Superoxide dismutase (SOD) is an enzyme that ensures detoxification against oxidative stress in extracellular components. We aimed to evaluate the impact of SOD3G362A polymorphism (rs 2536512) on the impairment of seminal SOD activity and its risk for idiopathic male infertility in Algeria, as well as to investigate the association between sperm DNA integrity, standard semen parameters, and seminal SOD activity.
MethodsIn this case–control study, we included 111 infertile men with idiopathic infertility and 104 fertile controls from Algeria. Semen analyzing was done according to the World Health Organization manual. Seminal SOD activity was measured using a commercially colorimetric method (Randox Laboratories Ltd., UK). DNA fragmentation was evaluated using the Halosperm kit (Halotech DNA S.L, Spain) and SOD3G362A genotyping was assessed by polymerase chain reaction-restriction length fragment polymorphism (PCR-RFLP).
ResultsSeminal SOD activity was significantly lower in the infertile group than in the control group (85.87±40.11 vs 154.24±48.456U/mL, p<0.0001), it also decreased in all infertile subgroups. We detected positive correlations between SOD activity and semen parameters (concentration, mobility, vitality, and morphology) (p≤0.05). There was no association between the risk for male infertility and DNA integrity (p>0.05) and SOD3G362A (OR=0.826, 95%CI: 0.439–1.55, p=0.554). For GA vs GG and (OR=0.639, 95% CI: 0.305–1.340, p=0.235) for AA vs GG.
ConclusionsSeminal SOD evaluation can be a beneficial indicator for sperm quality and risk for idiopathic male infertility in Algeria, while sperm DNA integrity, as well as SOD3G362A genotypes, are not.
La superóxido dismutasa (SOD) es una enzima que asegura la desintoxicación contra el estrés oxidativo en los componentes extracelulares. El objetivo fue evaluar el impacto del polimorfismo SOD3G362A (rs 2536512) en el deterioro de la actividad de la SOD seminal y su riesgo de infertilidad masculina idiopática en Argelia, así como investigar la asociación entre la integridad del ADN del esperma, los parámetros estándar del semen y la actividad seminal de la SOD seminal.
MétodosEn este estudio de casos y controles, se incluyeron 111 varones con infertilidad idiopática y 104 controles fértiles de Argelia. El análisis del semen se realizó de acuerdo con el manual de la Organización Mundial de la Salud. La actividad seminal de la SOD se midió utilizando un método colorimétrico comercial (Randox Laboratories Ltd, Reino Unido). La fragmentación del ADN se evaluó utilizando el kit Halosperm® (Halotech DNA SL, España) y la genotipificación de SOD3G362A se evaluó mediante polimorfismo del fragmento de longitud de la restricción de la reacción en cadena de la polimerasa (PCR-RFLP).
ResultadosLa actividad seminal de la SOD fue significativamente menor en el grupo infértil que en el grupo de control (85,87±40,11 versus 154,24±48,456U/ml; p<0,0001), y también se redujo en todos los subgrupos infértiles. Detectamos correlaciones positivas entre la actividad de la SOD y los parámetros del semen (concentración, movilidad, vitalidad y morfología) (p≤0,05). No hubo asociación entre el riesgo de infertilidad masculina y la integridad del ADN (p>0,05) o el polimorfismo SOD3G362A (OR=0,826; IC del 95%: 0,439-1,55; p=0,554) para GA versus GG y (OR=0,639; IC del 95%: 0,305-1,34; p=0,235) para AA versus GG.
ConclusionesLa evaluación de la SOD en plasma seminal puede ser un indicador beneficioso para la calidad del esperma y el riesgo de infertilidad masculina idiopática en Argelia. La integridad del ADN del esperma, así como los genotipos SOD3G362A, no lo son.
The generation of reactive oxygen species (ROS) in the male reproductive tract has become a real concern because of their potential toxic effects at high levels on sperm quality and function.1 Spermatozoa are particularly susceptible to ROS induced damages for several reasons related to the cell itself or to environmental factors such as; the lack of DNA repair mechanisms in sperm cells, the high levels of polyunsaturated fatty acids (PUFAs) contained in spermatozoa membrane, the ability of Spermatozoa to produce ROS, the very low levels of cytoplasmic antioxidant enzymes in Sperm and the fact that Sperm spend long periods as isolated cells in both male and female genital tracts.2,3
Superoxide dismutase (SOD) is an antioxidant enzyme that catalyzes the conversion of superoxide into oxygen and hydrogen peroxide; it protects the organism by scavenging both extracellular and intracellular superoxide anion and prevents lipid peroxidation of the plasma membrane.4 SOD includes three distinct mammalian isoforms: copper-and-zinc-containing superoxide dismutase (CuZn-SOD: SOD1), manganese superoxide dismutase (Mn-SOD: SOD2), and extracellular superoxide dismutase (EC-SOD: SOD3).5 SOD3 is the only antioxidant enzyme that scavenges superoxide radicals in the extracellular space and must be conjugated with Catalase (CAT) or Glutathione peroxidase (GPX) to exercise its function.6 In a previous study, we investigated the relation between CAT levels in seminal plasma, CAT-262C/T genotypes, and the risk for male infertility, the study has revealed that CAT plays an essential role in maintaining normal sperm parameters, and clearly showed the deleterious effect of CAT-262T allele on seminal CAT activity.7
The present study is placing a special emphasis on EC-SOD, which accounts or 25% of enzymatic antioxidants in human seminal plasma,8 because of its importance as the only antioxidant that converts superoxide radicals in seminal fluid, to which spermatozoa are particularly reliant for protection.9 The purpose of this work was to evaluate the extracellular activity of SOD in infertile men and fertile controls and to examine the relation between seminal SOD activity and standard semen parameters as well as the DNA fragmentation percentage. As it is known, single nucleotide polymorphisms (SNPs) account for a significant proportion of observed genetic mutations and might alter the expression levels and functions of the affected genes. The most known SOD3 SNPs are: rs 2536512, rs 1799895 rs 699473.10 rs 2536512 polymorphism or SOD3G362A, results in a threonine-to-alanine conversion that replaces a polar hydrophilic amino acid with an aliphatic hydrophobic amino acid at position 58 of the SOD3 protein, eliminating a PKC delta phosphorylation motif and is considered to be essential for tetramerization.11 However, little is known with regard to the effect of SOD3G362A on SOD activity. Thus, this SNP was further genotyped to identify whether there was an association between SOD3G362A genotypes and seminal SOD activity and also the risk for unexplained male infertility in Algerian population.
Material and methodsSubjects, semen collection and processingA total of 170 men suffering from male infertility and 104 fertile controls were volunteered and recruited from the University Hospital of Batna and private laboratories from the same city in the Eastern part of Algeria. A detailed medical history and examination were performed for each participant. Also detailed questionnaires were given to eliminate any female, lifestyle, or family history contribution in these cases.
By making very rigorous inclusion criteria, we avoided any possible causes affecting sperm quality and seminal antioxidant status. These criteria included age and BMI matched cases and controls, potentially sufficient size of the studied samples, which came from the same homogeneous population that originated from the same geographic region, and finally by matching the abstinence time to three days for all the included men. We excluded from the study infertile men who had recognizable causes of male infertility; cancer (n=9), cryptorchidism (n=11), Klinefelter's syndrome (n=3), Y chromosome microdeletions (n=1), obstructive azoospermia (n=9), sperm agglutination (n=4), genitourinary infections and leukocytospermia (n=12), altered hormonal status (n=10). After exclusion of these cases, 111 men with idiopathic infertility remained for the study. All the included controls were healthy, presented normal semen parameters, and have had recently fathered a healthy child without any complications. None of the participants was on any medication or under any kind of antioxidant supplementation that may affect sperm parameters and SOD activity.
Semen samples were collected in sterile containers by masturbation, after 3 days of sexual abstinence. Evaluation of semen parameters was performed after liquefaction for 30min at 37°C, the samples were then centrifuged at 3500rpm to separate the seminal plasma which was liquated and stored at −80°C till use. Semen sampling and quality analyzing were done according to the World Health Organization manual.12
The study was approved by the institutional scientific and review board of Batna 2 University: 2012/BIO/LMD 3/096. Informed written consent was obtained from each participant, the protocols and procedures used in this research were in compliance with the Declaration of Helsinki.
DNA integrity evaluationDNA integrity was evaluated using Halosperm kit (Halotech Dna S.L, Spain 2/2008.13.06) for DNA fragmentation assay according to the instructions of the manufacturer.
SOD activity measurementSOD activity was measured using a commercially available colorimetric method (Randox Laboratories Ltd, UK SD 125). This method employs xanthine and xanthine oxidase to generate superoxide radicals which reacts with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazoliumchloride (I.N.T.) to form red formazan dye. The SOD activity is then measured by the degree of inhibition of this reaction using calibration curve of inhibition percentage for each standard against Log10 of standards and SOD activity was expressed as U/mL.
Blood samples and DNA extractionFive mL of venous blood were collected in EDTA tube from each of the participated subjects, then immediately separated in aliquots and stored at −80°C until use. Genomic DNA was extracted from frozen blood according to the manufacturer's instructions using the “PureLink Genomic DNA Mini Kit” Thermo Fischer Scientific (Waltham, MA, USA, K1820-02). DNA concentration and purity were measured before storing the DNA at 4°C until molecular analysis.
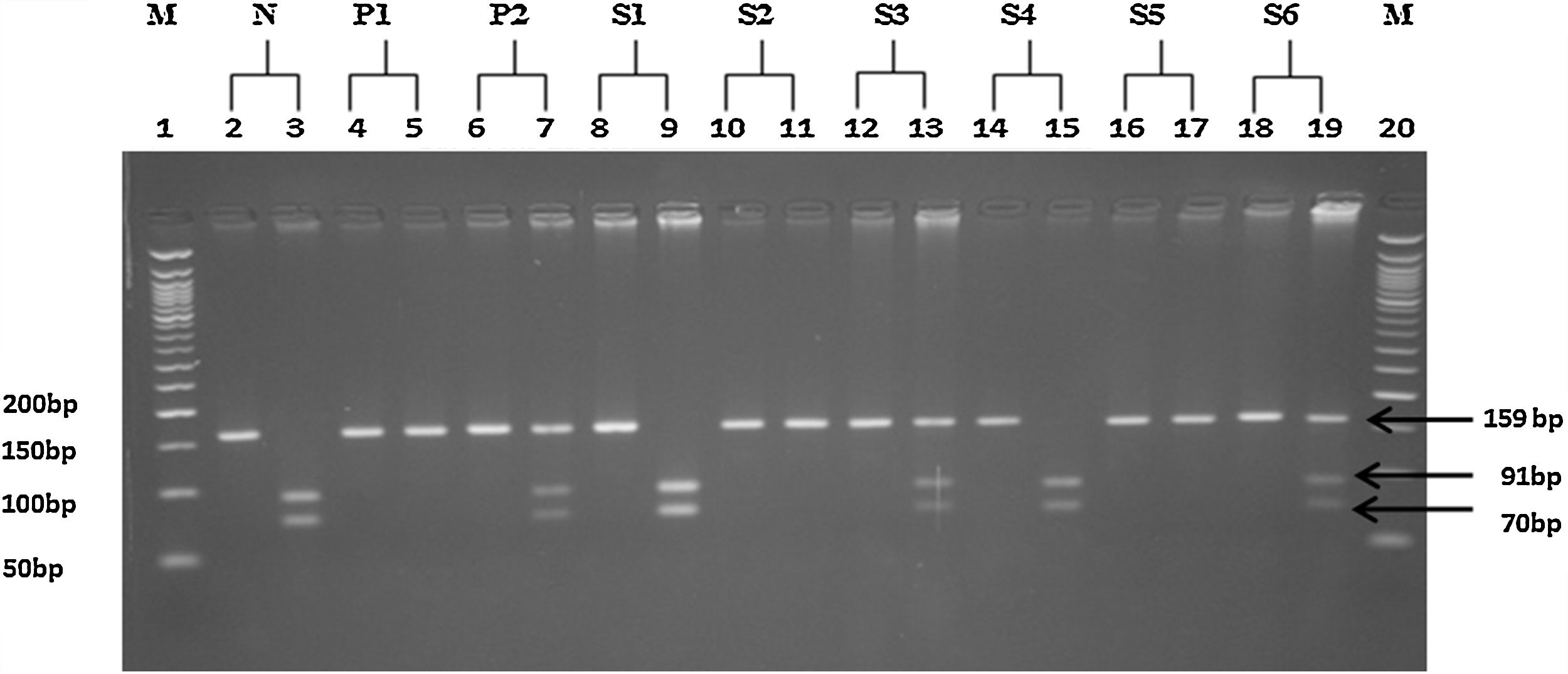
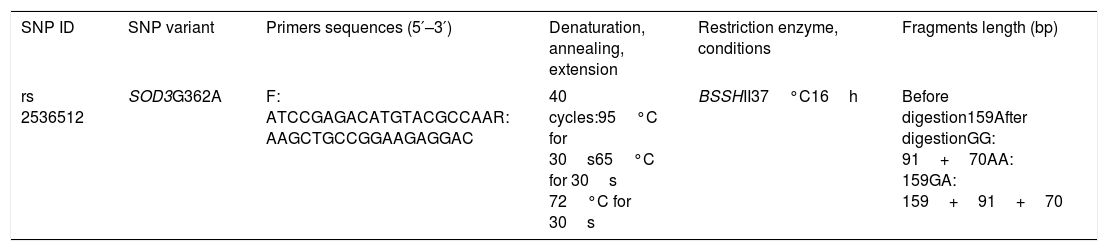
Genotyping of SOD3G362ASOD3G362A genotypes were determined by PCR amplification and subsequent digestion with BSSHII restriction enzyme (New England Biolabs Ltd., Hitchin, UK) (Fig. 1). Table 1 summarizes the genotyping conditions and requirements, which includes the primers used for DNA amplification (Gene link, Hawthorne, NY, USA), PCR conditions and size of obtained products, restriction conditions and the sizes of the digested DNA fragments.
Gel image showing PCR-RFLP representative 3% agarose gel of the examined SOD3G362A polymorphism. Lanes 1 and 20: 50bp DNA ladder. N: negative control; P1: homozygous positive control; P2: heterozygous positive control. S1 through S6 represent results from this study, where the left lane of each sample is the undigested PCR product, while the right lanes are the digested products of the respective samples.
Primers and conditions used for genotyping analysis of SOD3G362A.
| SNP ID | SNP variant | Primers sequences (5′–3′) | Denaturation, annealing, extension | Restriction enzyme, conditions | Fragments length (bp) |
|---|---|---|---|---|---|
| rs 2536512 | SOD3G362A | F: ATCCGAGACATGTACGCCAAR: AAGCTGCCGGAAGAGGAC | 40 cycles:95°C for 30s65°C for 30s 72°C for 30s | BSSHII37°C16h | Before digestion159After digestionGG: 91+70AA: 159GA: 159+91+70 |
SNP: single nucleotide polymorphism.
For amplification, 25μL reactions were used containing 40ng of genomic DNA, 0.16μM of each primer and 1× PCR master mix (Promega, San Luis Obispo, CA, USA). Restriction reactions were performed according to the manufacturer instructions (New England Biolabs Ltd., Hitchin, UK). Amplified and restricted DNA fragments were resolved by electrophoresis on 3% high resolution agarose and visualized under UV light after staining with ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA). This set of primers and BSSHII digestion enzyme were designed and used for the first time in the study of SOD3G362A polymorphism.
Statistical analysisStatistical analyses were performed with IBM SPSS statistics 20 for Windows (SPSS Inc., Chicago, IL, USA). Differences between the means of the two continuous variables were evaluated by the Student's t-test. Pearson correlation was used to search for possible associations between the different semen parameters, SOD activity and DNA fragmentation percentage. Chi-square χ2 test was used for categorical variables and to evaluate the genotype distribution and allele frequencies of the studied polymorphisms, and whether individual variants were in the Hardy–Weinberg equilibrium (HWE). The association between male infertility and the studied genotypes was estimated based on an odds ratio (OR) and a 95% confidence interval (CI) using a multivariate logistic regression analysis. The difference in seminal SOD activities between the three genotypes carriers was analyzed by one-way ANOVA and Tukey post hoc. p-Value of <0.05 was considered statistically significant.
ResultsIn the present case–control study, we evaluated for the first time, seminal SOD activity of age matched 111 infertile men (cases) and 104 healthy fertile controls from Batna city in the eastern mountainous part of Algeria, and its association with semen parameters and DNA fragmentation. We studied as well the possible effect of SOD3G362A polymorphism on this enzyme activity and male factor infertility. The main strength of this work is the consistent, precise and reliable data that highlights the significance of the coming results. It was based on very rigorous inclusion criteria to eliminate the implication of any possible factors in the pathological conditions leading to male infertility or to the variations in the enzymatic activity of SOD.
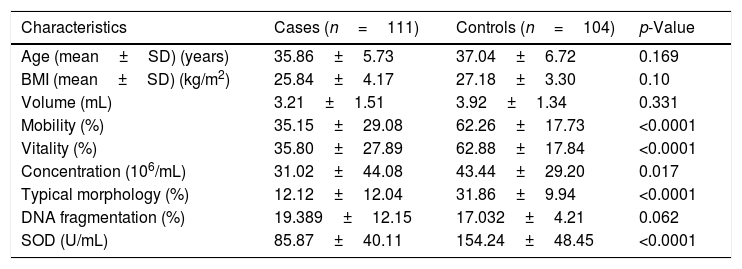
Table 2 shows demographic characteristics, semen parameters, DNA fragmentation and seminal SOD activity of cases and controls. There were nonsignificant differences between cases and controls for age and body mass index (BMI). Regarding semen parameters, all the tested criteria were significantly different between the two studied groups. The percentage of spermatozoa with fragmented DNA in infertile men compared to controls was nonsignificant. The comparison of seminal SOD activity between infertile men and fertile controls showed highly significant differences between the two groups (p<0.0001).
Demographic characteristics, semen parameters and seminal SOD activity.
| Characteristics | Cases (n=111) | Controls (n=104) | p-Value |
|---|---|---|---|
| Age (mean±SD) (years) | 35.86±5.73 | 37.04±6.72 | 0.169 |
| BMI (mean±SD) (kg/m2) | 25.84±4.17 | 27.18±3.30 | 0.10 |
| Volume (mL) | 3.21±1.51 | 3.92±1.34 | 0.331 |
| Mobility (%) | 35.15±29.08 | 62.26±17.73 | <0.0001 |
| Vitality (%) | 35.80±27.89 | 62.88±17.84 | <0.0001 |
| Concentration (106/mL) | 31.02±44.08 | 43.44±29.20 | 0.017 |
| Typical morphology (%) | 12.12±12.04 | 31.86±9.94 | <0.0001 |
| DNA fragmentation (%) | 19.389±12.15 | 17.032±4.21 | 0.062 |
| SOD (U/mL) | 85.87±40.11 | 154.24±48.45 | <0.0001 |
p-Value significant when ≤0.05.
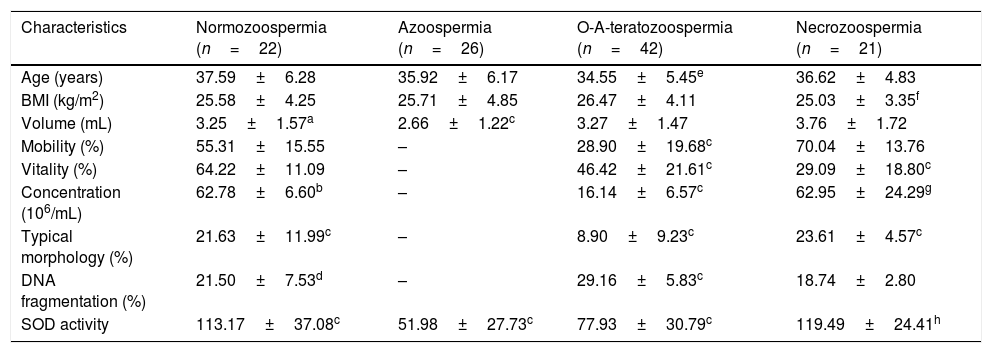
After classification of infertile cases according to semen values, four subgroups were obtained: normozoospermia (n=23), azoospermia (n=26), oligo-astheno-teratozoospermia (n=42) and necrozoospermia (n=21). Table 3 shows that the decrease in SOD was a general trend in our population, a significant low SOD activity was observed in all subgroups including normozoospermia.
Demographic criteria, semen characteristics, DNA integrity and SOD activity in infertile men subgroups and results of their comparison with fertile controls.
| Characteristics | Normozoospermia (n=22) | Azoospermia (n=26) | O-A-teratozoospermia (n=42) | Necrozoospermia (n=21) |
|---|---|---|---|---|
| Age (years) | 37.59±6.28 | 35.92±6.17 | 34.55±5.45e | 36.62±4.83 |
| BMI (kg/m2) | 25.58±4.25 | 25.71±4.85 | 26.47±4.11 | 25.03±3.35f |
| Volume (mL) | 3.25±1.57a | 2.66±1.22c | 3.27±1.47 | 3.76±1.72 |
| Mobility (%) | 55.31±15.55 | – | 28.90±19.68c | 70.04±13.76 |
| Vitality (%) | 64.22±11.09 | – | 46.42±21.61c | 29.09±18.80c |
| Concentration (106/mL) | 62.78±6.60b | – | 16.14±6.57c | 62.95±24.29g |
| Typical morphology (%) | 21.63±11.99c | – | 8.90±9.23c | 23.61±4.57c |
| DNA fragmentation (%) | 21.50±7.53d | – | 29.16±5.83c | 18.74±2.80 |
| SOD activity | 113.17±37.08c | 51.98±27.73c | 77.93±30.79c | 119.49±24.41h |
p-Value significant when ≤0.05.
The study of correlations shows that seminal SOD activity in the infertile group was positively correlated with sperm concentration (p<0.0001, r=0.478), vitality (p<0.0001, r=0.444), mobility (p<0.0001, r=0.557), and percentage of typical morphology (p<0.0001, r=0.578). However, sperm DNA fragmentation was not correlated with this enzyme activity (p=0.334, r=0.093) or with any of the tested semen parameters (p>0.05).
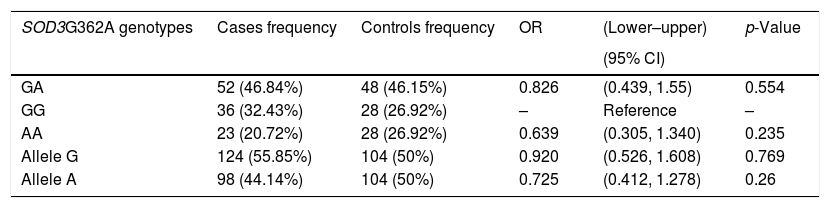
Allelic frequencies were in Hardy–Weinberg equilibrium. The genotypes and alleles distribution with the odd ratios (ORs) for the infertile males (cases) and controls are summarized in Table 4, the frequencies of both alleles and the different genotypes in cases and the controls were statistically not different (p>0.05). There was no significant association between genotypes (GA and GG) and risk for male infertility among infertile males subjects (For GA vs GG: OR=0.826, 95%CI: 0.439–1.55, p=0.554; For AA vs GG: OR=0.639, 95% CI: 0.305–1.340, p=0.235).
Genotypes and allele frequencies of SOD3G362A polymorphism in the studied population.
| SOD3G362A genotypes | Cases frequency | Controls frequency | OR | (Lower–upper) | p-Value |
|---|---|---|---|---|---|
| (95% CI) | |||||
| GA | 52 (46.84%) | 48 (46.15%) | 0.826 | (0.439, 1.55) | 0.554 |
| GG | 36 (32.43%) | 28 (26.92%) | – | Reference | – |
| AA | 23 (20.72%) | 28 (26.92%) | 0.639 | (0.305, 1.340) | 0.235 |
| Allele G | 124 (55.85%) | 104 (50%) | 0.920 | (0.526, 1.608) | 0.769 |
| Allele A | 98 (44.14%) | 104 (50%) | 0.725 | (0.412, 1.278) | 0.26 |
p-Value significant when ≤0.05.
Then, we examined the variation in seminal SOD activity between the carriers of different SOD3G362A genotypes, we found that it was slightly different, but this difference did not reach a statistically significant level neither in the infertile group (GG: 88.715±39.408U/mL, GA: 84.471±37.893U/mL, AA: 87.824±45.051U/mL, p=0.875) nor in the controls (GG: 155.164±46.557U/mL, GA: 154.880±50.556U/mL, AA: 152.227±48.318U/mL, p=0.968).
DiscussionOur results showed that SOD activity in seminal plasma was decreased in infertile men compared to fertile controls which support a lot of researchers’ findings.13–16 However, few authors did not report such a difference.17–19 In addition, we found that SOD activity was positively correlated with the studied semen parameters, which is in agreement with the study of Macanovic et al.20 and merges the results mentioned in Table 3, where all subgroups of infertile men presenting different sperm abnormalities had a lower seminal SOD activity compared to controls with regular sperm parameters. The important seminal SOD activity in the control group, the positive correlations observed between this enzyme and sperm quality, and the results of SOD according to subgroups show the ability of this antioxidant to protect spermatozoa from free radicals, precisely O2≷−, and its important role in preserving their fertilizing potential.
Male infertility could be attributed to either defective sperm function or abnormal sperm morphology. Since, on one hand, human spermatozoa are rich in polyunsaturated fatty acids and therefore are susceptible to free radicals attack mediated by membrane lipid peroxidation, and on the other SOD is one of the major enzymes of the defense system which scavenges the ROS and prevents peroxidation of PUFAs in cytoplasmic membrane,16 it seems that the decrease of SOD activity in seminal plasma of infertile men can be in fact the cause for the reduced Sperm function and the damaged morphology of spermatozoa; which may explain the idiopathic male infertility cases.
As we have shown in a previous study,7 seminal catalase activity was significantly decreased in all the infertile groups compared to controls, we reported as well significant correlations between standard semen criteria and this enzyme activity. Putting together the earlier results with the present findings, we can conclude a firm relationship between the low enzymatic activity and impaired semen parameters leading to male infertility in our population, especially that SOD and CAT act coordinately to eliminate hydrogen peroxide (H2O2) from seminal plasma.
In this study, DNA damage as measured by the Halosperm kit does not appear to be a marker for sperm dysfunction or male infertility, this result supports those reported by Sellami et al.21 who found no association between the degree of sperm chromatin condensation and sperm concentration, motility, and viability. But, recent research conducted by Park et al.22 using the same test, noted a strong correlation between high DNA fragmentation index and each of: sperm mobility, vitality, and count. In our protocol, Halosperm kit assesses the capacity of sperm chromatin to disperse under hydrochloric acid denaturation. The level of fragmentation is estimated based on the size of the nuclear dispersion and visualization of halo was done using optic microscopy after Diff-Quik staining. In this case, the peripheral limit of the halo in some samples may not be accurately discriminated from the background which can cause confusion when quantifying halo types and can be considered as limitation leading to the contradictory results reported by each study. On the other hand, sperm DNA damage evaluated by more robust tests such as the SCSA and TUNEL has been shown to correlate more strongly with semen criteria.22,23
In the present work, sperm DNA fragmentation was not correlated with seminal SOD activity. This result disagrees with those reported by Yan et al.23 who found a significant negative correlation between this enzyme activity and DNA damage, as assessed by TUNEL assay. In fact, the origin of sperm DNA damage seems to be multifactorial and may occur from internal and/or external factors. The absence of any association between DNA fragmentation and the low seminal SOD in our study can be justified by the external activity of the evaluated enzyme that exhibits its function outside the sperm cells, so, even if it protects the spermatozoa from environmental ROS, it cannot be directly implicated in the protection of DNA.
To our knowledge, our study is the first to search for a possible link between SOD3G362A polymorphism and idiopathic male infertility in the Algerian population. The observed lack of association between SOD3G362A genotypes and male infertility cases in this work can be related to ethnic, genetic and lifestyle factors. This result is however in agreement with the only research by Ji et al.24 regarding Chinese population. The same authors studied the relation between polymorphisms in SOD2 gene and male infertility, they found that SOD2Val16Ala (rs4880) genotypes were associated with a higher risk for infertility cases. Another study of SOD2 polymorphisms revealed that the presence of at least one Ala-MnSOD allele (rs4880) increased the risk of infertility in the French population.25
Our work is in agreement with the only one conducted by Yan et al.23 about the association between SOD3G362A polymorphism and seminal SOD activity, the authors reported no influence of the different SOD3G362A genotypes on this enzyme activity in seminal plasma, but according to them, it seemed that SOD2Val16Ala (rs4880) was associated with low levels of SOD2. The distinct subcellular location of SOD is particularly important for compartmentalized redox signaling.26–29 SO3 is found chiefly in seminal plasma and is the only antioxidant that converts superoxide radicals in this extracellular compound,9,30 so even if this study does not show any effect of SOD3G362A on the impaired seminal SOD activity or on idiopathic male infertility cases, other polymorphisms of the SOD3 gene must be investigated to search for a potential genetic origin of the decreased SOD activity and male infertility cases related to this enzyme deficiency.
ConclusionOur work showed and confirmed the importance of SOD enzyme in the detoxification process of seminal plasma. The seminal activity of SOD in infertile group was significantly associated with sperm quality including concentration, vitality, overall motility and percentage of typical morphology which clearly indicate that SOD has an essential role in maintaining normal sperm criteria and male fertility potential. Besides, this study showed that DNA fragmentation, as measured by the halo test, as well as the SOD3G362A polymorphism were not correlated with idiopathic male infertility in Algeria.
Although the evaluation of antioxidants is not yet a routine in clinical practice, our research concluded the importance of seminal SOD assessment and supported the possibility that this enzyme provides a useful marker for the diagnosis of idiopathic male infertility and can be employed routinely in male infertility screening in the future. The main strength of our work is the fact that it presents the first data on genotypes and allele frequencies of SOD3G362A in Algeria and could provide a base for further research regarding this polymorphism in our population. However, the authors recognize that more studies including men from various geographic areas and different ethnicities in Algeria are warranted to determine whether the frequencies of SOD3G362A genotypes and the activity of seminal SOD are much variable in other infertile populations.
Ethics statementThe present study protocol was reviewed and approved by the institutional scientific and review board of Batna 2 University: 2012/BIO/LMD 3/096. Informed consents were approved and signed by each participant.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis work has been partially supported by grant number 15/2016 for M. Sadiq from the Deanship of Scientific Research and Higher Studies, Yarmouk University, Irbid, Jordan.
Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this paper.
The authors would like to acknowledge all volunteered patients and controls for their participation in this study; National Research Center in Biotechnology, Constantine, Algeria, for insuring the DNA extraction from all the blood samples. CHU of Batna and Laboratory of El Farabi, Batna, Algeria, for their guidance and help in collecting semen samples and assistance in examining semen parameters, Dr Smadi Adnane for his precious help.