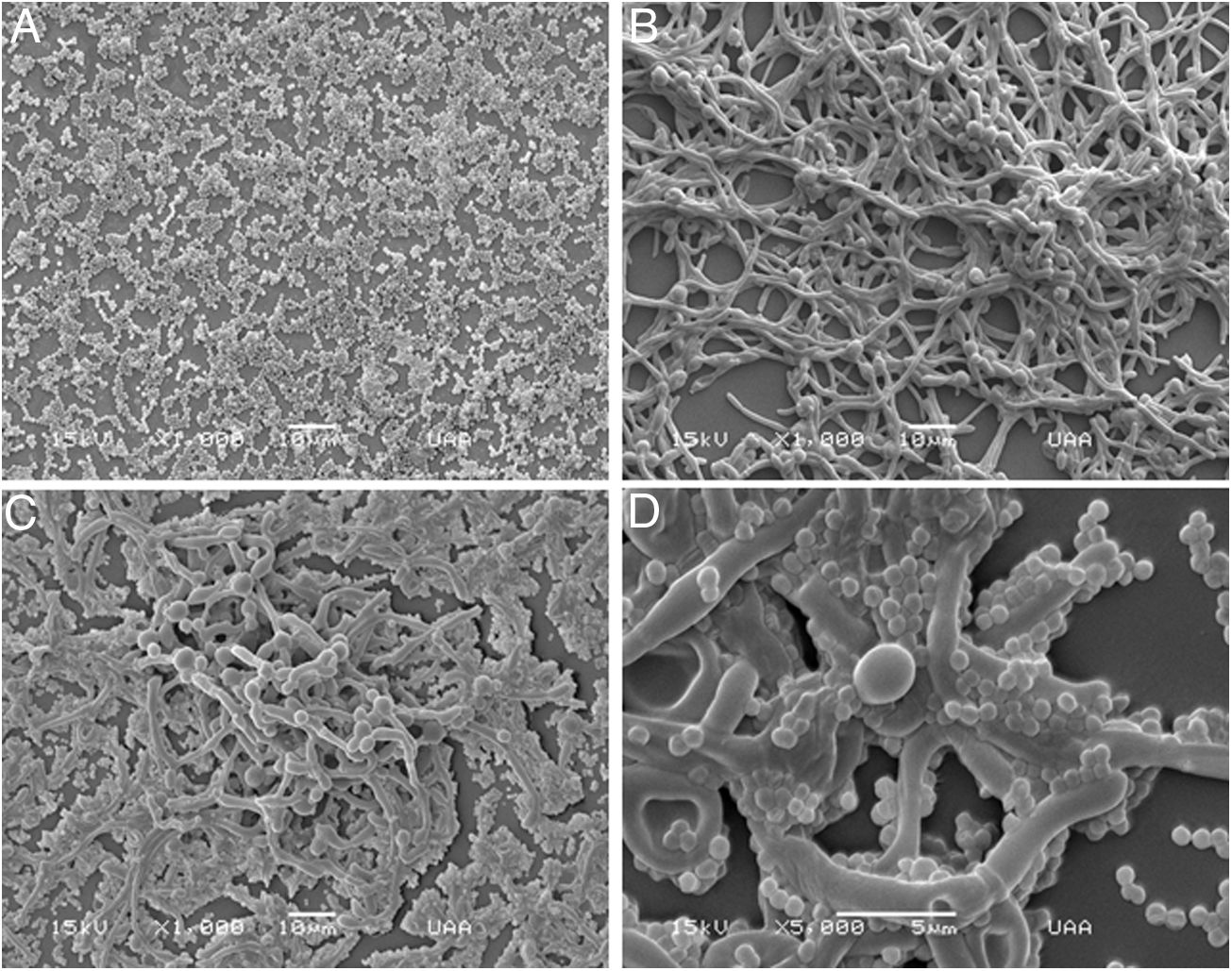
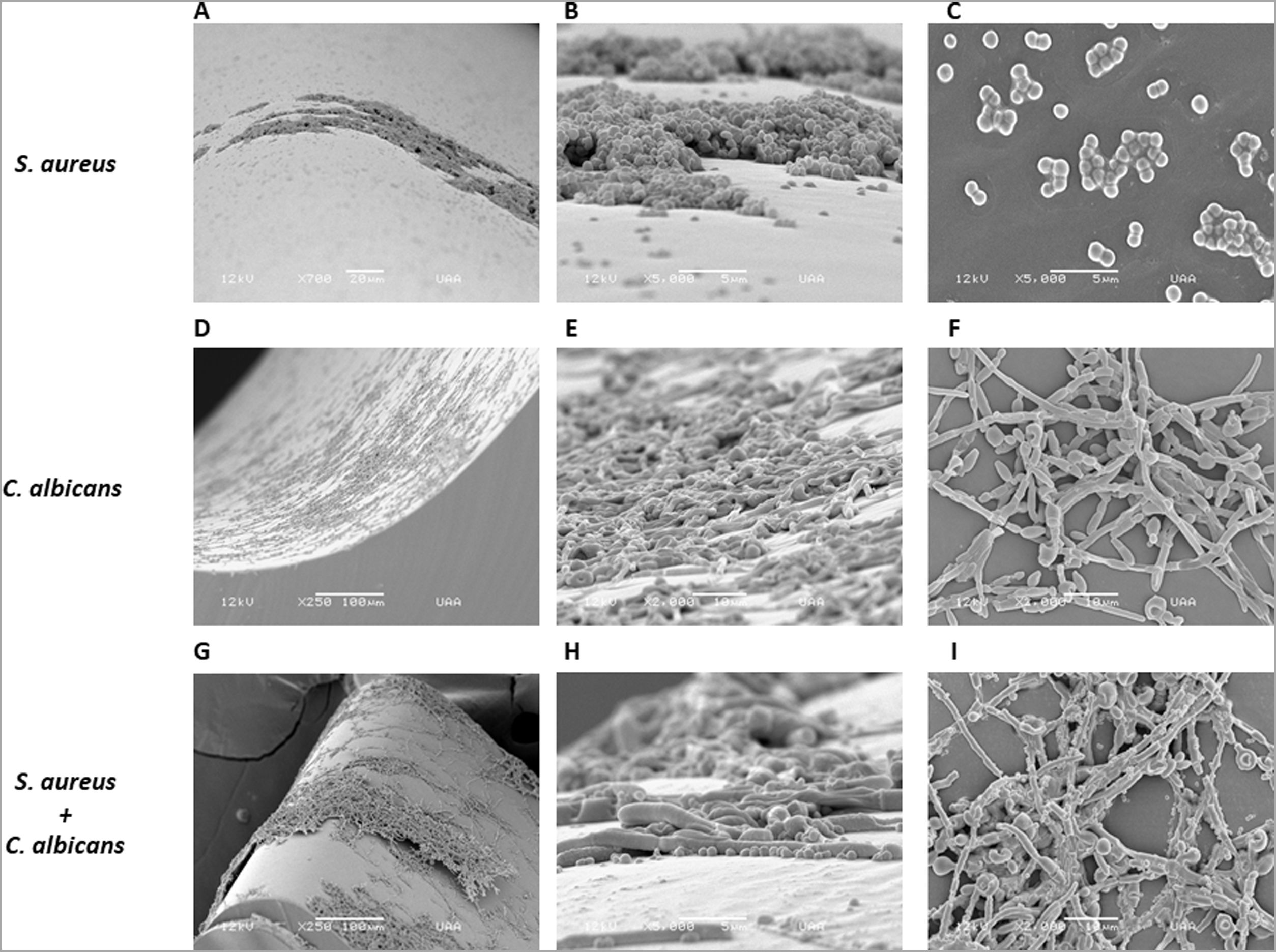
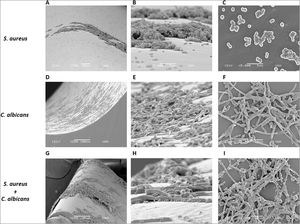
As S. aureus has been isolated along with C. albicans from bloodstream infections, we analyzed the biofilm formation of both microorganisms in a catheter model. We used a rotating mixer to accomplish a more dynamic system simulating a contaminated fluid passing through the catheter. In contrast with the static model in the Lab-Tek system aforementioned, the monomicrobial biofilm formation was visually lower in the catheter model for both microorganisms. However, C. albicans seemed to have better adherence and a more homogeneous biofilm-like structure in comparison with S. aureus, that adhered mainly as individual or grouped cocci, and biofilm-like structures were only observed in very few places throughout the catheter (Fig. 3A–F). On the other hand, the polymicrobial biofilm was more complex and structured, with C. albicans adhered to the internal surface of the catheter and S. aureus attached on the different morphological forms of the fungus (Fig. 3G–I).
Analysis by SEM of the biofilm formed by S. aureus and/or C. albicans in a catheter model. (A, B and C) S. aureus monomicrobial biofilm at 700×, 5000×, and 5000×. (D, E and F) C. albicans monomicrobial biofilm at 250×, 2000×, and 2000×. (G, H and I) C. albicans and S. aureus polymicrobial biofilm at 250×, 5000×, and 2000×. This experiment was repeated twice in triplicate.
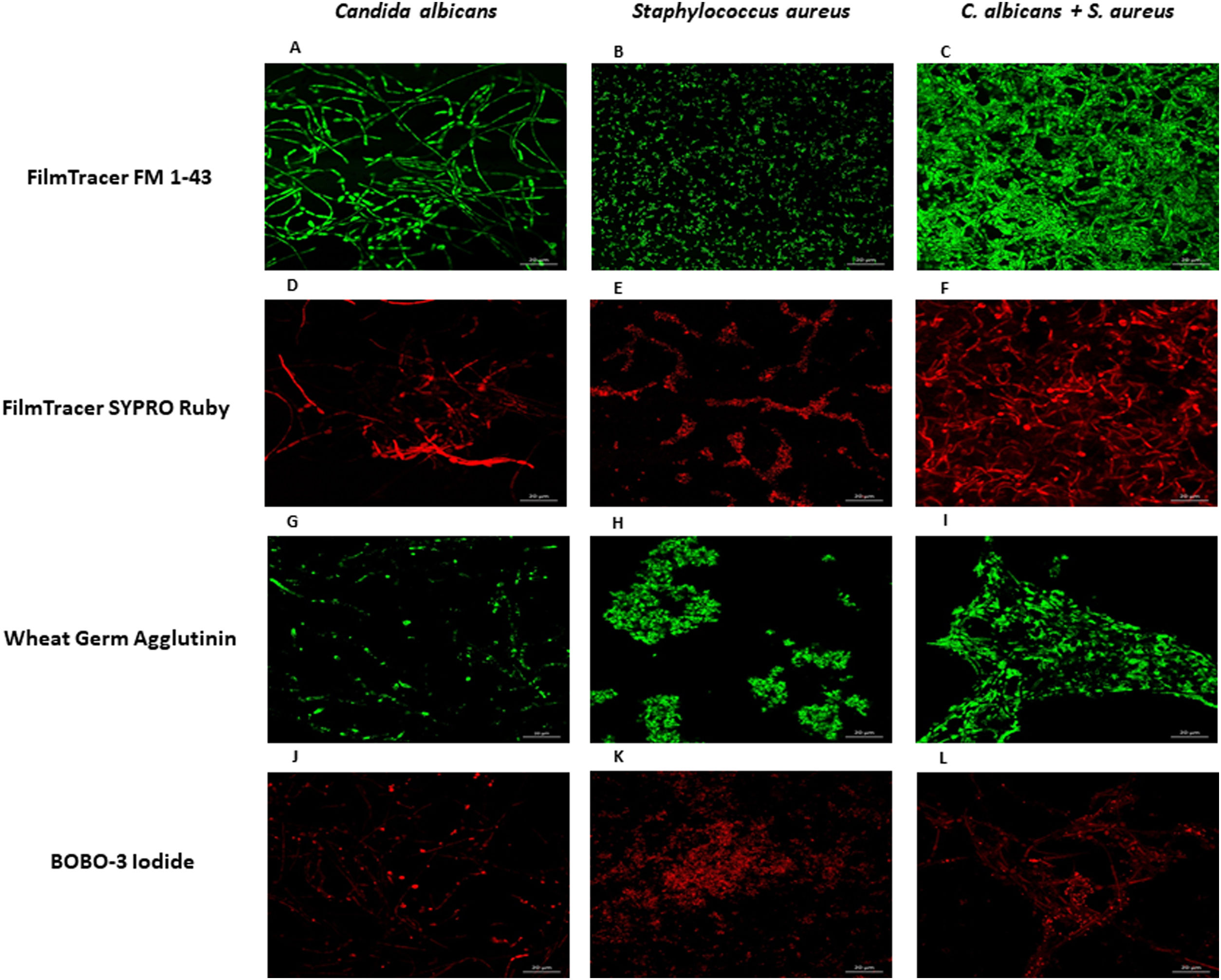
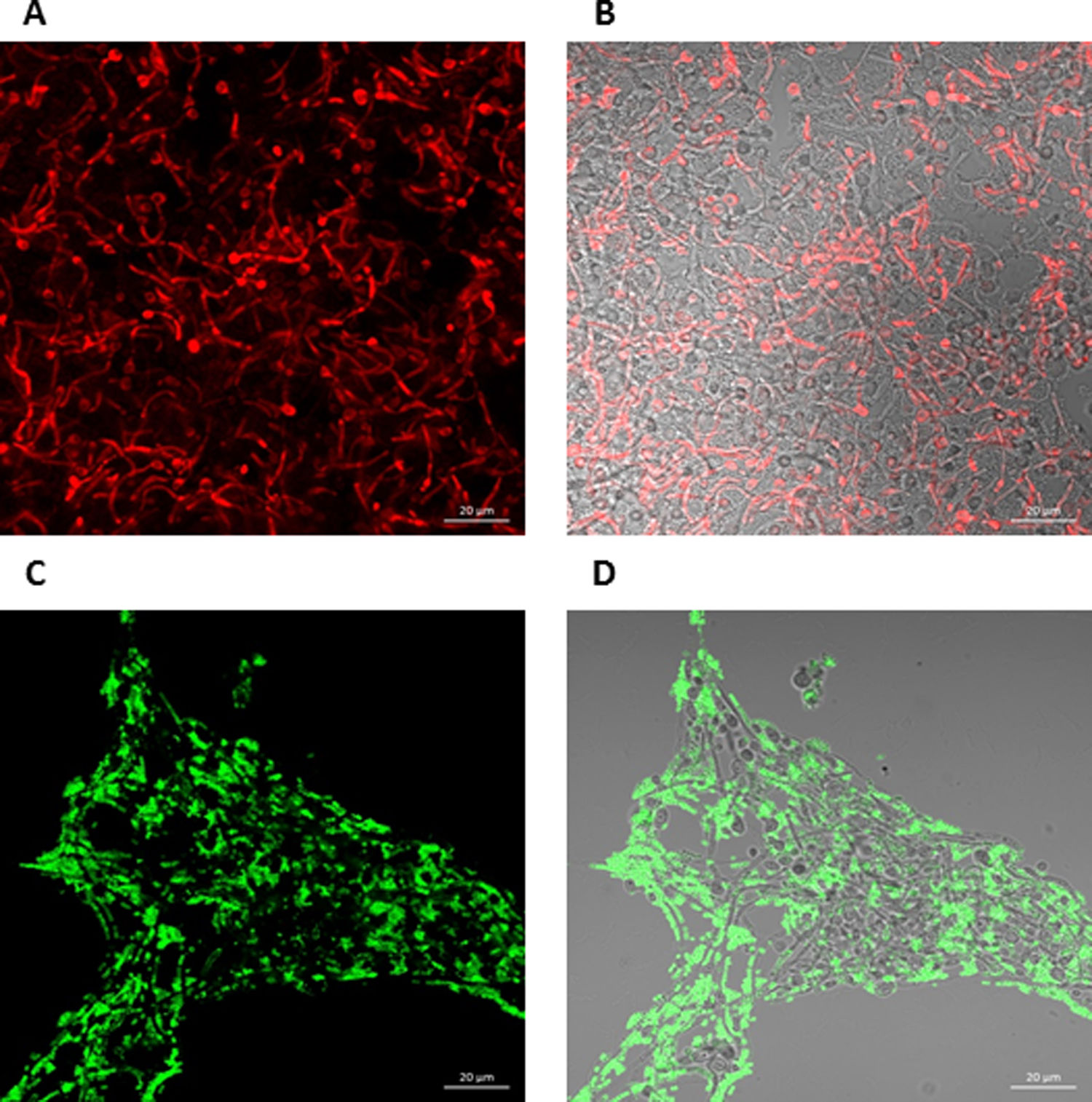
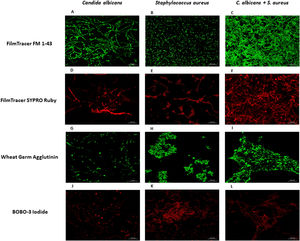
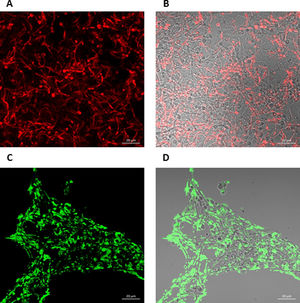
To further analyze the biofilms, both microorganisms were incubated again in Lab-Tek II chamber slides. After the biofilms were formed, the slides were stained with different fluorescent reagents and observed by confocal microscopy. With FilmTracer 1–43, a dye that stains cell membranes, we observed a more complex biofilm when both microorganisms were present, with C. albicans having a better adherence to the slide surface and S. aureus attaching on the hyphal phase of the fungus (Fig. 4A–C). FilmTracer SYPRO Ruby stains most types of proteins; interestingly, unlike the monomicrobial biofilms, in the polymicrobial one the dye stained preferentially C. albicans rather than the bacteria (Fig. 4D–F, Supplemental Fig. 2A, 2B). In contrast, S. aureus was preferentially stained in the polymicrobial biofilm with WGA (Fig. 4G–I, Supplemental Fig. 2C, 2D) and BOBO-3 (Fig. 4J–L), fluorescent reagents that stain glycoproteins and extracellular DNA, respectively.
Analysis by confocal microscopy of the S. aureus and C. albicans mono- and polymicrobial biofilms. Biofilms were stained with the next reagents: (A, B, and C) FilmTracer FM 1-43 Green Biofilm cell stain. (D, E, and F) FilmTracer SYPRO Ruby biofilm matrix stain. (G, H, and I) wheat germ agglutinin, Alexa Fluor 488 Conjugate. (J, K, and L) BOBO-3 Iodide. This experiment was repeated twice in triplicate.
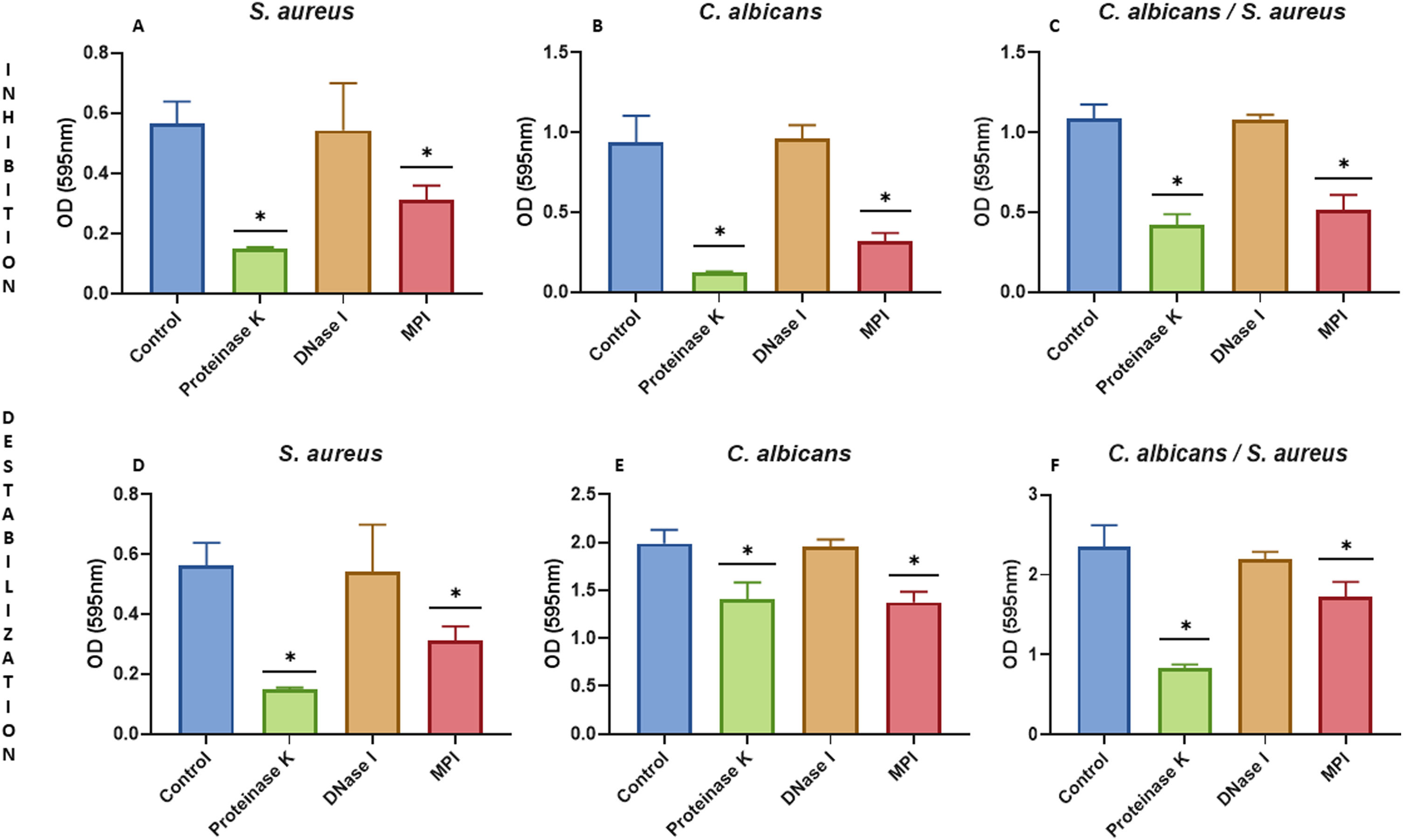
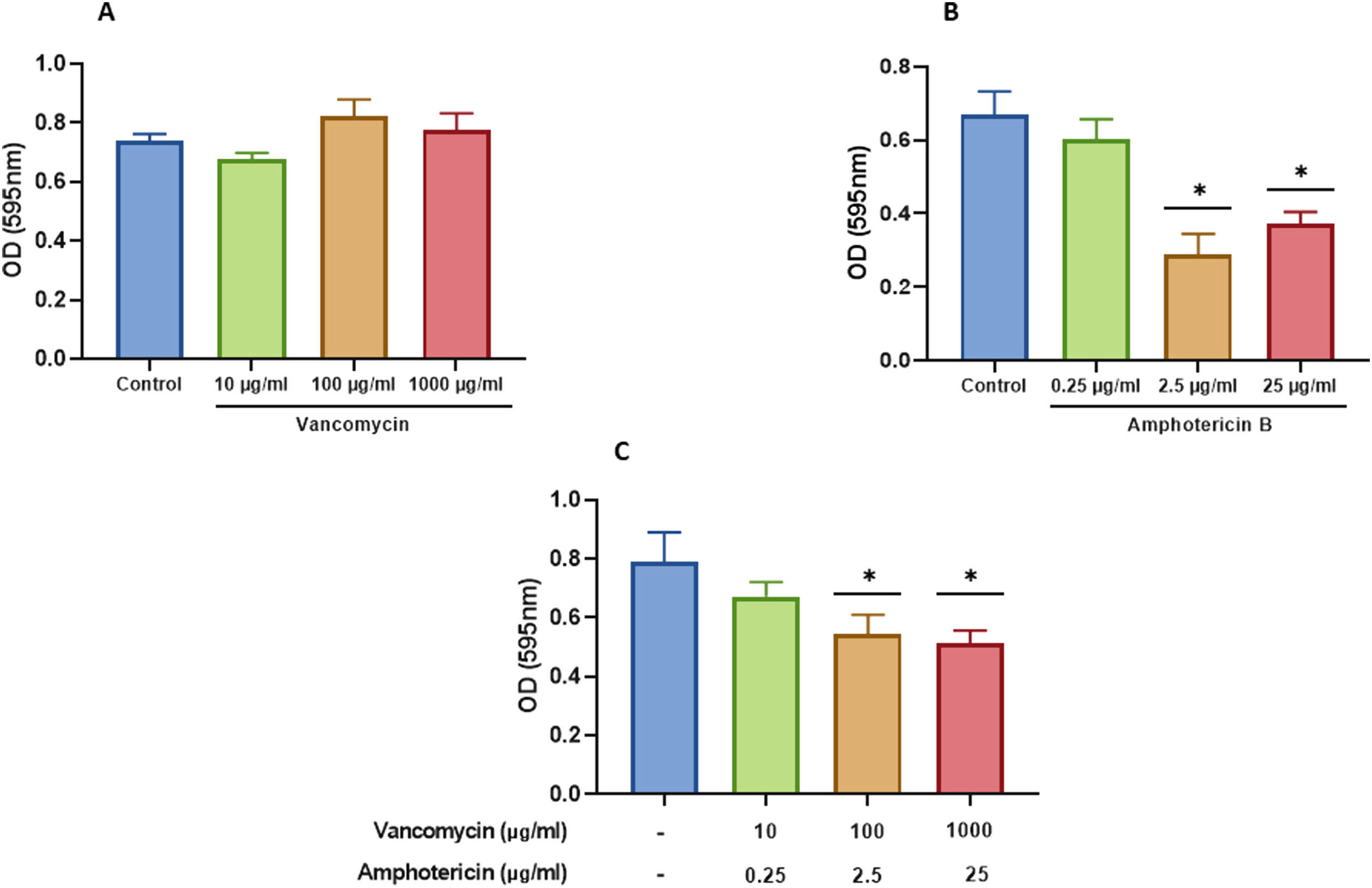
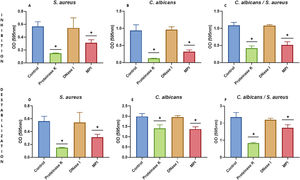
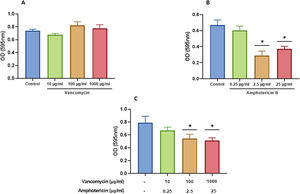
We characterized the biofilms using destabilizing chemical agents and antimicrobials, and found that when the reagents were used in the RPMI-1640 medium during the biofilm formation (24h), proteinase K strongly inhibited biofilm formation of both C. albicans and S. aureus. Sodium (meta)periodate (MPI) resulted in an inhibition of the C. albicans monomicrobial biofilm, but surprisingly had no effect on the biofilm of S. aureus (Fig. 5A–C). Interestingly, DNase I treatment clearly affected the monomicrobial S. aureus biofilm (Fig. 5A), but had no effect on the C. albicans biofilm or the polymicrobial one (Fig. 5B, C). We also found that the polymicrobial biofilm was inhibited by proteinase K and MPI (Fig. 5C). In order to rule out the cytotoxicity of the compounds or the inhibition of the microbial adhesion, we used them for three hours in the pre-formed biofilms. Destabilization of the monomicrobial and polymicrobial biofilms occurred with the treatment of proteinase K and MPI only, while the treatment with DNase I did not lead to biofilm destabilization (Fig. 5D–F). Finally, we tested the stability of the pre-formed polymicrobial biofilm using vancomycin and/or amphotericin B. A destabilization of the biofilm was observed when amphotericin B was present, while the treatment with vancomycin did not result in a clear destabilization of the biofilm (Fig. 6A–C).
Inhibition or destabilization of the mono- and polymicrobial biofilm formed by S. aureus and C. albicans using proteinase K, DNase I, and sodium (meta)periodate. Biofilm formation was inhibited for: (A) S. aureus monomicrobial biofilm, (B) C. albicans monomicrobial biofilm, and (C) S. aureus and C. albicans polymicrobial biofilm. Pre-formed biofilms were destabilized for three hours for: (D) S. aureus biofilm, (E) C. albicans biofilm, and (F) S. aureus and C. albicans polymicrobial biofilm. Dunnett's multiple comparison test (one-way ANOVA) was used to analyze statistical significance (comparison with an untreated control, p≤0.05). This experiment was repeated three times in triplicate.
Destabilization of the S. aureus and C. albicans polymicrobial biofilm with amphotericin B and vancomycin. Polymicrobial biofilms were treated for 24h with: (A) vancomycin at 10, 100, and 1000 μg/ml; (B) amphotericin B at 0.25, 2.5, and 25μg/ml; and (C) amphotericin B and vancomycin at the aforementioned concentrations. Dunnett's multiple comparison test (one-way ANOVA) was used to find statistically significant differences (p≤0.05). This experiment was repeated three times in triplicate.
S. aureus and C. albicans are common microorganisms in several locations of our body that may also be responsible for many hospital-acquired infections. These microorganisms have been co-isolated from several biofilm-associated diseases such as denture stomatitis, periodontitis, burn wound infections, and also from medical devices.1,4,11,13 It is known that S. aureus does not produce biofilms so easily on abiotic surfaces.5 In our study the density of S. aureus biofilm was lower when compared with that of C. albicans biofilm in the different media tested. RPMI medium has been used to study the interaction between S. aureus and C. albicans.23,33 However, there is no information regarding the use of other culture media to study the changes in biofilm formation by these microorganisms. It has also been reported that C. albicans biofilm development is influenced by carbon sources.21 This may have a clinical relevance as the presence of C. albicans was found to be higher in the oral cavity of patients with diabetes in comparison with non-diabetic individuals.26 Also the presence of glucose in relation to C. albicans biofilm development has been analyzed using RPMI medium.12,29
Thus, we tested the biofilm formation capacity of both S. aureus and C. albicans using RPMI and DMEM supplemented or not with glucose and/or FBS. In terms of biofilm formation, the addition of FBS reduced the capacity of biofilm formation in S. aureus and the polymicrobial biofilm in all media. Bacterial adhesion and cell growth, important biological processes involved in biofilm formation, were analyzed. The ability of S. aureus to adhere to the polystyrene wells was severely reduced in the presence of FBS. This may be the reason behind the reduction in biofilm formation, even when FBS highly promoted the in vitro proliferation of the bacteria. We suggest that the adhesion of S. aureus to the wells was altered by the binding to serum proteins such as albumin. It has been shown that the ability of S. aureus to adhere to central venous catheters is drastically reduced in the presence of human blood plasma or serum albumin.10 In blood stream infections plasma proteins could act as an interface, dragging bacterial cells adhered to catheters and releasing them to the bloodstream.
In the case of C. albicans biofilm, even when FBS also decreased the adhesion of C. albicans, biofilm density seemed to be not affected. We found that a concentration of 1×106 cells per well was better than 1×107 cells. Thus, after reaching a certain level of adhesion, cell growth is a key matter for biofilm development. In this regard, glucose addition increased the biofilm density only with DMEM, while with RPMI supplementation with glucose and FBS increased significantly biofilm density. It is worth mentioning that glucose promoted a higher cell growth rate than FBS in C. albicans. Differences in C. albicans biofilm formation in both culture media may come from differences in the content of some chemical compounds. It is known that the development of C. albicans biofilm involves several steps, including adhesion, proliferation, morphological changes, extracellular matrix production, and cell dispersion.17 We need to further characterize the influence of the microbial density and the effect of nutrients over the steps involved in C. albicans biofilm formation.
Polymicrobial biofilm was similar in optical density to the monomicrobial biofilm of C. albicans, and with some media it was slightly lower. However, the analysis with SEM showed a complex polymicrobial biofilm, with C. albicans adhered to the surface of the Lab-Tek II slides and S. aureus adhered preferently to C. albicans. This complexity in the polymicrobial biofilm was also observed in our catheter model, in which C. albicans seemed to have a better capacity to adhere to this abiotic surface. This relation between both microorganisms unveiled by SEM could be analyzed in terms of the mechanisms the bacteria display to adhere to the fungal layer.
Fluorescent staining methods, along with confocal microscopy, enable us to observe that S. aureus attached to the hyphal phase of the fungus. Interestingly, SYPRO Ruby, that stains most classes of proteins, stained preferentially C. albicans while WGA, that stains glycoproteins, stained preferentially S. aureus. It has been shown that the production of an adhesin named polysaccharide intercellular adhesin (PIA) or poly-N-acetylglucosamine (PNAG) encoded in the icaADBC operon by methicillin-sensitive S. aureus (MSSA) is the main mechanism of adhesion and biofilm formation in these bacteria.16,18 We suggest that in the polymicrobial biofilm the adhesion to the slide surface comes mainly from C. albicans, where proteins play a major role on this process, while the contribution of glycoproteins in the biofilm formation could be more important for S. aureus. Of further interest is the role of the adhesin PIA of S. aureus in the adhesion to the hyphal form of C. albicans.
The composition of C. albicans biofilm in the dry weight extracellular matrix includes proteins (55%), carbohydrates (25%), lipids (15%), and extracellular DNA, as well as mannans, the most abundant polysaccharides, associated with linear β-1,6 glucans.24 Thus, it is not surprising that proteinase K and MPI, a reagent used to oxidize hydroxyl groups of polymeric hexosamines such as those of the PNAG, inhibit the monomicrobial and polymicrobial biofilms of C. albicans. However, in the case of S. aureus, MPI did not inhibit the monomicrobial biofilm. As PIA (PNAG) has been proposed as the main mechanism of adhesion in MSSA, we suggest that S. aureus may use a different adhesion mechanism when MPI is present in the RPMI medium; in this respect, other mechanisms of biofilm formation in MSSA have been described.16 The stability of the C. albicans preformed biofilm was also greater than that of S. aureus biofilm. This result was expected as the biofilm of C. albicans showed a much higher optical density in the crystal violet assay; however, preformed biofilms were also affected by proteinase K and MPI. Thus, proteins and β-1,6-linked polysaccharides seem to have a prominent role in the biofilm structure.
Finally, destabilization of the polymicrobial biofilm was only evident when using amphotericin B, while vancomycin had no effect. Once again, we suggest that C. albicans contributes the most to the final structure and stability of the polymicrobial biofilm.
ConclusionsWe characterized the in vitro polymicrobial biofilm of C. albicans and S. aureus as they have been found together in biofilm-related infections. We found interesting the fact that FBS affects the adhesion of both microorganisms, as well as S. aureus biofilm formation and the polymicrobial biofilm formation. We suggest that plasma proteins, such as albumin and fibrinogen, may act as an interface, binding to biofilm-derived microorganisms in colonized catheters. C. albicans contributed the most to the whole density of the biofilm, in which β-1,6-linked polysaccharides seem to be important components. Finally, C. albicans adhered preferentially to all the surfaces tested, while S. aureus adhered mainly to the surface of C. albicans. The requirement of nutrients that may be involved in all the steps of biofilm formation, such as adhesion, cell growth, extracellular matrix formation, and dispersion, should be analyzed in the future.
FundingThe Autonomous University of Aguascalientes through the internal project program with code number PIB 21-5 supported this work.
Conflict of interestThe authors declare that they have no conflict of interest.