The pharmacological effects of ketamine are being discovered constantly, and new mechanisms involving epi-genetics could account for some of its clinical responses. Nowadays, it is regarded as a pharmacological tool in translational research and has the potential to revolutionise the therapy of complex patient conditions such as pain and depression.
Medical interest in exploring the drug's properties has grown, as is demonstrated by the increased investigation over the last five years, according to research sites such as Pubmed. These publications draw attention to the multifaceted roles of ketamine as an anaesthetic, analgesic (in multiple contexts, chronic, acute, refractory and breakthrough pain), antihyperalgesic, neuromodulator, bronchodilator and/or antidepressant. It is therefore important to review the pharmacological characteristics and their implications in current treatments.
Los efectos farmacológicos de la ketamina están siendo continuamente descubiertos y nuevos mecanismos que involucran a la epi-genética podrían explicar algunas de sus respuestas clínicas. Hoy día se considera una herramienta farmacológica en la investigación translacional y tiene el potencial de revolucionar la terapéutica de condiciones complejas para el paciente como son el dolor y la depresión.
El interés médico en conocer las propiedades del fármaco en diferentes contextos ha aumentado y prueba de ello es que en los últimos cinco años, ha habido un aumento significativo en la investigación según sitios como Pubmed. Estas publicaciones, muestran los roles multifacéticos de la ketamina como anestésico, analgésico (en múltiples contextos como dolor agudo, crónico, refractario e irruptivo), antihiperalgésico, neuromodulador, broncodilatador y/o antidepresivo. Por ello es importante revisar las características farmacológicas y sus implicaciones en la terapéutica actual.
Ketamine is a drug that is becoming increasingly more consolidated in anaesthetic practice. It was synthesised by Calvin Stevens in 1962 and was first used by Domino and Corssen in 1965.1 It was introduced into Mexico in 1970.
It is an arylcyclohexylamine derivative whose structure is related to phencyclidine and cycloheximide, the main antagonist of N-Methyl-d-Aspartate (NMDA), an excitatory amine, by blocking the receptors of the same name.2
The properties of this molecule allow it to act on different levels and thus achieve effects such as sedation, analgesia and anaesthesia; it also has anti-inflammatory, antidepressant and neuroprotective properties.
The purpose of this review is to emphasise its current role, indications, pharmacokinetics, pharmacodynamics and different clinical uses.
Pharmacological propertiesIt is a white crystalline, water-soluble salt that is stable at room temperature. It has a molecular weight of 238, with a PK of 7.5.3
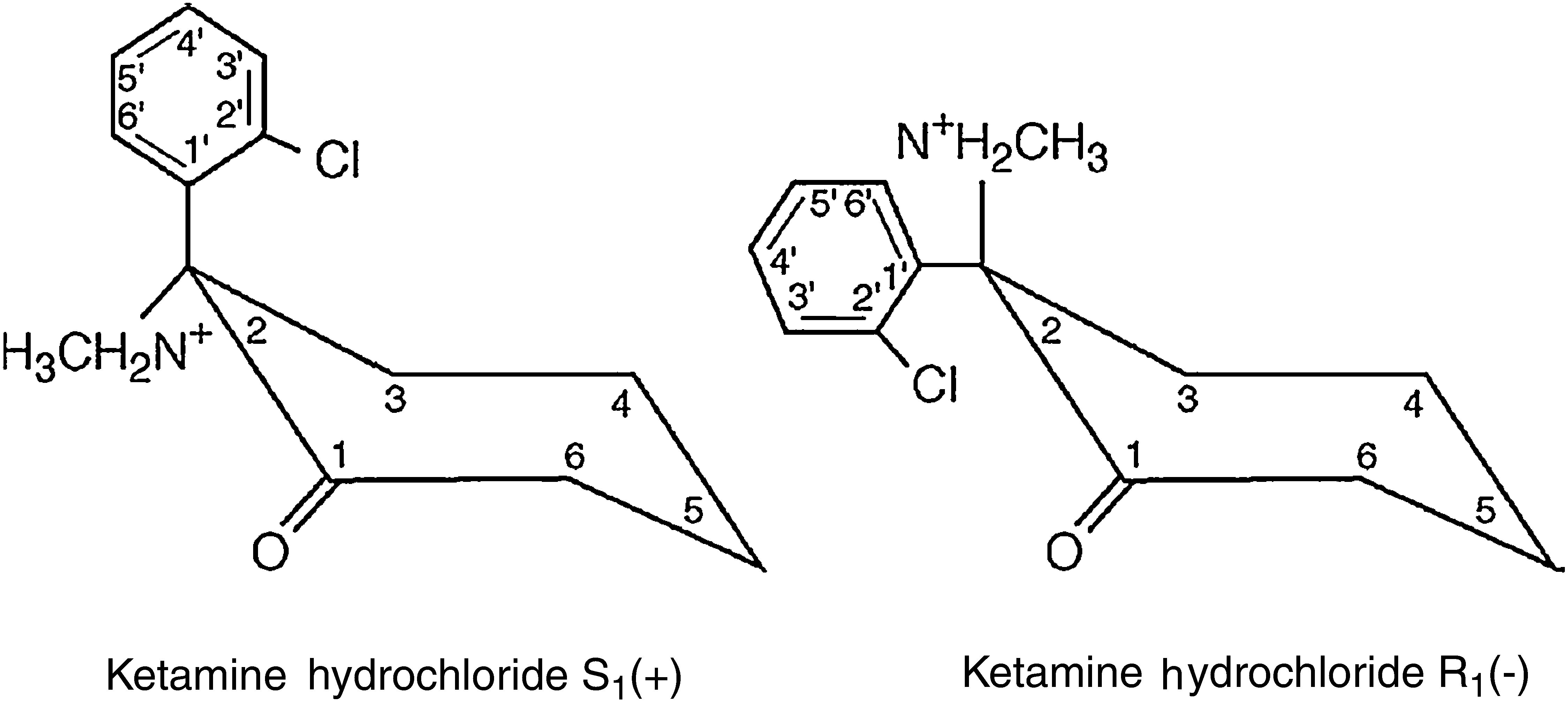
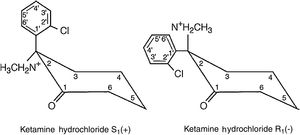
The ketamine molecule contains a chiral centre that produces two optical isomers, of which the S isomer (+) has a major advantage over the R isomer (−) as its anaesthetic and analgesic properties are four times more potent (Fig. 1).3,4
It is marketed in formulated concentrations containing 10, 50 and 100mg of base ketamine with phemerol or benzethonium chloride as preservative, and the pharmaceutical presentations are slightly acid (pH 3.5–5.5).5
Intravenous administration at 2mg/kg of weight produces unconsciousness after 20–60s,5,6 making it the inductor with the fastest onset of action, an advantage which is accounted for by its high Ke0 (0 elimination constant) of 1.3L/min and its t1/2 (elimination half-life) Ke0 of 0.53min.7
Its bioavailability when given intramuscularly is 93%, yielding plasma concentrations five minutes after administration. Orally, only 17% is available due to the first-pass effect. It binds to proteins at 40–50%, with a volume of distribution of 0.3ml/kg and a plasma concentration therapeutic window of 0.5–2.5mcg/ml.7
Metabolism takes place in the hepatic microsomal system, when it becomes Norketamine (N-desmethyl ketamine), with a potency of 1/3 to 1/10 with regard to the initial molecule. Clearance is 18ml/kg/min, with an elimination t1/2 of 2–3h.8
Intact renal excretion is 4%, and 16% in different forms. It is important to point out that joint administration with benzodiazepines prolongs the elimination half life due to the competitive inhibition of N-desmethyl ketamine.9
A suitable analgesic effect may be obtained with levels from 40ng/ml, which can easily be reached with IV doses of 0.2–0.75mg/kg or IM of 2–4mg/kg.10
Mechanism of actionThe main central action site is the thalamocortical projection system. It thus selectively depresses neural function, particularly in areas of association and the thalamus, while simultaneously stimulating the limbic system, including the hippocampus, which creates a functional disorganisation of non-specific pathways in the midbrain and the thalamic area. It also depresses the transmission of impulses in the medullary reticular formation, an important structure in the transmission of the emotional affective components of pain perception, from the spinal cord to the higher centres of the brain, thus giving rise to the term “dissociative anaesthesia”.11,12 This function depends on the interaction with the NMDA (N-methyl-d-Aspartate) receptors, which may be blocked by drugs such as phencyclidine and ketamine. There is evidence of opioid receptor occupation in the brain and spinal cord (the S enantiomer acts on the mu receptor), which could account for some of its analgesic effects on the central nervous system (brain and spinal cord).8,13–15 Therefore, due to the existence of a common receptor, cross tolerance may be expected between opiates and ketamine.16 This theory would gain greater credence if naloxone reversed its effects in human beings, although Stella et al., in a study with 68 adults premedicated with naloxone for general surgery, found dysphoric reactions to emersion. One possible theory is the stimulation of the sigma opioid receptors.16,17
There is also evidence of stimulation of the muscarinic/nicotinic cholinergic receptors, serotonin, dopamine and norepinephrine receptors, type-L calcium channels and sodium channels.18
Pharmacological actionsEffects on the central nervous systemKetamine produces a cataleptic condition different from the general anaesthesia produced by other agents that simulates normal sleep.19
These patients have profound analgesia, but their eyes remain open and they maintain many reflexes (corneal, tussigenic, swallowing), which should not be interpreted as being protective; the pupils dilate moderately and nystagmus appears, there is tearing, salivation and movements of the head and limbs; however, practically none of these effects appears when it is used at sub-anaesthetic doses for the treatment of chronic pain.19,20
It increases cerebral metabolism, cerebral blood flow and intracranial pressure (ICP).21 Pfenninger et al. studied mechanically-ventilated pigs with increased ICP in which the ICP was measured, with no increase found in ICP with doses of 0.5–2.0mg/kg of the intravenous medicinal product.22
It provokes clear changes in the EEG, with a reduction in the activity of alpha waves and with increases in beta, delta and theta waves.23 It is difficult to draw conclusions on its anticonvulsant properties.24 Although there is an epileptiform EEG pattern in the limbic and thymic regions of human beings, there is no evidence that it affects cortical regions.23,24
Psychic phenomena have been reported, such as the sensation of floating, vivid dreams, hallucinations and delirium. These phenomena are more common in patients above the age of 16 years, women, after short procedures, large doses and rapid administration.25 The benzodiazepines, and more particularly midazolam, have proven to be the most efficacious agents in the prevention of these symptoms. In this regard, clinical studies comparing diazepam and midazolam have been conducted, with the latter having been found to be enormously superior, as well as reducing anaesthetic recovery time in the surgical setting.26
Effects on the cardiovascular systemThe capacity to stimulate the cardiovascular system is one of its main outstanding characteristics versus other intravenous anaesthetic agents.27 The benzodiazepines can attenuate cardiostimulatory response.
It is the only anaesthetic with sympathomimetic action, which produces stimulation at cardiac level and in the peripheral resistances. An increase in all the haemodynamic constants has been described: heart rhythm, systemic blood pressure, systemic vascular resistances, pulmonary arterial pressure and pulmonary vascular resistances due to an increase in catecholamines caused by re-uptake inhibition.28
The acute haemodynamic effects in children were studied by Morray et al. two minutes after the application of 2mg/kg, with minimum changes found, and independent of intracardiac short circuit, PaCO2 and PaO2. With the same dose, Hickey et al. found no changes in pulmonary vascular resistance, systemic vascular resistance and cardiac index in intubated and mechanically-ventilated infants.27–29
Effects on the respiratory systemDuring the initial phase after administration, there is a slight depression of respiration (greater in newborns), although permeability of the respiratory tract, skeletal muscle and diaphragm tone are conserved; for this reason, it does not interfere with ventilatory mechanics.30
Its bronchodilator reaction, well-known since the first clinical studies, is of particular importance, and it is effective in the prevention of bronchoconstriction caused by circulating catecholamines. The aforementioned reasons make it the drug of choice for the induction of anaesthesia in asthmatic patients.30
Acute post-operative painNowadays, satisfactory post-operative pain control is one of the most important as-yet unresolved challenges in the surgical setting and has a major impact on patients and on the health system in general. Pain is a warning function that triggers protective responses and seeks to minimise tissue damage. When tissue damage is unavoidable, a cascade of changes takes place in the central and peripheral nervous system that is responsible for pain perception.31
The reported therapeutic concentrations of ketamine for analgesia are 200ng/ml, and a suitable analgesic effect is obtained with levels starting from 40ng/ml, IV doses of 0.2–0.75mg/kg or IM doses of 2–4mg/kg.32
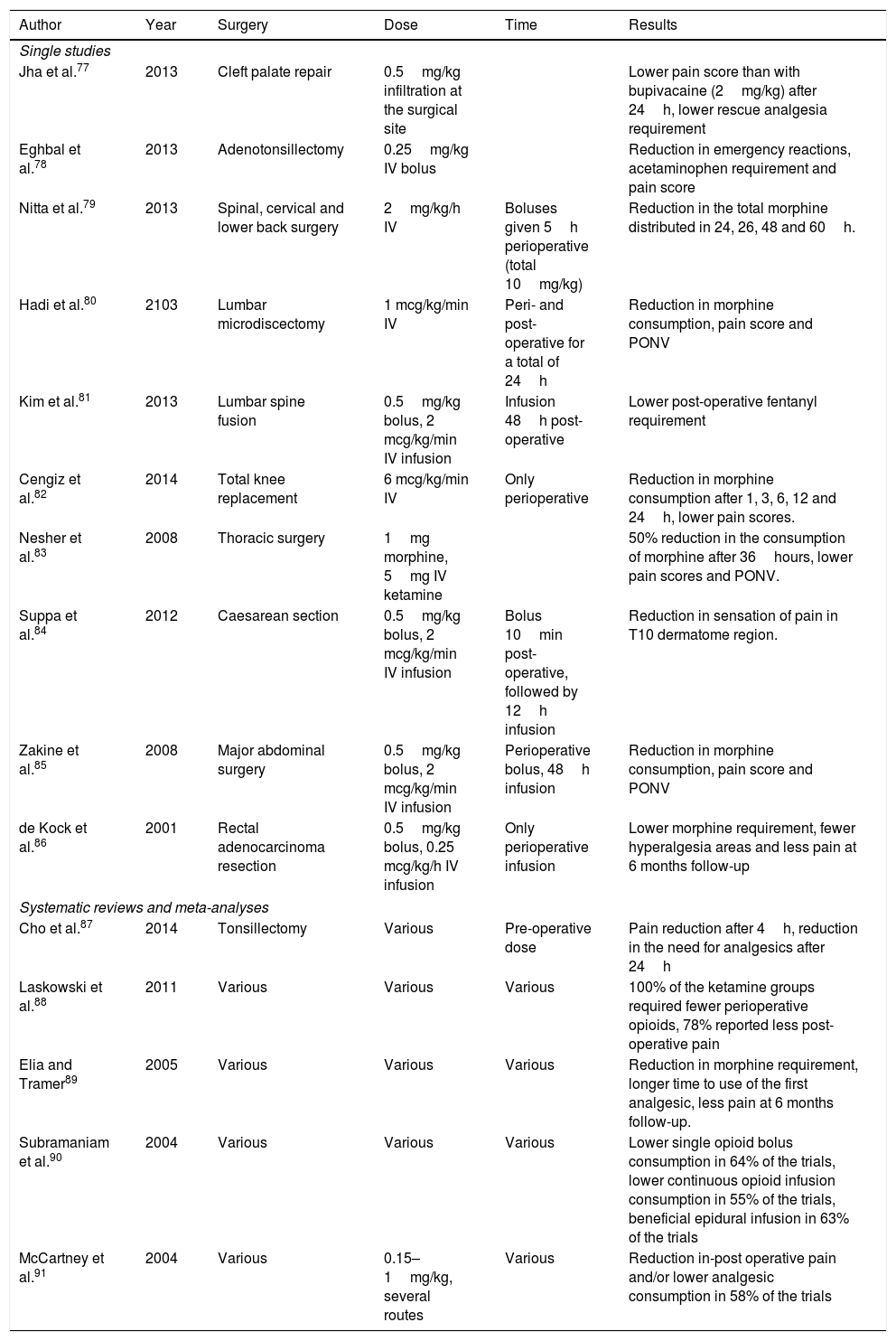
Ketamine has proven its effectiveness in the reduction of post-operative pain in several types of surgery (Table 1).
Studies in which the use of ketamine for pain was significant.
| Author | Year | Surgery | Dose | Time | Results |
|---|---|---|---|---|---|
| Single studies | |||||
| Jha et al.77 | 2013 | Cleft palate repair | 0.5mg/kg infiltration at the surgical site | Lower pain score than with bupivacaine (2mg/kg) after 24h, lower rescue analgesia requirement | |
| Eghbal et al.78 | 2013 | Adenotonsillectomy | 0.25mg/kg IV bolus | Reduction in emergency reactions, acetaminophen requirement and pain score | |
| Nitta et al.79 | 2013 | Spinal, cervical and lower back surgery | 2mg/kg/h IV | Boluses given 5h perioperative (total 10mg/kg) | Reduction in the total morphine distributed in 24, 26, 48 and 60h. |
| Hadi et al.80 | 2103 | Lumbar microdiscectomy | 1 mcg/kg/min IV | Peri- and post-operative for a total of 24h | Reduction in morphine consumption, pain score and PONV |
| Kim et al.81 | 2013 | Lumbar spine fusion | 0.5mg/kg bolus, 2 mcg/kg/min IV infusion | Infusion 48h post-operative | Lower post-operative fentanyl requirement |
| Cengiz et al.82 | 2014 | Total knee replacement | 6 mcg/kg/min IV | Only perioperative | Reduction in morphine consumption after 1, 3, 6, 12 and 24h, lower pain scores. |
| Nesher et al.83 | 2008 | Thoracic surgery | 1mg morphine, 5mg IV ketamine | 50% reduction in the consumption of morphine after 36hours, lower pain scores and PONV. | |
| Suppa et al.84 | 2012 | Caesarean section | 0.5mg/kg bolus, 2 mcg/kg/min IV infusion | Bolus 10min post-operative, followed by 12h infusion | Reduction in sensation of pain in T10 dermatome region. |
| Zakine et al.85 | 2008 | Major abdominal surgery | 0.5mg/kg bolus, 2 mcg/kg/min IV infusion | Perioperative bolus, 48h infusion | Reduction in morphine consumption, pain score and PONV |
| de Kock et al.86 | 2001 | Rectal adenocarcinoma resection | 0.5mg/kg bolus, 0.25 mcg/kg/h IV infusion | Only perioperative infusion | Lower morphine requirement, fewer hyperalgesia areas and less pain at 6 months follow-up |
| Systematic reviews and meta-analyses | |||||
| Cho et al.87 | 2014 | Tonsillectomy | Various | Pre-operative dose | Pain reduction after 4h, reduction in the need for analgesics after 24h |
| Laskowski et al.88 | 2011 | Various | Various | Various | 100% of the ketamine groups required fewer perioperative opioids, 78% reported less post-operative pain |
| Elia and Tramer89 | 2005 | Various | Various | Various | Reduction in morphine requirement, longer time to use of the first analgesic, less pain at 6 months follow-up. |
| Subramaniam et al.90 | 2004 | Various | Various | Various | Lower single opioid bolus consumption in 64% of the trials, lower continuous opioid infusion consumption in 55% of the trials, beneficial epidural infusion in 63% of the trials |
| McCartney et al.91 | 2004 | Various | 0.15–1mg/kg, several routes | Various | Reduction in-post operative pain and/or lower analgesic consumption in 58% of the trials |
PCA, patient-controlled analgesia; PONV, postoperative nausea and vomiting.
The combination with intrathecal dexmedetomidine delivers superior post-operative analgesia and prolongs analgesic time with a reduction in the total consumption of morphine without greater side effects in comparison with each drug separately.33
In combination with local anaesthesia for modified pecs block, it prolongs the time to the first request for analgesia and gives rise to a reduction in total opioid consumption without serious side effects in patients undergoing modified radical mastectomy.34
Antidepressant effectKetamine has recently emerged as an effective treatment in refractory depression. Depression is associated with the loss of neurons, a reduction in the number of synapses and dendritic dearborisation. The drug can potently induce mechanisms that reverse these neurodegenerative processes, not only by blocking the glutamate receptor, but also by activating the eukaryotic elongation factor (eEF2). This, in turn, activates the synthesis of proteins derived from the brain-derived neurotrophic factor (BDNF), which appears to provide the foundations for lasting benefits.35
Analgesia in burn patientsIntravenous ketamine has demonstrated its analgesic efficacy in burns, with a reduction in secondary hyperalgesia, in comparison with opioid monotherapy. The combination of ketamine plus morphine resulted in the abolition of hyperalgesia in a study of 67 burn patients.36
Opioid-induced anti-hyperalgesicOpioid-induced anti-hyperalgesia is related to high doses, prolonged administration or the abrupt interruption of opioid infusion. Ketamine is known to significantly attenuate this condition by means of an intra-operative bolus or infusion37 due to opioid-induced tolerance and hyperalgesia, there being no totally effective treatment for pain caused by this condition. The mechanism postulated is the activation of the NMDA receptor. Ketamine's antagonistic activity on the NMDA receptor has been shown to regulate opioid-induced hyperalgesia. A review in PubMed showed 4 published articles about ketamine in the management of pain caused by sickle-cell disease with opioid-induced hyperalgesia, which found that the patients that received a ketamine infusion presented a reduction in pain intensity and a significant reduction in opioid doses.38
Avoiding opioid dependence and toleranceThe endogenous opioid peptides (EOP) are strongly related to other systems for the development of tolerance and dependence. These phenomena also involve dopamine and oxytocin, blocking the onset of tolerance versus beta-endorphins and enkephalins. The NMDA block the onset of tolerance through interaction with the mu or delta opioid receptors which are related to this phenomenon. Recent studies suggest that the NMDA and CCKB receptors also mediate opioid tolerance by means of a convergent nitric oxide pathway as the second messenger.39
For the treatment of chronic painThe following indications have been proposed for ketamine: oncological neuropathic pain, post-herpetic neuralgia, chronic trauma, amputation, spinal cord lesion, central origin pain secondary to cerebrovascular accident, phantom limb pain, restless legs syndrome, chronic orofacial pain, fibromyalgia, etc.40
Its capacity to reduce neuropathic pain in some studies is reported as superior to opioids, predominantly as an improvement in pain intensity in cancer patients presenting a neuropathic pain component.41
The current treatments for central neuropathic pain syndrome are still inadequate. The use of an intravenous infusion of ketamine is a therapeutic alternative. The report suggests that ketamine modulates pain by returning the NMDA receptor to the resting state, whereby the propagation of the pain-inducing signal towards the brain is interrupted, permitting the restoration of the balance between the inhibition and facilitation of pain.42
Ketamine as treatment of acute pain in the emergency roomIntravenous opioids are the main form of management of severe acute pain in the emergency room. Although the opioids deliver rapid and effective pain relief, the doses required to produce suitable analgesia may generate adverse effects such as over-sedation and respiratory depression.43 Low doses are effective and safe for the treatment of acute pain in emergency rooms, either alone or in combination with opioid analgesics, although their use is associated with greater rates of minor, well-tolerated, adverse side effects.43,44
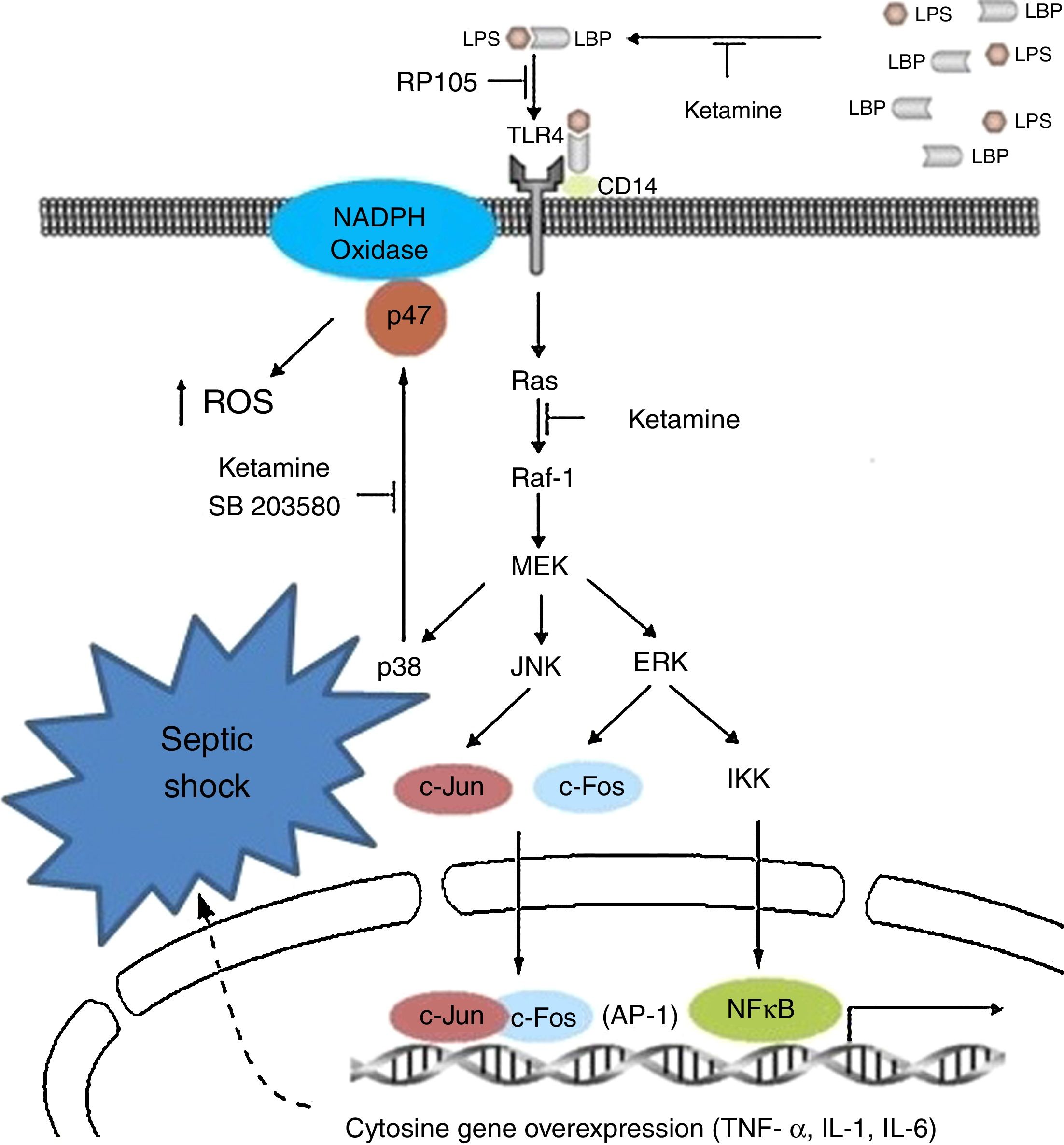
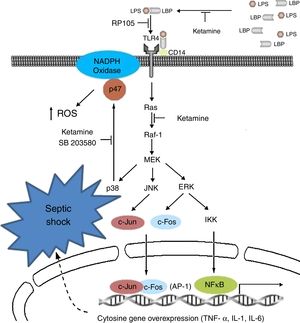
Immunoinhibitory effectsIt is probably the most studied characteristic in recent years, in which immune inhibitory effects have been observed in vitro in the laboratory and in the clinical setting of patients with sepsis; particularly, anti-inflammatories, in modulating innate immunity and pro-inflammatory signalling with down-regulation of the increase in the expression of sepsis-induced TLR2/4.45–47
Clinically speaking, ketamine can increase arterial pressure and maintain circulatory stability, mainly as a result of the inhibition of pro-inflammatory cytosine secretion.48–50 Moreover, it acts upon the metabolism of tryptophan and bioproducts such as kynurenine; a product known to be a modulator of the NMDA receptor functions,51 and on the production of cortisol.52
It could reduce the synthesis of Tumour Necrosis Factor-alpha (TNF-a) and of Interleukin 6 (IL-6), reducing mitochondrial membrane potential53,54 by suppressing the production of endotoxin-activated macrophages (LTA) of gram-negative organisms by inhibiting the expression of genes for the production of these two substances.55 They suppress macrophage function by means of the inhibition of phagocytic activities, oxidative capacity and the synthesis of mRNA (messenger RNA), TNF-a and IL-1b, reducing the activation of nuclear factor (NF)-kB;56 and the synthesis of IL-6 at transcriptional level, in view of the reduction in the phosphorylation of the ERK1/2 complex mediated by the transduction signal of TLR-2 and TLR-4 and RAGE.57
The modification of this signalling pathway is probably one of the multiple ways that down-regulate the activation of nuclear factor NF-kB and the genetic expression of TNF-a and IL-6. Other studies have postulated an impact on critical physiological and psychological functions mediated by the suppression of induction in the activity of nitric oxide synthetase and suppression of the expression of endotoxin-generated proteins.58 The medicinal product dose-dependently inhibits the inflammatory response induced by lipopolysaccharides (LPS).59,60 The NMDA receptor also inhibits the extracellular signals regulated by the kinase pathway and the proliferation of carcinomatous cells by cell cycle arrest.61,62 Another way of inhibiting lymphocyte, NK cell and neutrophil function is by suppressing chemotactic activity, with a reduction in the production of oxidants.63–65
Hudenz et al. concluded that single doses of ketamine of 0.5mg/kg applied during induction were associated with low levels of C-reactive protein (CRP) and a low incidence of delirium and cognitive dysfunction following cardiac surgery performed with extracorporeal circulation pump.66
Ketamine induces immune responses in patients that will undergo surgery, since surgical trauma may induce a complex cytokine cascade with different effects on the patient. Some pro-inflammatory cytokines in the immune system are over-stimulated, whereas cell-mediated immunity is suppressed.67,68 For this reason, certain undesirable effects, such as low blood pressure, shock and multiple organ failure, may occur and compromise patients if the inflammatory response is disproportionate.69 In view of the known existence of interactions and regulations between pain perception and pro-inflammatory cytokines,70 it is important to reduce the suppression of lymphocyte-mediated immune response and attenuate the reaction of the pro-inflammatory cytokines to surgery by effective pain management, reducing the response to surgical stress in the post-operative period.71Fig. 2 shows the cytokine cascade in which ketamine intervenes during a septic process.
Molecular mechanisms of ketamine that induce down-regulation of the genetic expression of proinflammatory cytokines. AP, activator protein; ERK, extracellular signal-regulated kinase; IKK, inhibitor of nuclear factor kappa-B kinase; IL, interleukin; JNK, c-Jun N-terminal kinase; LPB, lipopolysaccharide binding protein; LPS, lipopolysaccharide; NADPH, nicotinamide adenine dinucleotide phosphate; NK-kB, nuclear factor kappa-B; TLR, toll-like receptor; TNF, tumour necrosis factor.
Roytblat et al. also reported that the administration of small doses of ketamine in patients undergoing cardiopulmonary bypass surgery during induction anaesthesia significantly reduced IL-6 serum levels over the 7-day post-operative period,72 as well as TNF-a and IL-6.73–75
Recently, anti-cytokine therapy has been applied to control sepsis using an extracorporeal haemoperfusion device that specifically absorbs mediators such as the cytokines by means of a neutral micropore resin. Very low levels of IL-6 and IL-8, significant increases in cardiac index, systemic vascular resistance, greater speed in the withdrawal of vasoactive agents, as well as a reduction in heart rate were found in these patients compared to the control group.75,76
Although data are limited, particularly in studies in human beings, the existing results call for further and better studies with a greater number of cases exploring the relationship between IL-6 and morbidity and mortality. A greater and continuous effort is called for in order to clarify, in detail, the mechanisms of this suppression in the pro-inflammatory cascade and its influence in the future.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThere is no funding.
Conflict of interestsThere are no conflicts of interest.