A 56-year-old woman with no medical history presented to the emergency department complaining of oppressive chest pain of three hours of duration triggered by walking. An electrocardiogram (ECG) performed at hospital admission showed ST-segment depression and T-wave inversion in left precordial and inferior leads. Serum troponin I levels were above the normal values. Non ST-segment elevation acute coronary syndrome (NSTEACS) was diagnosed and an invasive approach with a percutaneous coronary intervention was performed. No obstructive coronary lesions were observed. However a tortuous coronary fistula emerging from the left-anterior descending coronary artery to the pulmonary artery was observed. Coronary arterio-venous fistulas are present in 0.002% of the general population and in 0.25% of patients undergoing cardiac catheterization for any cause. Most of them are asymptomatic. Ischemic symptoms may develop but are infrequent in patients with no atherosclerotic disease.
Un hombre de 56 años sin antecedentes médicos se presentó en el departamento de urgencias quejándose de dolor torácico opresivo de tres horas de duración desencadenado al caminar. El electrocardiograma (ECG) tomado al ingreso mostró depresión del segmento ST e inversión de las ondas T en derivaciones precordiales izquierdas e inferiores. Los niveles de troponina I sérica se encontraron arriba de los valores normales. Se diagnosticó síndrome coronario agudo sin elevación del segmento ST (SCACEST) y se realizó un abordaje invasivo mediante intervención coronaria percutánea. No se observaron lesiones coronarias obstructivas. Sin embargo, se observó una fístula coronaria tortuosa emergiendo de la arteria coronaria descendente anterior hacia la arteria pulmonar. Las fístulas arterio-venosas coronarias se encuentran presentes en 0.002% de la población general y en 0.25% de los paciente sometidos a cateterismo cardíaco por cualquier causa. Muchos de ellos son asintomáticos. Síntomas isquémicos se pueden desarrollar, pero son infrecuentes en pacientes sin enfermedad aterosclerosa.
Coronary arterio-venous fistulas (CAVF) are rare major vascular malformations in which there is an abnormal connection between a coronary artery and any of the four chambers of the heart or any of the great vessels (superior vena cava, pulmonary artery, pulmonary veins or coronary sinus), by-passing the coronary bed.1–5,7–13
It is estimated that CAVF are present in 0.002% of the general population, represent 0.4% of all cardiac malformations and are visualized in nearly 0.25% of patients undergoing catheterization.1,12 At least 75% of coronary artery fistulas found incidentally are small and clinically silent. However the true incidence of coronary artery fistulas is highly speculative since many may be small and approximately 50% remain asymptomatic, or are only detected incidentally with imaging for another indication.1,2,6,7,9–12
CAVF was first described by Krause in 1865 and the first successful surgical closure was reported by Bjork and Crafoord in 19471–7 in a patient with a preoperative diagnosis of patent ductus arteriosus,2 but their diagnostic triad was not described until 1978 by Haller and Little, who characterized these lesions by the presence of an abnormal continuous murmur similar to that of a patent ductus arteriosus, a left-to-right shunt, and a large coronary artery with evidence of fistula on angiography.3–5
CAVF may be congenital or acquired.1–3,5,10,12 The congenital form is by far the most frequent and represents an arterial anomaly of termination (it terminates into an abnormal structure). It can be found in any age group and has no predilection among both sexes. Acquired CAVF are extremely rare and usually iatrogenic, posttraumatic or caused by Takayasu arteritis or chest irradiation.5,12
Congenital CAVF may arise due to persistence of sinusoidal connections between the lumens of the primitive tubular heart that supply myocardial blood flow in the early embryologic period.2,10 Spontaneous closure of the fistula secondary to spontaneous thrombosis has been reported, although it is very uncommon (1–2% of cases).1,2,4,5,7
Two major groups have been identified; solitary and multiple CAVF.7 Single fistulas are more common, ranging from 74 to 90%. Multiple fistulae are present in up to 16%, and fistulas originated from both coronaries in 5%.5,12 The solitary form is that observed in acquired CAVF.7 3% of cases are associated with an absence of the contralateral coronary artery.12 Some CAVF might disappear spontaneously during childhood,1,2,12 specially with small or medium size fistulas,11 although this is rare.4
Approximately 10–30% of patients with a CAVF also have another congenital cardiovascular anomaly. The most commonly seen defects include tetralogy of Fallot, patent ductus arteriosus, and atrial septal defect.5,6,11,12
CAVF are classified according to the chamber or vessel to which it drains: type 1, draining to the right atrium; type 2, draining to the right ventricle; type 3, draining to the pulmonary artery; type 4, draining to the left atrium; and type 5, draining to the left ventricle.6 Drainage into the left-sided chambers is less frequent. Fistulous drainage occurs into the right ventricle in 40%, right atrium in 26%, pulmonary artery in 17%, left ventricle in 3%, coronary sinus in 7%, and superior vena cava in 1%.1,3,5,6,10
Case reportA 56-year-old woman with no medical history presented to the emergency department complaining of oppressive chest pain and dyspnea three hours lasting, triggered by walking.
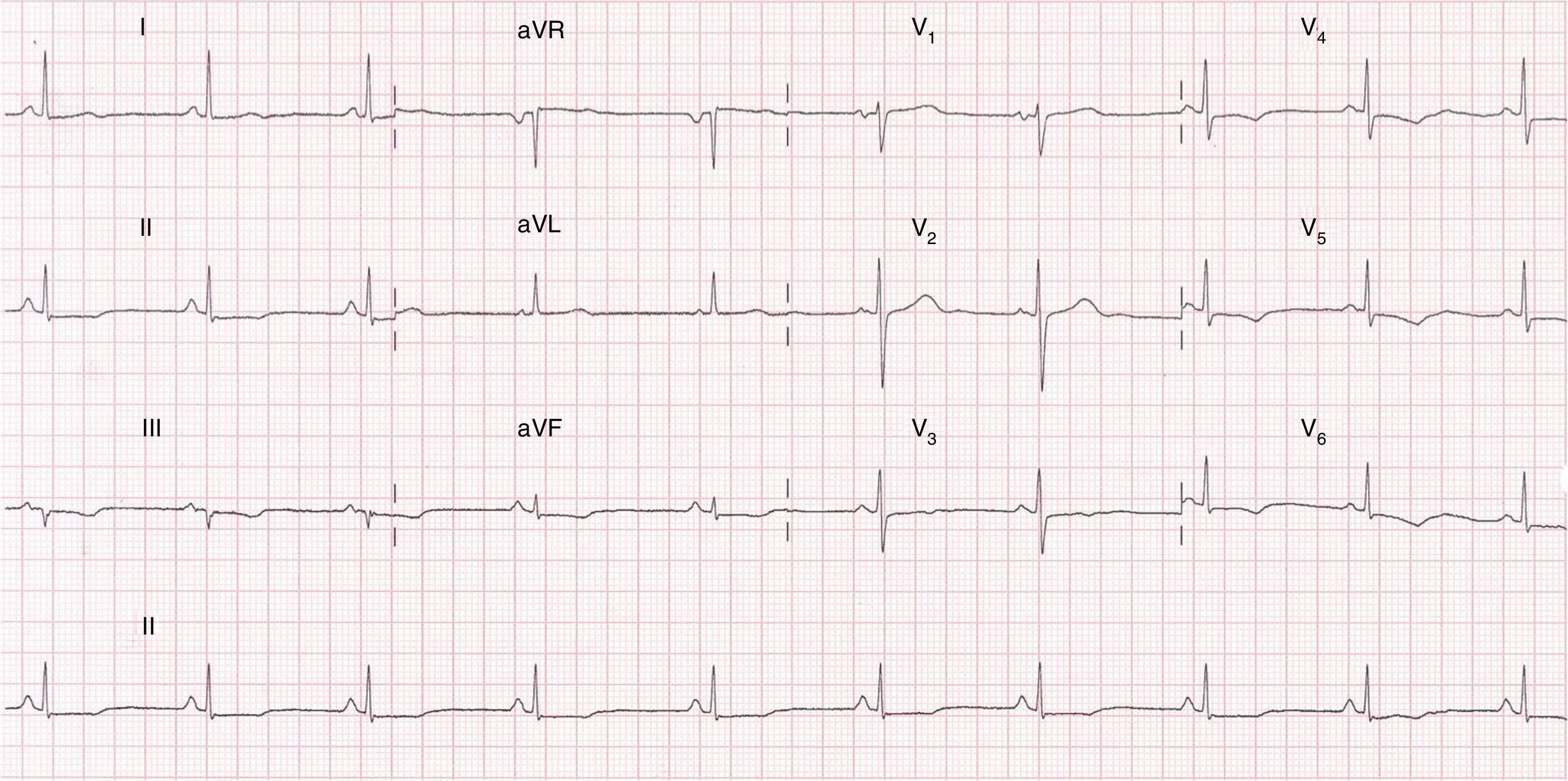
An electrocardiogram (ECG) performed at hospital admission showed sinus rhythm; and 0.1mm ST-segment depression and T-wave inversion in precordial leads from V-3 to V-6 and in the inferior leads Dll, Dlll and aVF (Fig. 1). Blood samples were obtained and serum troponin I levels were 1.5ng/ml which was above the 99th percentile upper reference limit. No other remarkable findings were reported.
Non ST-segment elevation acute coronary syndrome (NSTEACS) was diagnosed and an invasive approach with a percutaneous coronary intervention was performed at the second hour from hospital admission. No obstructive coronary lesions were observed. However a tortuous coronary fistula with multiple emerging branches from the left-anterior descending coronary artery to the pulmonary artery was visualized (Fig. 2A and B).
(A) Left coronary artery in RAO view in which a fistula (arrow) is observed emerging from the proximal coronary anterior descending artery to the pulmonary artery (PA); multiple small branches (asterisks) to feed the large fistula can be visualized more proximal to the main branch, no atherosclerotic lesions are observed. (B) Right coronary artery in LAO view without evidence of obstructive lesions in any segment.
An interventional approach for the correction of the fistula was first intended, however given the complexity of the vascular malformation, a surgical approach was offered to the patient. For no medical reasons surgery was rejected by the patient and a medical treatment was implemented.
DiscussionThe physiologic derangement of the CAVF depends on the location and size of the abnormal communication, the severity of the left-to-right shunt and the resistance of the fistulous connection and on the site of fistula termination. The resistance is determined by the size, tortuosity, and length of the pathway. Flow from the coronary artery to a venous structure or right-sided cardiac chamber occurs throughout the cardiac cycle. Blood follows the lower-resistance pathway through the fistula rather than traversing the smaller arterioles and capillaries of the myocardium. With larger fistulas, a diastolic runoff occurs, drawing blood away from the normal coronary pathway with a widened pulse pressure and a coronary steal.2,3,5,7,10,12
Over time, the coronary artery leading to the fistulous tract progressively dilates, which may progress to aneurysm formation, intimal ulceration, medial degeneration, intimal rupture, atherosclerotic deposition, calcification, side-branch obstruction, mural thrombosis, and rarely, rupture.10,11
If the left-to-right shunt is significant, complications such as pulmonary hypertension, heart failure, bacterial endocarditis, arrhythmias, or myocardial ischemia due to the aforementioned coronary steal may ensue.3–7,10–12
When left untreated, CAVF cause clinical symptoms in 19% of patients aged younger than 20 years and in 63% of older patients.2,3,11 Symptoms include exertional dyspnea, fatigue, palpitations and ischemic chest pain with heart failure.9 Most patients who experience symptoms related to a coronary artery fistula present during the 4th through 6th decades of life.8
Myocardial ischemia, mainly during exercise, has been reported in 3% of adult patients with large coronary fistulas.13 Onset of symptoms such as chest pain and dyspnea is a primary indication for closure of the fistula.7 Angina pectoris may be rarely seen in patients without arteriosclerotic coronary artery disease. Patients with angina pectoris are mostly older than 40 years old and they have coexistent coronary artery stenosis and/or have large or multiple fistulas.10,12
Diagnosis of CAVF should be considered in any patient presenting with cardiac murmurs, regardless of symptoms. Differential diagnosis includes patent ductus arteriosus, pulmonary arteriovenous fistula, ruptured sinus of Valsalva aneurysm, aorto-pulmonary window, prolapse of the right aortic cusp with an associated ventricular septal defect, internal mammary artery to pulmonary artery fistula, and systemic arteriovenous fistula.1 The typical murmur of a moderate or large coronary artery fistula is continuous with diastolic accentuation, but isolated systolic or diastolic murmurs have been described.11
The electrocardiogram (ECG) is normal in about 50% of patients. In the rest, it may show right or left ventricular hypertrophy and/or volume overload. An ischemic pattern is more likely if the coronary steal involves a major branch of the left circumflex artery.2
Two-dimensional transthoracic echocardiography or transesophageal echocardiography may establish the diagnosis, demonstrating the origin and drainage site, or provide clues such as coronary dilation or chamber enlargement. A markedly enlarged coronary artery can usually be detected with both transthoracic echocardiography and TEE.2,7,10 In cases of fistulous termination into a right-sided heart chamber or systemic venous structure, there is left-to-right shunting and right- and left-sided heart volume overload.8
Although echocardiography is often used, detailed evaluation may be difficult in some patients. Multidetector computed tomography (MDCT) allows excellent anatomical delineation with high resolution.10 It is the preferred noninvasive method for evaluating patients with suspected coronary anomalies. Magnetic resonance angiography (MRA) can also be used for evaluation of the coronary arteries. It has high temporal resolution and does not involve ionizing radiation or use of iodinated contrast medium. However, MRA has a long acquisition time and lower spatial resolution, than that of MDCT. MRA may be useful for assessing the proximal coronary arteries, but assessment of the distal coronaries is difficult.5,7
Coronary angiography remains the gold standard for imaging the coronary arteries.1,2,7,9,10 Medium-to-large coronary artery fistulae can be identified by the associated dilation of the coronary artery (which carries the normal coronary myocardial flow plus the abnormal fistulous flow). This coronary dilation is proximal to the site of fistulous connection into the low-pressure cardiac receiving chamber or central venous structure.8
The current treatment options for CAVF include surgical ligation alone (with or without cardio-pulmonary by-pass) or accompanied by coronary artery bypass grafting, and transcatheter closure.2 The surgical obliteration of the fistula, since described by Biörck and Crafoord in 1947, remains until now the most effective treatment,2 with epicardial or endocardial ligations being safe and effective with good reported success.1 However, catheter-based closure methods, first described in 1983, have become the preferred treatment option, if technically feasible.3,4,11,12
Three main surgical techniques are used. The most common consists of an epicardial approach with ligation of the fistula. The other approaches are from within the lumen of the dilated proximal coronary artery by means of an arteriotomy close to the fistula site, or by an exposure of the fistula from within the cardiac chambers or pulmonary artery, with direct suture or with autologous pericardial patch.2
In patients with atherosclerotic coronary artery disease presenting with acute coronary syndrome, surgical coronary revascularization can be safely associated with ligation excision of the CAVF. In patients with normal coronaries should be considered for closure by interventional cardiology technique but surgical closure can be considered in cases of high complexity with extreme tortuosity of fistulous tract and/or aneurysm formation, or by patient request.7,9
In the most recent (2009) American College of Cardiology/American Heart Association Guidelines for the Management of Adults with Congenital Heart Disease, percutaneous or surgical closure is a Class I recommendation with level of evidence C for large fistulae regardless of symptoms and for small- to moderate-size fistulae with evidence of myocardial ischemia, arrhythmia, systolic or diastolic dysfunction, ventricular enlargement, or endarteritis. While patients with small asymptomatic CAVF should not undergo closure of the defect.14
Recent results of transcatheter and surgical approaches indicate a good prognosis from both techniques. Life expectancy is considered normal.2
The most frequent complication associated with catheter based closure is embolization of the occlusion device, and in one study this complication occurred in 17% of patients.4–7,11–13 Mortality related to surgical closure of isolated coronary artery fistulas is rare, <1%.11 A 6.1% incidence of arrhythmias has been reported.5
The natural history of the CAVF is variable. Some authors recommend its closure even in asymptomatic patients to prevent fistula related complications such as heart failure, endocarditis and myocardial ischemia, which increase with age.1
The case of our patient with a type III CAVF, did not show the typical pattern of clinical presentation of these vascular malformations, which as mentioned before are asymptomatic in the most part of cases and detected only incidentally. Although previous reports exist of ischemic symptoms being the initial manifestation of the CAVFs, just a few were acute coronary syndromes.
A corrective treatment of the defect was mandatory for this case, since associated symptoms were present. Given the complexity of the fistula with multiple origins from the coronary vasculature and its tortuosity, a surgical treatment was proposed by the medical team as the best approach for this case. For non-medical reasons the patient refused surgery and only pharmacological anti-ischemic therapy was implemented. There was a favorable clinical evolution and hospital discharge was possible at the fifth day of hospitalization. However, although the natural history is variable, we believe that once symptoms have presented, there will be a recurrence as CAVFs tend to grow during the time.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThere is no conflict of interest.