The aim of this study was to summarize the evidence of radiofrequency electromagnetic radiation (RF-EMR) exposure from wireless devices on total motile sperm count (TMSC) and identify gaps in the literature that could help clarify this link.
Materials and methodsA literature search was conducted using PubMed/MEDLINE to find relevant studies examining the effects of EMR on male fertility, with a specific focus on TMSC, published from 2000 to 2019. R was used for data analyses.
ResultsMotility was identified as the parameter linked to TMSC that was most negatively impacted by EMR exposure. Many gaps were found including geographic and lack of standardization with EMR factors such as exposure time and operating frequency.
ConclusionThe EMR emitted by wireless devices may negatively affect TMSC, which is one of the better predictors of achieving pregnancies and impairs male fertility. Our findings highlight the need for clinicians to explore wireless device usage to help guide treatment decisions in men or couples with subfertility concerns.
El objetivo de este estudio fue resumir la evidencia de la exposición a la radiación electromagnética (EMR) por radiofrecuencia de dispositivos inalámbricos en el recuento total de espermatozoides móviles (TMSC) e identificar brechas en la literatura que podrían ayudar a aclarar este vínculo.
Materiales y métodosSe realizó una búsqueda de literatura en PubMed/MEDLINE para encontrar estudios relevantes que examinaran los efectos de la EMR en la fertilidad masculina, con un enfoque específico en el TMSC, publicados desde 2000 hasta 2019. Se utilizó el programa R para el análisis de datos.
ResultadosLa motilidad se identificó como el parámetro vinculado al TMSC que se vio más negativamente afectado por la exposición a EMR. Se encontraron muchas lagunas, incluyendo la estandarización geográfica y la falta de estandarización con factores EMR, como el tiempo de exposición y la frecuencia de funcionamiento.
ConclusiónLa EMR emitida por dispositivos inalámbricos puede afectar negativamente al TMSC, que es uno de los mejores predictores para lograr embarazos y afecta la fertilidad masculina. Nuestros hallazgos ponen de relieve la necesidad de que los médicos exploren el uso de dispositivos inalámbricos para ayudar a guiar las decisiones de tratamiento en hombres o parejas con problemas de subfertilidad.
Studies have shown a trend in decreasing sperm quality globally.1,2 Although the exact reason for this decline in sperm quality, and by extension fertility, is unknown, in recent years, the impact of modifiable factors, such as lifestyle has attracted more attention.3 One lifestyle factor that has gained attention over the years is the increased use of wireless devices which emit electromagnetic radiation (EMR), and it is necessary to better understand whether this is due to the advancement of mobile communication devices. Radiofrequency electromagnetic radiation (RF-EMR) may have both thermal and non-thermal effects on biological tissue.4 Recent studies have shown a correlation between EMR exposure and semen parameters and provide a link as to how EMR may negatively affect fertility potential.5–8
Of note have been the rapid developments in the field of mobile communications. The effect of EMR on living organisms depends on the signal frequency, intensity, and duration of exposure. Mobile phones emit RF-EMR, at frequencies ranging between 800 and 2200MHz, which are absorbed by the human body.1 The rate at which electromagnetic (EM) energy is absorbed by tissues is defined as the specific absorption rate (SAR), which is expressed in Watts per kilogram (W/kg). According to the International Commission on Non-Ionizing Radiation Protection (ICNIRP), the recommended threshold SAR limit of mobile phones is 2.0W/kg,4 and many current models have SAR values of about 1.4W/kg.1 It has also been implied that mobile phones and other EM devices that emit RF-EMR are harmful to human fertility.2 Recent evidence has also shown that Wi-Fi from laptops negatively affects sperm quality.9,10 The oscillating current and transfer of energy generated by RF electric fields can result in rapid heating, which could influence sperm quality.11 EMR exposure contributes to the cumulative effect of modern-day environmental sources of exposure that may collectively reduce sperm quality, which may explain current trends in infertility.
The male reproductive tract is extremely sensitive to EMR because of the mechanisms involved in the development and maturation of sperm within the testicles.3 During testicular development, the EMR penetration depth, exposure time, the number of developing sperm cells exposed to microwaves and the water content of the organ are important factors. Wireless devices operate at different frequencies in various countries and continents, and the exposure to RF energy depends on the frequency of the device in question. Although the emission of electromagnetic waves (EMWs) is constant, the field strength, frequency and power vary for different technologies, e.g. 2G, 3G, and 4G. The effects of EMR on human sperm have not yet been fully understood, but it is necessary to clarify the relationship between sperm exposure to various EMR sources and geographic location. A better understanding of the collective influence of EMR on sperm quality, and subsequently fertility, will help to improve treatment, advice and support for individuals seeking fertility treatment.
Recent studies have shown that total motile sperm count (TMSC) is a better predictor of male factor fertility than the World Health Organization (WHO) classification system that is typically utilized.12,13 As such, this comprehensive review aims to explore the link between EMR from wireless devices and TMSC parameters. We also aim to determine discrepancies between studies, as well as gaps in the literature, which could help to guide future studies.
Materials and methodsInformation discoveryA literature search was conducted using PubMed/MEDLINE and Google Scholar to find relevant studies examining the effects of EMR on male fertility published between 2000 and 2019. The search terms used were ‘phone’ OR ‘laptop’, OR ‘tablet’ OR ‘wi-fi’ OR ‘electromagnetic’ OR ‘radiofrequency radiation’ AND ‘semen’ OR ‘sperm’ OR ‘fertil*’ OR ‘male reproduction’. The search was limited to studies using human and animal subjects, as well as those that reported information on basic semen parameters.
Inclusion and exclusion criteriaArticles were only included if they were written in English, published in the last two decades, reported on human and animal subjects and did not use workplace RF-EMR exposure. Both in vitro and in vivo studies were incorporated if they met certain inclusion criteria (maximum SAR value of 2.0W/kg, frequency of 800–2200MHz, etc.) based on earlier literature.1 The studies that did not meet the inclusion criteria were excluded.
Data miningParameters used to generate TMSC (Eq. (2)) are the most widely accepted indicators of male factor infertility assessment. Some of the studies supplied data on all these outcome measures, while others focused on just some of them. All were recorded and their discrepancies considered.
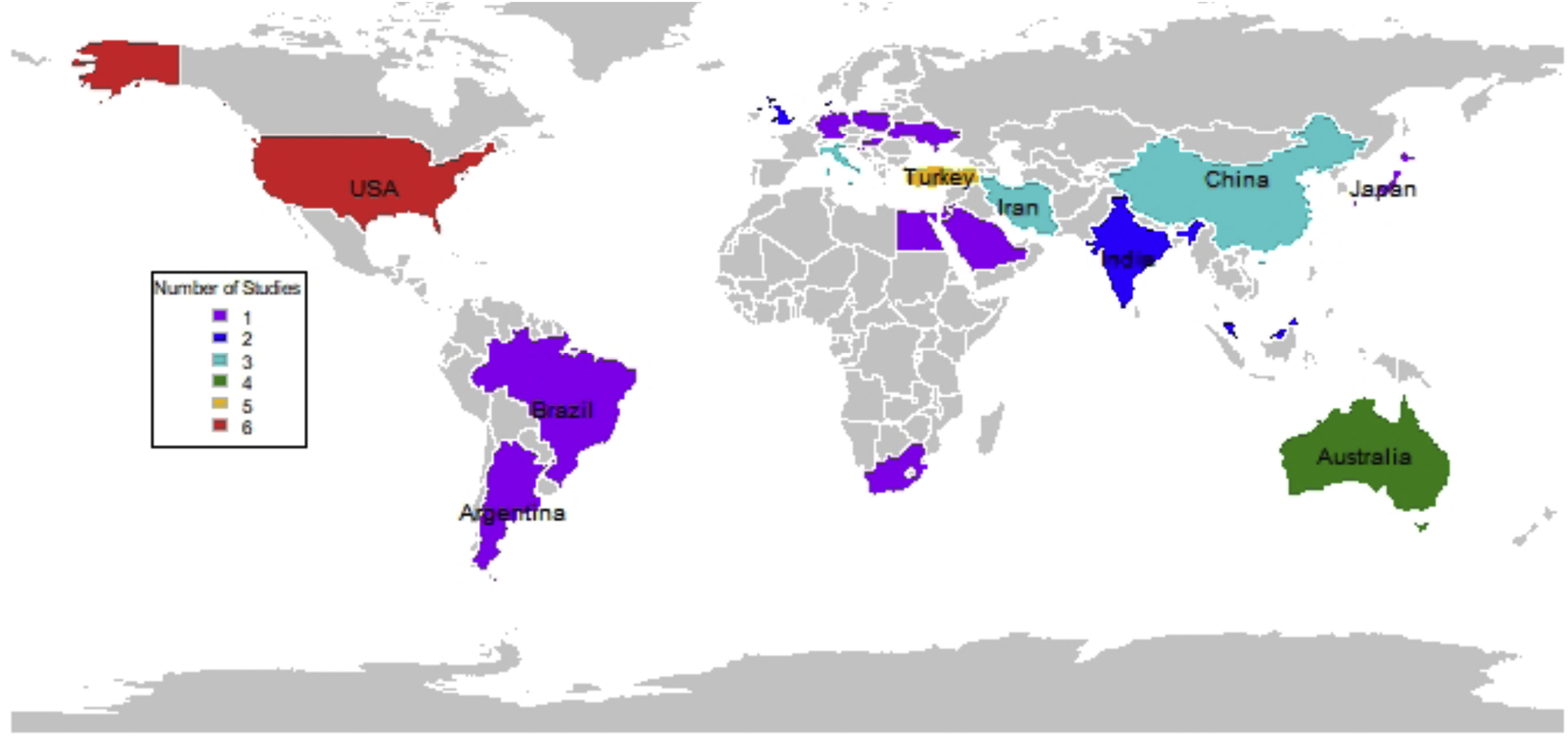
The study design, sample size, operating frequency, SAR, exposure time and findings for the studies looked at were all recorded to obtain a comparative perspective. The statistical software R was used to create a World map highlighting the locations that have peer-reviewed publications on EMR and TMSC parameters over the past two decades (see Fig. 1). Equations used to determine the SAR and TMSC are presented.
EquationsThe impact of RF-EMF on the human body is measured by the SAR, which is determined by:
where E is the electric field strength, sigma (σ) is the conductivity of the tissue and rho (ρ) is the tissue density. The measured electric field strength values and SAR distribution are 1 and 10g mass averaged SAR values.The TMSC is determined by:
ResultsLiterature miningThe initial literature search retrieved 1688 articles, of which 704 articles were from MEDLINE, 932 articles from PubMed and 52 articles were from Google Scholar. Another 976 articles were removed due to duplication. Subsequently, 642 articles were excluded after reviewing the titles and abstracts as they did not match the aim of this review paper. Full papers were obtained for the remaining 70 articles and a thorough review process was conducted. Five articles were further excluded because they were not written in English. In total, 65 papers were reviewed. However, only 30 articles were eligible for data extraction owing to the focus on EMR and TMSC parameters.
Study characteristicsAnimal studiesOf the 30 eligible articles, 14 took an in vivo approach and they reported on the effects of RF radiation on rodents, of which 10 were rats and 4 were mice (Table 2). The age of the animals used in the studies varied from prenatal to adult.
The studies varied in terms of distance of samples to the RF generator and duration of exposure. The shortest duration was 20min/day for 1 month, while the longest was 24h/day for 12 months. Despite the differences in the setting, all the studies reported SAR values below the limits recommended by the United Stated Federal Communications Commission (US FCC), ICNIRP and the WHO.
Most of the studies defined their control group as the group that received no RF-EMR exposure when the generator was switched off, and only received cage restriction stress.14 On the other hand, the control group in the study done by Mahmoudi et al. (2015) was exposed to a non-energizing Wi-Fi router, which refers to a switched-on Wi-Fi router without any data exchange between the linked devices.
Human studiesFifteen articles reported the effects of RF-EMR exposure on human subjects, with 12 being in vitro studies and three in vivo studies. In three of the experimental studies, semen ejaculates were placed a few centimetres away from a laptop, which was actively connected to Wi-Fi,7,9,16 while one study used a Wi-Fi modem.17 The closest distance was 3cm, while 60cm was the farthest distance. The duration of exposure ranged from 5min to 16h.
Two studies clearly stated the characteristic of the samples subjected to the exposure.9,16 Meanwhile, three studies distinctly characterized chronic diseases, presence of infection and azoospermia as the exclusion criteria for the semen samples, and were excluded from the study as those underlying factors would have affected the results.7,16,18
Similar to the animal studies, all the SAR values reported in these human studies conformed to the recommended limits set by the US FCC, ICNIRP and the WHO. In all the studies, sperm motility was significantly affected by the exposure. However, changes in sperm concentration were inconsistent between studies. In addition, two studies showed increased reactive oxygen species (ROS) level, a nucleic acid damage marker, and a decreased antioxidant enzyme in the semen, indicating that oxidative stress may be linked to the harmful effects of RF-EMR on semen.16,18 A summary of the data for the animal and human studies is depicted in Table 2.
In several studies, the sample size was unclear as 23% did not mention that factor. However, although the majority of the studies did report their sample sizes, this was not standardized throughout; hence, sample size details required to sufficiently evaluate statistical inferences were lacking. As such, it was difficult to determine the suitability of the results because most did not state the reason for using a particular sample rate or source of the population (fertile, infertile or general). The ages of the study participants were rarely mentioned, and this is a confounder of male fertility.19 Of note, 23% of the studies took their samples from men seeking infertility investigations, and the remainder were obtained from the healthy male population.20–22
Of note were the thermal effects that may have led to a decrease in certain sperm parameters. Most studies did not address this limitation; however, it was not an issue in others as cooling methods and temperature control measures were implemented in their incubators prior to data collection.23
It was seen that 57% of the studies investigated this issue using an in vivo study design, 40% used in vitro and 3% took both approaches. From the perspectives explored, the EMR from wireless devices appears to influence TMSC parameters. However, the degree of impact varies due to several factors such as: the distance from the source of radiation, operating frequency, exposure time, and the SAR value of the wireless device.
Operating frequency, exposure time and SAR valueThe emission of EMWs is constant, but the field strength, frequency and power vary for different technologies (Tables 1a and 1b). It has been reported that EMR from wireless device exposure impairs the parameters associated with TMSC7,15; these are further verified by the results obtained in Table 2. Scrotal hyperthermia and oxidative stress are the main mechanisms by which the damage is generated.24 For the male reproductive system, the closer a wireless device is to the groin, the higher will be the exposure to EMR. The harmful effects of EMR have not been proven in human studies owing to inconsistencies in findings. It was noted that most studies tend to focus on human models as opposed to the previous focus on animals. In 50% of the identified studies carried out over the last two decades involving this topic, the focus was on human models, while the remaining studies focused on rats and mice.
Comparison of key parameters of different mobile technology generations.
| Comparison | 1G | 2G | 3G | 4G | 5G |
|---|---|---|---|---|---|
| Period | 1980–1990 | 1990–2000 | 2000–2010 | 2010–2020 | 2020–2030 |
| Transmit power | 3–5W | 1.27W | 0.5W | 0.25W | – |
| Operating frequency | 800MHz | GSM:900MHz1800MHzCDMA:800MHz | 2100MHz | 850MHz1800MHz | 3–300GHz |
| Characteristic | First wireless communication | Digital | Digital broadband, increased speed | High Speeds, All IP | Fastest data transmission, low latency services |
– denotes missing information.
Comparison of popular wireless standards.
| Comparison | Bluetooth | Wireless fidelity (Wi-Fi) | ||||
|---|---|---|---|---|---|---|
| Wi-Fi (a) | Wi-Fi (b) | Wi-Fi (g) | Wi-Fi (n) | Wi-Fi (ac) | ||
| IEEE standard | 802.15 | 802.11a | 802.11b | 802.11g | 802.11n | 802.11ac |
| Wireless generation | 2G | 3G | 2G | 3G | 4G | 5G |
| Year released | 1998 | 1999 | 1999 | 2003 | 2009 | 2014 |
| Transmit power | 0.01W | 0.1W | 0.1W | 0.1W | 0.1W | 0.1W |
| Operating frequency | 2.45GHz | 5GHz | 2.4GHz | 2.4GHz | 2.4GHz5.0GHz | 2.4GHz5.0GHz |
| Range | 10m | 30m | 30m | 30m | 50m | 80m |
Effect of electromagnetic radiation on total motile sperm count parameters.
| Study design | Sample size | Operating frequency (MHz) | SAR (W/kg) | Exposure time | Outcome | Reference |
|---|---|---|---|---|---|---|
| In vivo (in human) | 361 | – | – | Less than 2h/day2–4h/dayGreater than 4h/day | Decrease in sperm volume, concentration, and motility | Agarwal et al., 20085 |
| In vitro (in human) | 64 | 850 | 1.46 | 60min | Decrease in sperm motility | Agarwal et al., 200932 |
| In vitro (in human) | 27 | 900 | – | 5min | No effect on sperm volume, concentration, and motility | Erogul et al., 200625 |
| In vitro (in human) | 8 | 1800 | 0.4–27.5 | 16h | Sperm motility was significantly reduced. | De Iuliis et al., 20096 |
| In vivo (in mice) | 6 | 900 | – | 2h/day for 45 days | No effect on TMSC parameters | Kesari et al., 201214 |
| In vivo (in mice) | 5 | 900 | 0.09 | 12h/day for 7 days | No significant effect on sperm volume, concentration nor motility | Aitken et al., 200526 |
| In vivo (in human) | 2028 | – | – | – | Decrease in semen volume, sperm concentration and count | Zhang et al., 20167 |
| In vitro (in human) | 29 | 900 | – | 4h | Decrease in sperm motility | Avendaño et al., 20129 |
| In vitro (in human) | 40 | – | 1.3 | 50min | Mixed results in sperm motility in relation to different classes.No significant changes in sperm volume and concentration | Kamali et al., 201717 |
| In vitro (in human) | 270 | 1800 | 3.19 | 30min per day (n=89)31–120min per day (n=104)more than 121min per day (n=77) | No significant changes in TMSC parameters | Ding et al., 201818 |
| In vitro (in human) | 20 | – | 1–2.5 | 45min90min | Decrease in sperm motility | Ding et al., 201816 |
| In vivo (in mice) | 16 | 900 | 0.523.13 | 20min every day for 1 month | No adverse effect of cell phone exposure on measures of testicular function or structure. | Dasdag et al., 200327 |
| In vivo (in mice) | 16 | 2400 | 0.004880.002420.00102 | 24h/day for 12 months | No significant changes in total sperm parameters | Dasdag et al., 201531 |
| In vivo (in human) | 63 | – | – | No use<2h/day 2–4h/day >4h/day | Sperm count and progressive motility were barely altered. | Rago et al., 201333 |
| In vitro (in human) | 32 | 9001800 | – | 5h | Decreased progressively motile sperm | Gorpinchenko et al., 20148 |
| In vitro (in human) | 1082 | – | – | – | Decreased total motile sperm count and progressively motile sperm | Yildirim et al., 201534 |
| In vivo (in rat) | – | – | 0.091 | 4h/day for 7days2h/day for 7days2h4h | Significant decrease in Sperm concentration and motility | Mahmoudi et al., 201515 |
| In vivo (in rat) | 72 | 1950 | 0.4 0.8 | 5h per day, 5 weeks | No changes in TMSC parameters | Imai et al., 201135 |
| In vivo (in rat) | – | 9001800 | 1.2–30.01–0.05 | 2h per day, 90 days | Increased sperm motility | Ozlem Nisbet et al., 201236 |
| In vitro (in human) | 24 | 900 | 2.05.7 | 60min | No changes in TMSC parameters | Falzone et al., 200823 |
| In vitro (in human) | 371 | – | – | – | There were no changes in the total motility, concentration.Decreases in the proportion of rapid progressive motile sperm. | Fejes et al., 200528 |
| In vivo (in rat) | – | 1966 | 0.08–2.34 | 24h per day | No changes in TMSC parameters | Sommer et al., 200937 |
| In vivo (in rat) | – | 915 | 0.6 | 1h per day, 2 weeks | No changes to motility or count | Trosic et al., 201338 |
| In vivo (in rat) | – | 900 | 0.48 | 1h per day, 45 days | No changes in TMSC parameters | Tumkaya et al., 201339 |
| In vitro (in human) | 124 | 850 | – | 60min | Reductions to sperm motility of men with asthenospermia and oligospermia | Zalata et al., 201540 |
| In vivo (in rat) | 24 | 900 | 0.66 | 2h per day, 50 days | No changes in TMSC parameters | Liu et al., 201541 |
| In vivo (in rat) | – | 9001800 | 0.9 | 60min per day, 14 days | No changes in TMSC parameters | Al-Damegh, 201242 |
| In vivo (in rat) | 28 | 915–950 | – | 8h per day, 2–3 weeks | Decreased motility | Ghanbari et al., 201343 |
| In vivo (in rat) | – | 9001800 | – | 1h per day, 4 weeks | Reduced sperm motility, but not sperm count | Mailankot et al., 200944 |
| In vivo and in vitro | Varying | 2450 | Varying | Varying | Sperm volume, concentration and motility were affected negatively | Jaffar et al., 201910 |
– denotes information not stated.
In the studies that were identified, it was observed that the exposure time to the different signal frequencies and the SAR values influenced the sperm characteristics of each sample (Table 2). Of note, in some studies, the duration of exposure varied from minutes to hours and even days, which may have added to the discrepancies observed in the results reported.25–27 Other reports display a more standardized approach and show that overall, an increase in the exposure time of different radiofrequencies and SAR values has an increasingly negative effect on TMSC parameters (in particular motility and volume), while the effect on concentration was less clear.7,15,25,28
From the studies mined, 73% looked at the operating frequencies of the different wireless devices and how those frequencies may have affected male fertility; again, this was not standardized, and no cut-off limits were reported. Hence, the frequency at which there are hazardous effects on sperm quality remains unknown.
EMR and TMSC parametersSeveral reports have shown a correlation between cell phone use and decreased semen quality. For example, the use of cellular phones adversely affected the quality of semen in 361 men attending an infertility clinic,5,6 and the duration of cellular phone possession and the duration of daily transmission was negatively correlated with semen quality in 371 men.29 However, many of the studies showed that the sperm parameters linked to TMSC were most affected.
MotilityThe majority of studies reported a negative influence of EMR on sperm motility. Carrying GSM/2G phones in the trouser pocket or on the belt has been shown to decrease rapid progressive motility of sperm,28 which is in agreement with results of other studies presented in Table 2. In addition, the continuous use of mobile phones is associated with decreased motility.5 The results of a study showed that human spermatozoa exposed to RF-EMF exhibited decreased sperm motility (particularly rapid progressive motility), and other semen parameters such as sperm concentration, sperm survival and morphology, were less affected.30 These abnormalities appear to be related to the duration of cellular phone usage. An evaluation of semen quality showed that there was a decrease in motility following RF-EMR exposure.14,15,31 Conversely, Ozlem Nisbet et al. (2012) reported an increase in sperm motility.
VolumeSemen volume was shown to decrease when exposed to EMR in both human and animal models,5 as shown in Table 2. Of note, this decrease was not always quantified in most previous studies nor was it standardized in terms of operating frequencies, exposure time or SAR values (Table 2).
Sperm count/concentrationThe usage of wireless devices adversely affects semen quality by decreasing the sperm count, which contributes to male infertility.5 Many animal studies report a reduction in sperm count although these findings were not consistently reported.
The time spent sending or receiving messages was shown to be significantly associated with decreasing sperm count, and carrying a mobile phone in the trouser pocket was significantly associated with increasing immotile sperms.29 The extent of sperm damage and thus the degree of fertility impairment depends on the radiation dosage. Any EMR, including those emitted by cell phones, cell towers and laptops, may lead to detrimental effects on fertility.3 There have been several reports of a decrease in sperm count following RF-EMR exposure.14,15,31
Overall, the summarized reports indicate that the prolonged use of mobile phones is associated with a decrease in sperm concentration.5 However, the evidence is mixed, as one study reported no effect.28
Geographic indicatorsFig. 1 shows that most of the studies related to EMR and TMSC have been carried out in Europe and Asia. This trend correlates with the high mobile phone usage in these regions. Europe had the most impact from the previous two decadal periods analyzed; their mobile communication sector has developed rapidly during those same periods, so there may be some correlation between these two occurrences.
DiscussionThe world today has become increasingly more dependent on wireless technologies, whether cell phones, laptops, tablets, or even handheld gaming devices. As such, the need for an analysis into their usage and correlation with fertility is timely. This comprehensive review summarizes the evidence reported over the past two decades on the role played by EMR on parameters associated with TMSC. Overall, we found that the parameter which was most affected was the progressive motility. This review also identified geographic gaps, sample population factors, exposure time inconsistencies, non-standardization of frequencies and SAR values, as well as other literary disparities, which are important to understanding the influence of EMR on male fertility.
The finding that motility is the parameter most impacted by EMR is paramount as TMSC has been shown recent years to be one of the better predictors of achieving a pregnancy.12,13 Additionally, as modifiable lifestyle factors appear to have a greater negative impact on the motility in sub-fertile males,45 more studies are needed to see if this population also reacts differently to EMR. As such, case-control studies are needed to clarify the possible mechanism by which EMR exposure influences the motility.
Wireless devices operate at different frequencies in different geographical regions, and the exposure to RF energy depends on the frequency and transmit power of the device in question. Fig. 1 highlights the need for EMR and sperm quality investigations to be done in other regions of the world, such as the Caribbean, which has seen a drastic increase in the use of wireless devices in recent years.
The exposures seen in the in vivo studies are constrained by the legal limits placed on SAR values for mobile devices,4 and data on the maximum SAR values for mobile devices are available. However, all wireless devices have fluctuating SAR values, so better methods of monitoring participant exposure levels are urgently needed. The SAR values obtained were not clearly discussed in most articles, so it is difficult to assess this and determine their accuracy. Some were taken from the device manufacturer's specifications, while the researchers measured others. However, they did not specify how these values were obtained, and cannot be easily reproduced by other researchers.
It was surprising that few studies focussed on the distance between wireless devices and the testicles. This limitation may be important in analysing the effects of these devices on spermatogenesis and semen quality. When a biological body or tissue is exposed to RF-EMF, the RF energy is scattered and attenuated as it penetrates body tissues. Energy absorption is a function of the radiation frequency and the composition of the exposed tissue. There is a need to differentiate between the different technologies while investigating this issue, especially with the current push towards 5G. Although the 5G standard has not yet been finalized, theoretically, relatively higher frequencies will be used, and may thus lead to higher attenuation and greater penetration of body tissues. The EMR emitted from cell phones is higher in the body while making a cell phone call or using electromagnetic devices.3 As found in in vitro studies, EMR emitted at the same frequency as mobile phones decreased sperm motility6; the trends seen in this review are consistent with these effects.
There are some limitations to this study. Heterogeneity, which refers to variations between studies, is greater than expected owing to the sampling error. In addition, the possibility that other factors influenced the results of the studies cannot be ruled out. For example, the frequency of sexual activity, alcohol consumption and smoking status were not reported, so these may have affected the results of some studies as they are known to affect some semen quality parameters, including concentration and motility.46,47 Nevertheless, the inclusion of in vivo and the consistency of the results between the study types provide evidence that the other influencing factors were negligible. In addition, research involving males recruited from fertility clinics are not reflective of the general population, as they are likely to include a higher proportion of men with sperm parameters outside the WHO reference range.
Future research scopeEMR exposure appears to negatively impact sperm motility, volume and concentration, which are three of the most widely used indices for assessing sperm quality.48 Of note, sperm motility is estimated to be 10% lower in exposed groups compared with non-exposed groups. This is of clinical importance and warrants further research as EMR exposure may have a greater impact on sub-fertile men. Although gaining more attention in recent years, the number of available studies on wireless device exposure and sperm quality is limited. There is a need for additional studies that assess non-conventional semen parameters (such as those investigating sperm DNA integrity). Case-control studies involving standardized intensity and exposure values are also needed.
ConclusionWhile the advancement of wireless technologies is inevitable, it is accompanied by an increase in EMR from wireless devices. As fertile and sub-fertile males are known to react differently to various modifiable lifestyle factors, 45 it is expected that there will be a direct impact of EMR from wireless devices on male fertility.
Recent studies reveal that the EMR exposure from wireless devices has detrimental effects which may affect sperm parameters associated with TMSC, and which may impair male fertility and reduce the chances of achievable pregnancies. There are reports suggesting that these effects are linked to an over-production of ROS in exposed cells, increased oxidative stress and heating.
Studies indicate that the global decline in semen quality with RF-EMF exposure is related to physical parameters such as duration of radiation exposure, distance to the source of radiation, operating frequencies, SAR values, and depth of penetration of EMR. Presently, the mechanism of how RF-EMF radiation affects the male reproductive system remains unclear. Therefore, more studies are necessary to clarify the relationship between EMR and sperm and testicular functions. Our findings highlight the need for clinicians to explore wireless device usage to help guide treatment decisions in men or couples with subfertility concerns.
Availability of data and materialsAll material is available and relevant permissions have been given.
Ethics approval and consent to participateThis is a review paper and did not need ethical clearance.
Consent for publicationAll mentioned authors were involved in the writing of this review and gave consent for its publication.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial support and sponsorshipFor this review, no funding was received from any sponsor or funding organization.
Authors’ contributionDr. Louis-Ray Harris and Dr. Kamali Carroll envisaged this review paper; Mr. Lanceford Sterling prepared the manuscript; Dr. Louis-Ray Harris and Dr. Kamali Carroll critically reviewed the manuscript and added relevant information. All authors read and approved the final manuscript before publication.
Conflicts of interestThe authors declare that there are no conflicts of interest.
We are grateful to the researchers who conducted the studies included in this report and who made data available to us.