Parasites are known to be useful as biological tags in distinguishing fish stocks, but the parasite fauna method has received limited use in the fisheries of Mexico. Parasite fauna composition and their infection levels were quantified for Caranx caballus populations at 3 locations on the south central Pacific coast of Mexico. Three hundred eighty-eight fish were collected and examined between December 2009 and February 2012. Twenty-four species of parasites were identified: 2 monogeneans, 6 digeneans, 1 cestode, 5 nematodes, 8 copepods, and 2 isopods. At the component community level, species richness of parasites varied significantly from 9 to 18 (in 2011 and 2012, respectively, for Acapulco Bay), and was similar to previous reports for other species of Carangidae. The component communities and infracommunities of C. caballus exhibited a similar pattern: low species numbers, low diversity, and dominance by a single species. Multivariate discriminant analysis used to distinguish between the C. caballus populations, indicated that each location can be considered as a different fish stock, and that C. caballus, therefore, does not migrate between the studied locations, even though they are close from each other.
Se sabe que los parásitos son útiles como marcadores biológicos en la distinción de stocks de peces, aunque el método de la parasitofauna ha recibido un uso limitado en México. Se determinó la composición y los niveles de infección de la parasitofauna en poblaciones de Caranx caballus de 3 localidades del Pacífico mexicano. Se capturaron y examinaron 388 peces entre diciembre del 2009 y febrero del 2012, identificándose 24 especies de parásitos: 2 monogéneos, 6 digéneos, un cestodo, 5 nematodos, 8 copepodos y 2 isopodos. A nivel de comunidad componente, el número de especies de parásitos varió significativamente de 9 a 18 (en el 2011 y 2012, respectivamente, para la bahía de Acapulco), siendo similar a los registros previos obtenidos para otras especies de Carangidae. Las comunidades componentes, al igual que las infracomunidades de C. caballus presentaron un patrón similar: bajo número de especies, poca diversidad y dominancia de una sola especie. Los análisis multivariados utilizados para diferenciar poblaciones de C. caballus indicaron que cada localidad puede ser considerada como un stock de peces diferente y que por lo tanto C. caballus no realiza migraciones entre las localidades estudiadas, aunque sean cercanas una de otra.
Green jack Caranx caballus are widely distributed along the Pacific coast of the Americas, from southern California, USA, to Peru (Mair, Cipriani, Guzmán, & Usan, 2012; Robertson & Allen, 2008). By annual capture volume it is the third most important fishery resource on the coast of the state of Guerrero, Mexico (Flores-Garza, Flores, & García, 2009). Despite its importance, few data exist on vital biological aspects of this species. Data on the reproductive period, feeding behavior, and, particularly important for fishery management, the location of specific stocks along the Pacific coast of Mexico are almost non-existent.
The distinction between stocks of a species of fish is key for fisheries managers, because stocks can vary in size and growth rates over time, and therefore may react differently to fishing exploitation (Brickle & MacKenzie, 2007). Use of the parasite fauna method for marine fish stock identification has won wide recognition in recent years due to its usefulness for fisheries managers, and is now being applied worldwide in many countries (MacKenzie, 2002; MacKenzie & Abaunza, 2005; Timi, 2007). Two main approaches to the use of parasites as biological tags have been developed (MacKenzie & Abaunza, 2005). The first one involves selection of a small number of species of parasites based on specific criteria (MacKenzie, 1983; Williams, MacKenzie, & McCarthy, 1992) (called biological tags), while the second involves entire assemblages of parasites (parasite community and infracommunity), and more sophisticated statistical techniques (Brickle & MacKenzie, 2007). Although the parasite fauna method is clearly a useful tool for generating fisheries data, such as host species movements, and population structure (Braicovich, Luque, & Timi, 2012; George-Nascimento, 2000; Luque & Ramos, 2001; Timi, 2007), it is not yet been applied by governmental agencies in Mexico. The objectives of the present study were: (1) to identify the composition of the parasite fauna, and their infection levels in C. caballus populations at 3 locations along the Pacific coast of Mexico and (2) evaluate if some of the parasite species in its community can be used as biological tags for stock distinction in future fisheries studies.
Materials and methodsIn December 2009 and February 2012, a total of 388 specimens of C. caballus were collected from 3 locations along the south central Pacific coast of Mexico: Marquelia (16°33′N, 98°48′W, n=62), eastern coast of Guerrero, approximately 149km East from Acapulco Bay; Acapulco Bay (16°51′N, 99°53′W, n=264), central Guerrero, and Lázaro Cárdenas (17°57′N; 102°10′W, n=62), border between Guerrero and Michoacán, approximately 288km West of Acapulco Bay. Data from 3 samplings done in Acapulco Bay in different years (2009, 2011, and 2012) were used in a temporal comparative analysis. A complete necropsy was done of all specimens, and all parasites were collected from the internal and external organs (Vidal-Martínez, Aguirre-Macedo, Scholz, González-Solís, & Mendoza-Franco, 2001). Contents of the digestive tract were examined to identify prey items consumed by fish at each location; the diet was analyzed using the frequency of occurrence method (Lima-Junior & Goitein, 2001). Parasites were identified to the lowest taxonomic level possible. Specimens of most taxa were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City.
Infection levels for each parasite species at each location were described using prevalence (percent of hosts that were infected with a particular species), mean abundance (mean number of individual parasites of a particular species per fish that was examined) and intensity (range, minimum-maximum, number of a particular species that infected a population of fish) (Bush, Laferty, Lotz, & Shostak, 1997). Analyses were done at the levels of component community (i.e. total species of parasites in all fish that were collected per location) and infracommunity (i.e. total species of parasites in each individual fish) (Holmes & Price, 1986). Component community parameters included total species richness, total number of individuals of each species of parasite, the Shannon–Wiener index (H) as a measure of diversity, species evenness, and the Berger–Parker index (BPI) as a measure of numerical dominance (Magurran, 2004).
The qualitative Jaccard similarity index was used to evaluate similarity and difference in species composition of each parasite community between locations at this level. The Student t-test and x2 tests were applied to identify differences between component community parameters, and correlations were calculated using the Spearman range coefficient (rs). Infracommunities were described in terms of mean number of species of parasite per host, mean number of individuals of each species, and the mean Brillouin diversity index (H′) value per host. A one-way analysis of covariance (Ancova) was used to identify differences in infracommunity parameters between locations. Discriminant analyses, based on Mahalanobis distances, were used to identify differences between locations, and determine which species of parasite were responsible for these differences. Only species with a prevalence >10% in at least one of the locations (a component species; Bush, Aho, & Kennedy, 1990) were included in this analysis. Abundance data for these component species were computed using log (x+1).
ResultsSpecies compositionTwenty-four species of parasite were identified in 388 C. caballus specimens collected from 3 locations along the south central Pacific coast of Mexico: 2 monogeneans (adults), 6 digeneans (adults), 1 cestode (larva), 5 nematodes (3 adults and 2 larvae), 8 copepods, and 2 isopods (Table 1). Diversity was greatest among the copepods, which represented 33.3% of the total species recovered, followed by the digeneans (25% of the species recovered).
Parasite infection parameters of Caranx caballus in 3 locations from Pacific coasts of Mexico.
| Parasite | Site | CNHE | N/Location | P (%) | Total | Mean abundance | Intensity |
|---|---|---|---|---|---|---|---|
| Monogenea | |||||||
| Allopyragraphorus caballeroi | Gills | 63/B09 | 42.86 | 117 | 1.86±3.97 | 1–16 | |
| 148/B11 | 34.46 | 270 | 1.82±12.6 | 1–89 | |||
| 10005 | 53/B12 | 32.08 | 146 | 2.75±23.61 | 1–100 | ||
| 10007 | 62/L09 | 53.23 | 88 | 1.42±1.90 | 1–9 | ||
| 10006 | 62/M11 | 41.94 | 66 | 1.06±2.45 | 1–9 | ||
| Pseudomazocraes selene | Gills | 63/B09 | 39.68 | 194 | 3.08±12.1 | 1–44 | |
| 148/B11 | 34.46 | 199 | 1.34±6.4 | 1–37 | |||
| 10008 | 53/B12 | 58.49 | 266 | 5.02±12.3 | 1–5 | ||
| 10010 | 62/L09 | 3.23 | 3 | 0.05±0.71 | 1–2 | ||
| 10009 | 62/M11 | 45.16 | 47 | 0.76±0.82 | 1–4 | ||
| Digenea | |||||||
| Bucephalus varicus | Intestine | 63/B09 | 14.29 | 112 | 1.78±10.5 | 4–32 | |
| 148/B11 | 70.95 | 1,209 | 8.17±18.0 | 1–124 | |||
| 10111 | 53/B12 | 73.58 | 242 | 4.57±6.5 | 1–31 | ||
| 10013 | 62/L09 | 8.06 | 9 | 0.15±1.1 | 1–3 | ||
| 10012 | 62/M11 | 83.87 | 1,046 | 16.87±40.7 | 1–205 | ||
| Dactylostomum winteri | Intestine | 63/B09 | 12.70 | 12 | 0.19±0.76 | 1–3 | |
| 148/B11 | 12.16 | 32 | 0.22±1.40 | 1–6 | |||
| 10014 | 53/B12 | 7.55 | 6 | 0.11±1.0 | 1–3 | ||
| 62/L09 | 1.61 | 2 | 0.03 | 2 | |||
| 10015 | 62/M11 | 11.29 | 11 | 0.18±1.13 | 1–4 | ||
| Phyllodistomum carangis | Intestine | 10017 | 62/M11 | 1.61 | 1 | 0.02 | 1 |
| Stephanostomum megacephalum | Intestine | 10018 | 63/B09 | 7.94 | 18 | 0.29±2.8 | 1–7 |
| 10020 | 62/L09 | 19.35 | 18 | 0.29±0.80 | 1–3 | ||
| Stomachicola sp. | Intestine | 62/M11 | 1.61 | 1 | 0.02 | 1 | |
| Tergestia laticollis | Intestine | 63/B09 | 1.59 | 1 | 0.02 | 1 | |
| 10016 | 148/B11 | 2.70 | 4 | 0.03 | 1 | ||
| Cestoda | |||||||
| Nybelinia sp. | Esophagus | 10021 | 148/B11 | 2.03 | 3 | 0.02 | 1 |
| 10022 | 62/M11 | 33.87 | 44 | 0.71±1.14 | 1–5 | ||
| Nematoda | |||||||
| Anisakis sp. | Mesentery, stomach wall | 63/B09 | 19.05 | 26 | 0.41±1.11 | 1–4 | |
| 148/B11 | 31.76 | 73 | 0.49±1.02 | 1–5 | |||
| 53/B12 | 16.98 | 13 | 0.25±.73 | 1–3 | |||
| 62/L09 | 64.52 | 102 | 1.65±1.74 | 1–7 | |||
| 62/M11 | 66.13 | 233 | 3.76±18.8 | 1–122 | |||
| Cucullanus sp. | Intestine | 63/B09 | 3.17 | 4 | 0.06 | 2 | |
| Eustrongylides sp. | Stomach | 63/B09 | 1.59 | 1 | 0.01 | 1 | |
| 148/B11 | 1.35 | 2 | 0.01 | 1 | |||
| 62/L09 | 3.23 | 2 | 0.03 | 1 | |||
| Philometra sp. | Gonad | 148/B11 | 2.03 | 5 | 0.03±1.15 | 1–3 | |
| 62/L09 | 14.52 | 26 | 0.42±1.45 | 1–6 | |||
| 62/M11 | 14.52 | 15 | 0.24±0.71 | 1–3 | |||
| Spinitectus sp. | Intestine | 63/B09 | 3.17 | 3 | 0.05±0.71 | 1–2 | |
| 148/B11 | 5.41 | 14 | 0.09±1.16 | 1–4 | |||
| 62/L09 | 3.23 | 5 | 0.08±0.71 | 2–3 | |||
| 62/M11 | 12.90 | 11 | 0.18±0.52 | 1–2 | |||
| Copepoda | |||||||
| Caligus alalongae | Gills | 63/B09 | 25.40 | 36 | 0.57±1.34 | 1–6 | |
| 148/B11 | 52.70 | 145 | 0.98±1.24 | 1–6 | |||
| 53/B12 | 56.60 | 63 | 1.19±1.40 | 1–6 | |||
| 62/L09 | 64.52 | 77 | 1.24±1.07 | 1–5 | |||
| 62/M11 | 56.45 | 73 | 1.18±1.85 | 1–9 | |||
| Caligus mutabilis | Gills | 63/B09 | 4.76 | 3 | 0.05 | 1 | |
| 148/B11 | 3.38 | 5 | 0.03 | 1 | |||
| 53/B12 | 1.89 | 2 | 0.04 | 2 | |||
| 62/L09 | 8.06 | 6 | 0.10±0.45 | 1–2 | |||
| 62/M11 | 9.68 | 6 | 0.10 | 1 | |||
| Caligus robustus | Gills | 63/B09 | 3.17 | 5 | 0.08±2.12 | 1–4 | |
| 148/B11 | 6.08 | 10 | 0.07±0.33 | 1–2 | |||
| 62/L09 | 9.68 | 6 | 0.10 | 1 | |||
| 62/M11 | 8.06 | 8 | 0.13±0.55 | 1–2 | |||
| Bomolochus exilipes | Gills | 148/B11 | 0.68 | 1 | 0.01 | 1 | |
| Ergasilus sp. | Gills | 53/B12 | 3.77 | 2 | 0.04 | 1 | |
| Ergasilus c.f. gibbus | Gills | 63/B09 | 1.59 | 1 | 0.02 | 1 | |
| 148/B11 | 0.68 | 1 | 0.01 | 1 | |||
| 53/B12 | 1.89 | 1 | 0.02 | 1 | |||
| Lernanthropus giganteus | Gills | 63/B09 | 4.76 | 3 | 0.05 | 1 | |
| 148/B11 | 5.41 | 8 | 0.05 | 1 | |||
| 62/L09 | 12.90 | 10 | 0.16±0.46 | 1–2 | |||
| 62/M11 | 8.06 | 5 | 0.08± | 1 | |||
| Taeniacanthodes gracilis | Gills | 148/B11 | 0.68 | 1 | 0.01 | 1 | |
| 62/L09 | 1.61 | 1 | 0.02 | 1 | |||
| Isopoda | |||||||
| Gnathia sp. (larva) | Gills | 148/B11 | 0.68 | 1 | 0.01 | 1 | |
| Rocinella signata | Gills | 63/B09 | 1.59 | 1 | 0.02 | 1 | |
CNHE: accession number, Colección Nacional de Helmintos, Instituto de Biología, Universidad Nacional Autónoma de México. N: number of fish examined. Location: B: Acapulco Bay, 2009, 2011, 2012 collections; L09: Lázaro Cárdenas, 2009 collection; M11: Marquelia, 2011 collection. P%: prevalence; Total: total number of individual helminths collected; Abundance: mean number of parasites per examined fish±standard deviation; Intensity: range (i.e., minimum–maximum number of helminths present). Significantly different measurements of prevalence (G-test) and abundance (x2-test) are in bold (p<0.05).
Eight species of parasites (Allopyragraphorus caballeroi, Pseudomazocraes selene, Bucephalus varicus, Dactylostomum winteri, Anisakis sp., Caligus alalongae, C. mutabilis, and Lernanthropus giganteus) were widely distributed and occurred in the parasite communities at all locations. The prevalence of 7 of these species varied significantly between locations and sampling years. Infection percentages were highest for the monogenean, P. selene, in Acapulco Bay (2012), and Marquelia (2011) (G=46.4, p<0.05). Of the 2 most prevalent digeneans, B. varicus exhibited its highest prevalence in Marquelia (G=103.2, p<0.05), while D. winteri was most prevalent in Acapulco Bay (2009 and 2011) (G=10.4, p<0.05). Among the nematodes, Anisakis sp., had the highest infection percentages in Lázaro Cárdenas and Marquelia (G=58.5, p<0.05), while Spinitectus sp. was more prevalent in Marquelia (G=10.3, p<0.05). Among the copepods, C. alalongae and L. giganteus had the highest prevalences at Lázaro Cárdenas (Table 1). In contrast to the infection percentage results, only the digenean B. varicus exhibited significant variation in mean abundance between locations and sampling years; it was significantly more abundant in Marquelia, and Acapulco (2011, 2012) (x2=106.5, p<0.05) (Table 1). The prevalence values generally correlated positively with the mean abundance values, indicating that the more prevalent species were also the most abundant (rs=0.800, p<0.01).
Component communityThe species richness of parasites recorded by location and sampling year (Table 2) varied significantly from 9 (Acapulco Bay 2012) to 18 (Acapulco Bay 2011) (t=9.49, p<0.01). Total number of individual parasites ranged from 335 (Lázaro Cárdenas 2009) to 1,983 (Acapulco Bay 2011), with significant differences between locations (x2=1,908.7, p<0.05) and sampling years (x2=1,126.9, p<0.05). Three species of helminth dominated the parasite communities (Table 2). B. varicus was the only one that dominated at 2 locations, and with the same intensity (BPI=0.6, Table 2). The Shannon–Wiener diversity index values were generally low, ranging from 0.532 (Marquelia) to 0.819 (Lázaro Cárdenas), and varied between locations and sampling years (t=11.9, p<0.01). Qualitative similarity between the component communities at different locations and in different years ranged from 44.2% (Acapulco 2009–Lázaro Cárdenas 2009) to 76.2% (Acapulco 2011–Marquelia 2011). Similarity percentages were lower between the locations that were farther apart: 30.4% between Marquelia and Lázaro Cárdenas (437km separation); 44.17% in 2009 between Acapulco and Lázaro Cárdenas (288km separation); 76.2% between in 2011 between Acapulco and Marquelia (149km separation).
Characteristics of the parasite component communities and infracommunities in Caranx caballus from 3 locations in the Pacific coasts of Mexico. BPI: Berger–Parker index; H: Shannon–Wiener diversity index. Ani: Anisakis sp., Buc: Bucephalus varicus, Pse: Pseudomazocraes selene. Higher significance values are in bold (p<0.05).
| Date | Locality | Component communities | Infracommunities | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of fishes examined | Total length (cm) | Species richness | No. of parasites | BPI | Dominant species | H | Mean number of species | Mean number of individuals | Mean value of Brillouin index | ||
| December 2009 | Acapulco Bay | 63 | 23.5±4.3 | 16 | 537 | 0.361 | Pse | 0.767 | 2.7±1.4 | 12.2±12.3 | 0.90±0.34 |
| October 2011 | Acapulco Bay | 148 | 27.0±3.6 | 18 | 1,983 | 0.61 | Buc | 0.583 | 2.8±1.3 | 14.1±19.0 | 0.81±0.42 |
| February 2012 | Acapulco Bay | 53 | 23.8±1.4 | 9 | 741 | 0.359 | Pse | 0.614 | 2.6±.1.1 | 14.2±23.8 | 0.86±0.40 |
| December 2009 | L. Cárdenas | 62 | 30.9±1.3 | 14 | 355 | 0.287 | Ani | 0.819 | 3.0±1.2 | 6.5±3.6 | 1.00±0.35 |
| November 2011 | Marquelia | 62 | 36.2±2.6 | 14 | 1,567 | 0.668 | Buc | 0.532 | 4.1±1.6 | 26.1±41.8 | 1.06±0.45 |
The composition of the diet of C. caballus was widely different between locations and years. In Acapulco Bay, the diet included 12 items (mostly fish, peneids and crabs), but in Marquelia and Lázaro Cárdenas only 5 food items (mostly fish and crabs) were recorded. Body size (length) of fish differed between locations and sampling years (1-way Anova; F=155.8, p<0.001). The largest specimens (36.2±2.6cm) were collected from Marquelia and the smallest (23.5±4.3cm) from Acapulco Bay (2009). Only in the sample from Lázaro Cárdenas was body size positively correlated significantly with all infracommunity parameters: mean number of parasites (rs=0.331, p<0.05); mean richness (rs=0.380, p<0.05); and mean diversity (rs=0.414, p<0.01). Mean species richness of parasites ranged from 2.6±1.1 (Acapulco Bay 2012) to 4.1±1.6 (Marquelia 2011), and mean number of individual parasites from 6.5±3.6 to 26.1±41.8 (Table 2). Brillouin diversity index (H′) values varied from 0.81±0.42 to 1.06±0.45. Mean values for all infracommunity parameters were highest in Marquelia (2011) (1-way Ancova, p<0.001) (Table 2).
Multivariate analysesBecause abundance of the component species of parasites (prevalence>10%) did not vary significantly between years in the samples from Acapulco Bay (Table 1), data from this location were pooled for multivariate analyses. In the model constructed to identify stocks of C. caballus, the first 2 discriminant variables explained 100% of the variance, contributing 56.11% (eigenvalue=0.68) and 43.9% (eigenvalue=0.53), respectively. A significant overall group effect was observed (Wilks’ lambda=0.42, F337, p<0.001). Individual fish were mainly distributed along the first axis. Dimensionality tests showed that the 3 studied locations were significantly separate in both dimensions (x2=314.16, d.f.=16, p<0.01) (Fig. 1). Each fish was correctly classified to 1 of the 3 locations with an accuracy of 78.8%. Only 7 fishes from Marquelia, were assigned to Lázaro Cárdenas (Table 3). Of the 13 selected component species (i.e. prevalence>10%), only 8 were accepted by the model based on their lower Wilks’ lambda values.
Discriminant analysis classifications showing the numbers (in bold) and percentages of fish classified in each location (rows correspond to group memberships).
| Acapulco | L. Cárdenas | Marquelia | Percent* | |
|---|---|---|---|---|
| Acapulco | 191 | 23 | 12 | 84.51 |
| L. Cárdenas | 8 | 45 | 2 | 81.82 |
| Marquelia | 20 | 7 | 32 | 54.23 |
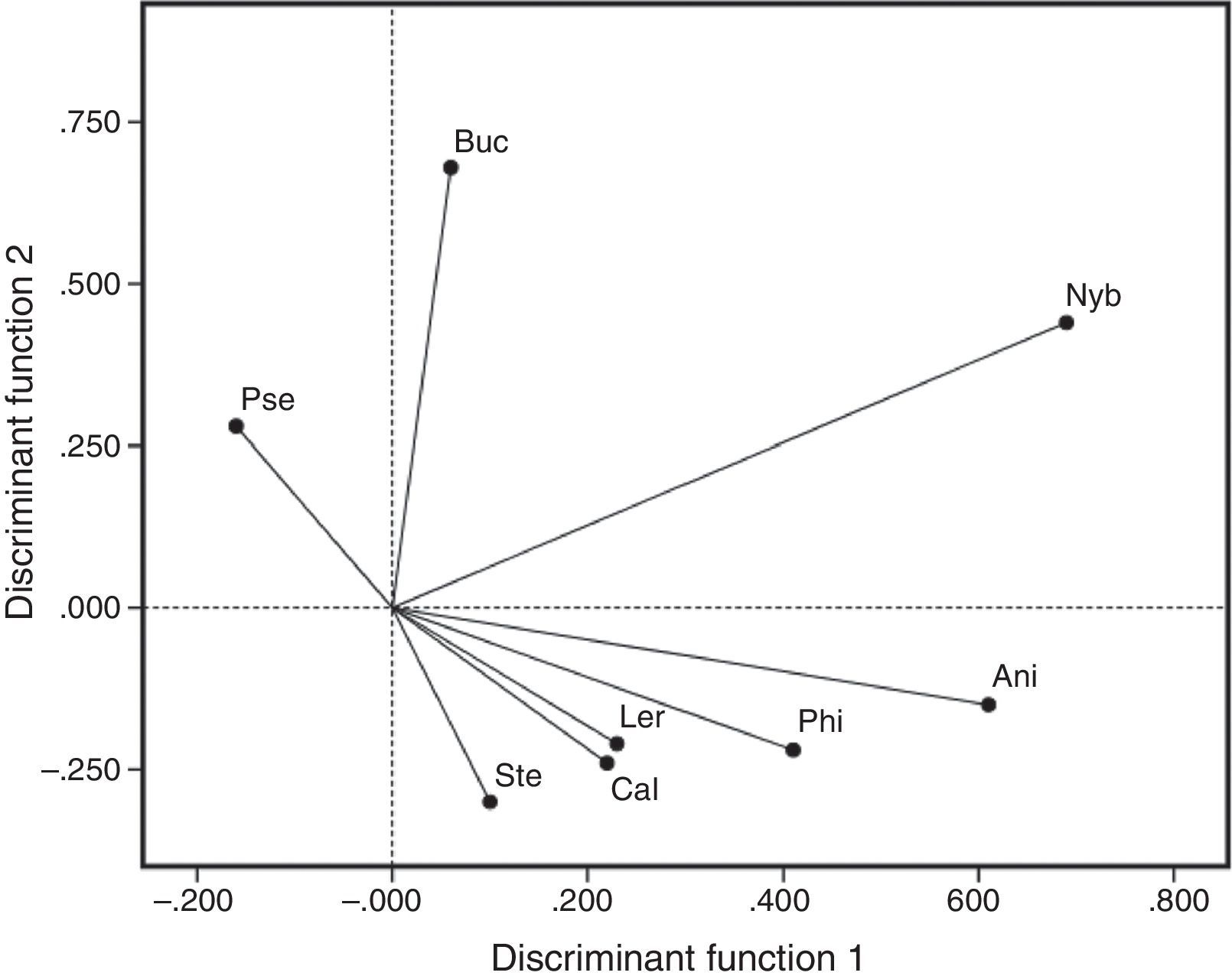
The importance of each species of parasite in distinguishing between groups (locations), evaluated as the contribution of each variable to the total sum of Mahalanobis distances, indicated that the cestode Nybelinia sp. and the digenean B. varicus were the most important helminth species in determining the position of the Marquelia sample (Fig. 2). The monogenean P. selene was characteristic of C. caballus from Acapulco Bay. Finally, the nematodes Anisakis sp. and Philometra sp., the copepods L. giganteus and C. alalongae, and the digenean Stephanostomum megacephalum were characteristic of the C. caballus from Lázaro Cárdenas (Fig. 2).
Canonical correlations between the first 2 discriminant functions, and the parasites of Caranx caballus selected as biological tags. Nyb, Nybelinia sp. and Buc, B. varicus determined the position of the Marquelia sample. Pse, Pseudomazocraes selene was characteristic of fishes from Acapulco Bay. Ani, Anisakis sp., Phi, Philometra sp., Ler, L. giganteus, Cal, Caligus alalongae, and Ste, Stephanostomum megacephalum were characteristic of the C. caballus from Lázaro Cárdenas sample.
All the species of metazoan parasites (14 helminths and 10 crustaceans) recorded in the 388 fish from the 3 locations (Table 1) are new geographical records for this host in Mexico. Carangid fish constitute one of the most intensely studied marine fish groups in Mexico, although attention has focused only on the taxonomy of their parasites (Pulido-Flores, 1997). Forty-eight species of helminth have been reported as parasites of 27 species from this family: 2 species of aspidogastreans, 29 monogeneans, 15 trematodes, and 2 acanthocephalans. However, only 14 species of helminths have been reported previously from C. caballus: the monogeneans A. caballeroi and P. selene, and the digeneans T. laticollis, D. winteri, and B. varicus were reported in C. caballus from Chamela, Jalisco, Mexico (Pérez-Ponce de León et al., 1999; Pulido-Flores, 1997). These species were also found in the present study.
Despite their belonging to a highly diverse group mainly parasitizing marine fish species, crustacean parasites have received very little attention in Mexico. In the Neotropics, copepods constitute the second largest group of parasites of marine fish, and the third largest group in freshwater fish (Luque & Tavares, 2007). In the present study, crustaceans represent 41.6% of the total species recovered, with copepods being the most diverse group (8 species) and the genus Caligus the best represented (Table 1). In a recent study done in Chamela Bay (Central Pacific coast of Mexico), Morales-Serna, Pinacho-Pinacho, Gómez, and Pérez-Ponce de León (2014) reported 16 species of Caligus from 19 species of fish, in which the parasites Caligulus robustus and C. mutabilis also were recorded in C. caballus in that study.
The parasite communities of C. caballus recorded in the present study, exhibited similar patterns at the component and infracommunity levels: low species numbers, low species diversity, and dominance by a single species (mainly the monogenean P. selene and the digenean B. varicus) (Table 2). However, the species richness observed at the component level (9–18 species, Table 2), was similar to that reported previously in other species of Caranx in the Americas, such as C. hippos (19 species) and C. latus (17 species) (Luque & Ramos, 2001). Hosts with a broad geographical distribution are exposed to larger numbers of species of parasite because they interact with many more species of intermediate host in the different regions of their distribution (Sasal, Morand, & Guégan, 1997). The present data support this suggestion since the overall recorded parasite fauna for C. caballus at the 3 sample location was 24 species, even though the highest species richness registered at the component level was 18 parasite species (Acapulco Bay 2011).
Further sampling of the parasite community of C. caballus at other locations along the Pacific coast of Mexico would add other species to the list of parasites found in this study. For example, some of the species reported previously in C. caballus from Chamela Bay (e.g., the monogeneans Protomicrocotyle manteri and Neomicrocotyle pacifica, and the digenean Pseudopecoelus priacanthi) were not observed at the 3 locations sampled as part of the present study (Pérez-Ponce de León et al., 1999; Pulido-Flores, 1997).
Similarity between component communities decayed with distance between locations, such that Marquelia and Lázaro Cárdenas had the least percent (30.4%) of shared species composition. Distance between sampling locations is one of the best predictors of similarity among parasite communities since hosts in sites nearer to each other are exposed to more geographically related parasite pools, which are more similar than those in locations further away (Poulin, 2003; Poulin & Morand, 1999).
The results of the discriminant analysis indicate that 8 parasite species can be used as biological tags to differentiate between stocks of C. caballus along the Southern Pacific coast of Mexico (Fig. 2). The cestode Nybelinia sp. and the digenean B. varicus were most abundant at Marquelia. Trypanorhynch metacestodes of the Nybelinia genus mature in elasmobranchs, often using teleost fish species such as Caranx sp. as intermediate or transport hosts. Most species of Nybelinia are commonly found in the stomach wall or the body cavity in several marine fishes (Palm, 1997). However, the Nybelinia specimens collected from C. caballus were found only in the esophagus (free in the mucus), suggesting high habitat specificity in this species of fish. A primary criterion for a parasite to be considered a tag species is that it must be easily detected in hosts that are examined. The exclusive location in the esophagus of C. caballus makes Nybelinia sp. easy to collect and therefore a promising tag species. The digenean B. varicus is a cosmopolitan parasite, using carangid fish as a final host. In Mexico, it has been reported in C. caballus and C. hippos (Pérez-Ponce de León et al., 1999). B. varicus was the second most important parasite for separation of the Marquelia population from those at Acapulco Bay and Lázaro Cárdenas (Fig. 2).
In Acapulco Bay, the monogenean P. selene was the most prevalent and abundant parasite species (Table 1). This monogenean parasitizes carangid fishes such as C. caballus, C. hippos and Trachinotus rhodopus along the Pacific coast of Mexico (Pérez-Ponce de León et al., 1999; Pulido-Flores, 1997). The second most abundant species of monogenean in C. caballus of Acapulco Bay was A. caballeroi. An advantage of using this species rather than P. selene is that it is larger and easily located in the gills, but its prevalence and abundance did not significantly differ between the sampled locations (Table 1), so it was rejected as a tag species in the discriminant analysis.
Of the 5 parasite species selected as typical of the Lázaro Cárdenas population (Fig. 2), the discriminant analysis identified the nematodes Anisakis sp. and Philometra sp. as the most important species. Nematode larvae of Anisakis spp. are the parasites most commonly used as biological tags because they are among the most common helminths in marine teleost fish (MacKenzie, 2002). This nematode was highly prevalent in fish from Lázaro Cárdenas and Marquelia, but was more abundant in the former (Table 1). Philometra sp. can be a more useful tag species because it was recorded in mature form, although only in the gonads of female fish. This pronounced preference for a specific infection site has been reported in other species of this helminth genus (Moravec & Justine, 2014).
In summary, of the 24 species of parasite recorded from C. caballus at the 3 locations, 8 can serve as biological tags. Difference in infection levels allowed differentiation between the 3 populations of fish. Based on these tag species, and the parasite component and infracommunity data, it appears there is no movement of C. caballus between the 3 locations, that is, this fish species does not migrate between locations.
Funds for this study were provided by “Fortalecimiento del Doctorado en Ciencias Ambientales de la Universidad Autónoma de Guerrero” (Fomix Conacyt-Guerrero, 2015), and “Indicadores para el Desarrollo Sustentable de la Pesca y su relación con el Turismo” Projects, en la comunidad de Barra de Potosí, Guerrero (Proyecto Semilla 2014, UAGro.). The authors wish to thank to students of the Marine Ecology Academic Unit (UAGro), for their assistance in field, and laboratory work, and two anonymous reviewers that provided important comments to improve this manuscript.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.