Schizophrenia is a clinically heterogeneous syndrome affecting multiple dimensions of patients’ life. Therefore, its treatment might require a multidimensional approach that should take into account the efficacy (the ability of an intervention to get the desired result under ideal conditions), the effectiveness (the degree to which the intended effect is obtained under routine clinical practice conditions or settings) and the efficiency (value of the intervention as relative to its cost to the individual or society) of any therapeutic intervention. In a first step of the process, a group of 90 national experts from different areas of health-care and with a multidimensional and multidisciplinary perspective of the disease, defined the concepts of efficacy, effectiveness and efficiency of established therapeutic interventions within 7 key dimensions of the illness: symptomatology; comorbidity; relapse and adherence; insight and subjective experience; cognition; quality of life, autonomy and functional capacity; and social inclusion and associated factors. The main conclusions and recommendations of this stage of the work are presented herein.
La esquizofrenia es un síndrome clínicamente heterogéneo que afecta a múltiples dimensiones vitales del individuo. Su tratamiento requiere un abordaje multidimensional en el que se deberían tener en cuenta la eficacia (la capacidad de una intervención para obtener el resultado pretendido en condiciones ideales), la efectividad (el grado en que se obtiene el efecto pretendido en condiciones de la práctica clínica habitual) y la eficiencia (el valor de la intervención con respecto al coste para el individuo o la sociedad). En una primera fase, un grupo de 90 expertos nacionales de todos los ámbitos, desde una perspectiva multidimensional y multidisciplinar de la enfermedad, definieron los conceptos de eficacia, efectividad y eficiencia en torno a 7 dimensiones clave: síntomas; comorbilidades; recaídas y adherencia; conciencia de enfermedad y experiencia subjetiva; cognición; calidad de vida, autonomía y capacidad funcional, e inclusión. Las principales conclusiones de esta fase se presentan en este trabajo.
Schizophrenia (schizophrenic spectrum disorders) is the most frequent psychotic disorder within non-affective psychotic syndromes, reported in DSM 5 under the heading “Schizophrenia and other psychotic disorders” and includes: schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, substance-induced psychotic disorder, psychotic disorder secondary to a medical illness, catatonia associated with a medical illness, and unspecified psychotic disorder.
This is a clinically heterogeneous syndrome that usually affects multiple dimensions of the individual's life, beginning frequently at the end of adolescence or early adulthood, and is associated with progressive functional deterioration in a significant percentage of cases, especially in cases where the optimal required treatment is not provided. Annual prevalence is currently 0.3% and the annual incidence is 0.8 cases per 10,000 inhabitants, which represents approximately 4000 new cases of schizophrenia diagnosed per year in Spain.1
According to the Global Burden of Disease Study in 2010, mental disorders account for 7.4% of the global burden of disease in terms of disability adjusted life years (183.9 million DALY); schizophrenia is considered to be responsible for 7.4% of that burden of disease, associated with mental disorders.2 The economic cost is a parameter that is becoming increasingly recognised in health policies and planning in research. To achieve a reduction in indirect costs, which are the majority of the costs associated with mental disorders in social, employment, family and personal terms, it should be assessed whether direct costs must be increased or not, something which is essential in the medium and long term, regardless of whether or not there is more and better social and healthcare investment in the short term to reduce the high levels of disability associated with the disease.3
The mortality rate of people with schizophrenia is twice that of the general population.4–6 The life expectancy of men and women with schizophrenia is 15 and 12 years, respectively, which is shorter than for those without schizophrenia.7
Today there is growing agreement on the influence of intensive treatments from the initial phases over the prognosis of the disease. Beyond the choice of antipsychotics for initial treatment, these intensive treatments broadly address the different needs of patients and their families.8
Early and optimal diagnosis and treatment of schizophrenia can reduce the risk of disability and increase the chances of the patient's functional recovery. The treatment of schizophrenia requires a multidimensional approach which should take into account the following parameters: efficacy (the ability of an intervention to obtain the intended result under ideal conditions), effectiveness (the degree to which the intended effect is achieved with normal clinical practice), and efficiency (the value of the intervention with respect to the cost for the individual or society). It is in this context where evidence demonstrates the relevance of implementing and developing optimised treatment approaches to the progression of the disease,9,10 and where the Rethinking movement arises.
Objectives and methodologyRethinking represents an initiative that, as its main objective, aims to draw up a number of expert recommendations on the fundamental treatment aspects of the best treatment and care in the real world for patients with schizophrenia and their caregivers. To this end, two specific objectives were proposed: 1) to assess the efficacy, effectiveness and efficiency of the treatment approaches currently adopted in our clinical environment; and 2) to identify needs which are not covered in the treatment of the disease and, therefore, propose specific action for improvement of the care and treatment of patients and caregivers. To achieve these objectives, a research methodology was designed that began with the formation of a multidisciplinary scientific committee (psychiatrists, managers, representatives of patient associations, representatives of scientific societies) from a selection of 90 recognised national experts with experience in the treatment of schizophrenia. These experts provided a multidimensional and multidisciplinary view of the disease, and committed themselves to sharing their experience in order to reach final recommendations on issues of interest.
This group of experts was selected on the basis of their professional recognition in the area of interest and the degree of experience and involvement in the treatment of those with schizophrenia in routine clinical settings. Considered and then included were professionals and groups from various different fields who were directly involved in the care and treatment of people with schizophrenia, and their caregivers: psychiatrists, psychologists, mental health nursing staff, health economics specialists, health managers, representatives of scientific societies, patient associations and patients’ relatives (Appendix A).
The scientific committee, in a first working meeting and through a reasoned group discussion, defined the overall working methodology to address the project, and posited 7 specific dimensions of the disease to work on the proposed objectives. These dimensions were as follows: symptoms; comorbidities; relapses and adherence to treatment; consciousness of illness and subjective experience; cognition; quality of life, autonomy and functional capacity, and inclusion.
The concepts of effectiveness and efficiency were specifically defined and analysed on the basis of these 7 key dimensions by the scientific committee. Working groups composed of experts from different disciplines were formed. Each working group was assigned a dimension and pairs were selected to evaluate, weight up and agree on the concepts of efficacy, effectiveness and efficiency in each dimension. In order to run this research, a critical and contrasted scientific review was undertaken to respond to the proposed topics. At a meeting where all the experts participated, the conclusions reached by each group were put together with the aim of producing a general document that would systematically record the evidence, suggestions for action and conclusions reached by the panel of experts on each of the clinical dimensions examined and analysed. The panellists, in their work, held interactive discussions, sharing their reasoned opinions and their commitment to reconsider some of these opinions by following the comments, arguments or suggestions of the other panellists in order to come to the final conclusions.
The Rethinking movement continued in a second phase in 2015, examining in greater depth the unmet needs identified by the professionals in their day-to-day work when dealing with the treatment of schizophrenia cases, and thus promote and implement areas for improvement. As a consequence of the analysis in this first part, it was considered necessary to include representatives of mental health nursing staff, due to their relevance in many of the treatment processes. The final document specifically highlights relevant and attainable intervention strategies that the experts’ group recommend by consensus, to improve the care of patients and caregivers. The results achieved were approved by the majority of the expert group, although there may have been some divergences on specific issues from a minority of the panel.
ResultsIn this article we thus present the main considerations and conclusions reached after the work done during these years by the panel of experts in order to define and describe a series of actions that would improve efficacy, efficiency and effectiveness in the current treatment of schizophrenia in our society.
SymptomsSymptoms are the determinants of diagnosis and, along with functionality, the aspect that has greatest weight in the choice of treatment and in the prognosis of the patients.
Positive symptoms are of prime importance in the diagnosis of the disease,11 with the most frequent being hallucinations (68%), delusions (65%) and conceptual disorganisation (50%).12 For their assessment, we have a number of validated instruments, both general (e.g., Brief Psychiatric Assessment Scale [BPRS],13 Positive and Negative Symptoms Scale for Schizophrenia [PANSS],14,15 Scale for the Assessment of Positive Symptoms [SAPS])16 as specific for different symptoms (e.g. Auditory Hallucinations Assessment Scale [PSYRATS]17,18). The efficacy of first and second generation antipsychotics for these symptoms is similar, providing better results if good treatment compliance is assured.19 In addition to this, cognitive-behavioural therapy adapted for patients with persistent positive symptoms has been shown to be a useful intervention, although the scale of the effect observed in clinical trials is, at best, moderate.20
It is noteworthy that in clinical practice, despite the usefulness it may have for objectifying the clinical status of patients, the routine use of these scales is minimal, their use being restricted to research environments.
At present, there is some controversy as to whether the symptoms of disorganisation (thought, language, behaviour) should be included in the positive dimension,21 or, on the contrary, they should make up an independent dimension.22 From the point of view of their assessment, there are no specific psychometric instruments validated in Spanish. Their assessment therefore falls on the items on the positive symptom scales, such as the PANSS and the SAPS, and the clinician-assessed item from the severity scale of the symptoms for the dimensions of psychosis.23
Negative symptoms are present in 58% of patients during the first episode.24 They are better predictors of progression than positive symptoms25,26 and, along with cognitive symptoms, have a major impact on functioning, lifestyle and somatic health.27 Several batteries and scales have been drawn up for assessing the psychopathology of negative symptoms, including the following scales: Brief Scale for Negative Symptoms (BNSS),28 the Interview for the Clinical Assessment of Negative Symptoms (CAINS),29,30 the Inventory for Deficient Schizophrenia Syndrome (SDS)31,32 or the Scale for the Assessment of Negative Symptoms (SANS).33,34 There are also scales such as the PANSS, which, in some of their sections, measure negative symptoms, or others that assess secondary negative symptoms such as motor symptoms (e.g. with the modified Simpson-Angus Scale35).
Among the limitations we have in evaluating the efficacy of antipsychotics for these types of symptoms is the difficulty, in clinical trials, of distinguishing between primary negative symptoms–directly related to the pathophysiology of schizophrenia–and symptoms which are secondary to psychotic, affective symptomatology, concomitant diseases or the side effects of some drugs. The main reason is that the scales usually used in research and clinical settings do not have the capacity to adequately identify this symptomatology, resulting in an under- or over-inclusion of symptoms and behavioural assessment rather than experiential assessment.36 There are no drugs that have demonstrated efficacy for primary negative symptoms,26,37 furthermore, it seems that drugs that block dopaminergic receptors, such as antipsychotics, can produce negative symptoms in healthy people.38 Although psychosocial interventions have been more studied for the management of positive symptoms, there is some evidence that cognitive-behavioural therapy, social skills training, and combined interventions are associated with continued improved negative symptoms for more than 6 months. There are, however, many questions to be clarified regarding these interventions, such as whether or not combined treatments can produce greater and more lasting improvement.39–41 There is currently no approved pharmacological approach for the treatment of primary negative symptoms, revealing a key unmet need in this area of treatment.42 The efficacy results of some new treatments aimed at increasing glutamatergic neurotransmission and associated with antipsychotics were promising in phase II, although the Phase III studies did not confirm these results.43,44 Negative symptoms secondary to positive symptoms, depressive symptoms or antipsychotic treatment, would require specific treatments.
Affective symptoms of schizophrenia have received little attention, although 25%–30% of patients present post-psychotic depression.45 Diagnosis is difficult, especially differential diagnosis with negative symptoms. For its assessment, we have the Calgary Depression Assessment Scale.46,47 Data on efficacy and efficacy of antipsychotics for these symptoms is in short supply and suggests limited efficacy, although this is probably higher for some second generation antipsychotics.48 With regard to manic symptoms, it is known that their presence contributes to the prognosis of schizophrenia. Moreover, DSM-5, in its deconstruction of the disease, includes mania in its severity scale of symptoms of psychosis dimensions, as assessed by clinicians.23 Another instrument that has been used to evaluate manic symptoms in schizophrenia is the Young Mania Rating Scale,49 although its validity for assessment in patients with schizophrenia has been little researched.50
Up to two thirds of patients with untreated schizophrenia have motor symptoms.51 Motor symptoms are also frequent in patients receiving antipsychotic treatment, with differences between different drugs.52 Several scales, such as the Simpson Angus Scale (SAS)35 the Abnormal Involuntary Movement Scale (AIMS),53 and Barnes’ Scale for Assessment of Akathisia (BARS)54 are available for assessment. There are virtually no efficacy studies to guide the treatment of these symptoms. The two most serious situations are catatonia and tardive dyskinesia. Benzodiazepines and electroconvulsive therapy can be used for catatonia.55 For tardive dyskinesia the treatment alternatives provide very limited results.
The prevalence of consummate suicide in patients with schizophrenia is 4.9%56 and the highest proportion of suicides occur during the first 2 years from the onset of the disease57. The Beck Suicide Intent Scale (BSIS) 58 or the Columbia Suicide Severity Rating Scale (CSSRS) can be taken for suicide risk assessment.59 Preventive treatment for suicide risk has been little researched and currently only one pharmacological treatment, clozapine, is considered efficient and effective.60,61 Suicide risk management is also important in the treatment of comorbidities such as depression or substance use disorders.
Along with the risk of suicide, in some cases patients with schizophrenia may exhibit aggressive behaviour. It is important to note that people with psychopathologically stabilised schizophrenia do not present violent behaviour more frequently than the general population.62–64 Aggressive behaviour is more frequent prior to diagnosis and has as risk factors positive symptoms, comorbidity with substance abuse disorders, lack of adherence to treatment, recent exacerbation, vital stressors in childhood and psychopathic traits of personality.62,65 The most widely used validated assessment tool is the Manifest Aggressive Scale (OAS).66 The treatment approach will depend on the paths leading to this aggressive behaviour; If aggression is related to psychotic symptoms, clozapine is an effective treatment, but if this behaviour depends, for example, on the presence of comorbidity with a substance abuse disorder, it may require other drugs or psychosocial interventions.67 Regarding the treatment of aggressive behaviour, it is possible to indicate that both first and second generation antipsychotics are effective, and clozapine seems especially useful in the management of these patients.67
Multiple unmet needs have been detected in relation to the symptoms of schizophrenia that affect efficacy, effectiveness and efficiency. The persistence of symptoms, especially negative or cognitive symptoms, from many of these dimensions in a significant proportion of symptomatic patients after initiating treatment–or resistance to the treatment of positive symptoms–advocates the need for more effective treatments and the search for new treatment targets with different mechanisms of action.26,68,69 Cognitive-behavioural therapy may be effective for certain symptoms and can lead to a substantial reduction in relapses; it improves the patient's adaptation to his environment and his overall functioning, although recent meta-analyses do not demonstrate greater efficacy for this type of therapy than other less sophisticated and less demanding interventions.70.71 Thus, further studies on the effectiveness and efficiency of the combination of psychopharmacology with different psychosocial interventions are needed.
Most research on the results of pharmacological interventions in schizophrenia has focussed more on improving symptomatology (especially positive symptoms) and not on how this translates into an improvement in functionality and the quality of life of the patient. In addition, variable compliance has not received the necessary attention and this variable affects many of the symptoms assessed in the studies. More pragmatic clinical trials are needed that would respond to clinical questions that are more focussed on not only positive symptoms (the need to improve treatments for primary negative symptoms) and which would enable assessment of outcomes under real-life conditions. These studies should include cost-effectiveness measures.
Comorbidities in schizophreniaAssuming remission and/or recovery as a treatment objective, the treatment of schizophrenia should go beyond the management of psychosis exclusively, also encompassing general health.
From the point of view of physical health, the most frequent comorbidities are those related to cardiovascular and metabolic risk factors.72–74 However, other medical co-morbidities such as oral problems, osteoporosis or pulmonary thromboembolism should be considered, to name but a few, which may also be present more frequently in these patients. Although this is associated with other comorbidities such as anxiety disorder or posttraumatic stress disorder, the most common comorbidities in patients with schizophrenia are obsessive compulsive disorder (prevalence 3.5–46%), substance abuse disorders (prevalence 47%), and depression (vital prevalence 81%).75
Diagnosis, especially early diagnosis, of physical comorbidities continues to be an important area of improvement in the management of these patients by all involved (patient, primary care physician and psychiatrist). The “Consensus on the physical health of the patient with schizophrenia on the part of the Spanish Societies of Psychiatry and Biological Psychiatry”76 has had a very limited impact on clinical practice77; unfortunately, this lack of follow-up of the guidelines for the management of psychotropic drugs extends to other specialties.78 Health promotion and prevention programmes require to be introduced for people with schizophrenia, and this entails greater coordination between health care networks and an assessment of their efficacy, effectiveness and efficiency, since the available information indicates a clear lack of evidence in this regard.72
The presence of physical comorbidities in patients with mental disorders increases costs. Specifically, schizophrenia is associated with higher costs per case than depression, in part because there is a higher risk of presenting physical comorbidities.79 This is a fundamental factor that has not been taken into account when assessing not only the efficiency but also the effectiveness and efficacy of the treatments of schizophrenia.
Just as we have indicated for diagnosis, the treatment of comorbidities, whether physical or mental, in some cases requires specific interventions (for example, treatment for cannabis abuse, smoking, guidance in healthy habits) and a prospective assessment of the usefulness and value of these interventions, all with a patient-centred approach. The initial results of some pragmatic clinical trials suggest that measures of that type could be effective and cost-efficient.80
In conclusion, the presence of comorbidities is one of the least developed fields of schizophrenia, and influences the efficacy, effectiveness and efficiency of the treatment of schizophrenia. It is therefore necessary to design education and awareness programmes for patients, primary care physicians and psychiatrists in order that all understand the importance of improving physical health, especially reducing cardiovascular and metabolic risk factors. Improving lifestyle and counteracting the side effects of treatments through specific intervention programmes are critical factors in this regard. Clinical practice and scientific evidence show the need to establish a comprehensive approach to health in patients as a way of achieving functional recovery.
Adherence to treatment and relapsesAdherence is defined as the extent to which a person's behaviour in terms of following prescribed treatment (pharmacological treatment, psychotherapy, lifestyle changes, etc.) corresponds to recommendations agreed with a healthcare provider. Partial adherence or complete withdrawal from treatment is often common in severe mental disorders; it is estimated that approximately half of the patients with schizophrenia usually stop taking 30% or more of the prescribed medication.81,82 Lack of adherence is associated with serious clinical consequences (increased risk of relapse, suicide or aggressive behaviour, among others) and high costs, both in monetary terms and psychosocial resources.83,84
In general, lack of adherence is of extraordinary relevance in all chronic diseases. It is estimated that between 33% and 69% of all hospital admissions in the U.S. are due to lack of adherence.85 The ability of clinicians and informal caregivers to address this problem is limited. Initiatives that improve adherence are key to progress towards more effective and comprehensive treatment plans.
Adherence assessment can be performed with direct methods, such as determining plasmatic levels of the drug or its metabolites, or indirect methods, such as medication count, patient interview or questionnaire use, although the only one validated in Spain is Morisky-Green's.86,87 The recommendation is to use both assessment methods as they provide complementary information. The most important factors associated with lack of adherence are lack of disease awareness, lack of adherence, presence of substance abuse disorder, lack of efficacy or poor tolerability of antipsychotic treatment, lack of family support, critical attitude and stigmatisation by the family.88–90 Possible strategies to improve adherence include: 1) pharmacological strategies, selecting the drug according to its profile of adverse effects, dosage and patient preferences, using long-acting intramuscular antipsychotics and avoiding combination therapy and 2) non-pharmacological strategies such as psychoeducation; strengthening the treatment alliance through decision-making techniques shared with the patient, and community assertive therapy, which have been shown to be efficacious, effective, and possibly efficient.91,92 In randomised clinical trials it has been observed that early intervention with family therapy reduces the severity of symptoms and improves access to treatment and compliance with it. Early intervention with cognitive-behavioural therapy reduces the severity of symptoms, with a low impact on relapses and hospital admissions.93
There is no universally accepted definition of relapse. The most frequently used in the literature is hospitalisation, although many others have been used that include scores on scales, subscales or items, behavioural changes or other operational criteria such as those of Csernansky and Andreasen.94 Among the factors that precipitate relapse are poor adherence to treatment, environmental stress, depression, substance abuse and history of relapse,94–96 and among protective factors: good adherence to treatment, duration of untreated psychosis less than 60 days and good response to treatment.97
Recent studies show the effectiveness of maintaining antipsychotic treatment with minimal effective doses for the prevention of relapses as a maintenance treatment.98,99 However, further maintenance treatment studies are required to compare with withdrawal of medication before it can be concluded that the best treatment strategy in patients with schizophrenia, following an acute psychotic episode, is indefinite maintenance treatment.
Relapses have relevant clinical and socioeconomic consequences. From the clinical point of view, it has been estimated that two-thirds of patients have a relapse and, of those who do, one in 6 does not recover from the episode.100 The costs of relapses, defined as hospitalisations, are high because they account for 38.5% of total costs of schizophrenia, only surpassed by the cost of informal care (47%) and well above the pharmaceutical cost (12.8%).101
Insight and subjective experienceAbsence of insight of illness and subjective experience is a key aspect in the prognosis of psychosis.102 The lack of insight into the existence of a chronic disease that needs treatment is a non-specific manifestation but is a characteristic of psychosis. This is a complex and multidimensional phenomenon that includes recognition of having a illness (what has been called cognitive insight), and awareness of clinical symptoms and the need for treatment (so-called clinical insight).103 Disease insight is often an indicator of state and not of trait, and is therefore a modifiable factor, which is relevant since it is an important predictor of functioning after a first psychotic episode.104,105 Subjective experiences are those related to the identification of, strictly speaking, subjective aspects of the mental states of the patient and which require the subject himself to be identified. It is necessary to distinguish between subjective experiences with taking antipsychotics, on the one hand, and subjective experiences as a vital situation, on the other, the latter being closer to the dimension of quality of life.
The assessment of both insight and subjective experience with medication will depend on the type of treatment intervention. If the objective is to assess response to medication, insight can be assessed using the Birchwood Insight Scale (IS)106,107 or the Scale to Assess Unawareness of Mental Disorders (SUMD).108 If the objective is the assessment of psychosocial treatment, the recommendation is to use the Beck Cognitive Insight Scale (BCIS)109,110 or the Personal Beliefs about Illness Questionnaire-revised (PBIQ-R).111 Similarly, for the assessment of subjective experience with treatment, the recommendation would be to use the Drug Attitude Inventory (DAI-30 or the abbreviated version DAI-10)112–114 if the objective is to assess pharmacological treatment, or the Subjective Well-Being under Neuroleptics Scale (SWN-K)115,116 to evaluate psychosocial interventions.
There are few assessable studies on the effect of antipsychotic medication on insight and with inconclusive results.117 With regard to psychosocial interventions, the promotion of support and social relations has been assessed, along with vocational rehabilitation, cognitive-behavioural therapy, motivational intervention therapy aimed at increasing adherence, training in social skills, and self-observation through video and comprehensive interventions.117 In general, these interventions have a modest effect on the modification of insight, with very heterogeneous results.117 On the other hand, there is still insufficient evidence to show that improved insight is associated with better health outcomes for the individual, and studies on this are required.
CognitionIn schizophrenia basically 7 domains of cognition are affected from the early stages of the disease: processing speed, attention, working memory, verbal and visual learning, reasoning and problem solving. 11,118 As a consequence, the productivity of patients, their quality of life and social functioning is affected. Therefore, it is considered important to evaluate the cognitive functioning deficit in patients with schizophrenia, especially at debut, in patients with a recurrent or refractory progression, or in those who complain of amnestic discomfort or functional deterioration. There are multiple instruments119 for the assessment of cognitive function in patients with schizophrenia, which include complete neuropsychological batteries such as MATRICS, developed for use primarily in clinical trials, or instruments that have been validated in our setting, such as the Brief Scale to assess Cognitive Impairment In Psychiatric Patients (SCIP)120,121 or the Behavioural Assessment of the Designative Syndrome (BADS).122,123 Cognitive interview tools have also been developed, such as the Schizophrenia Cognition Rating Scale (SCoRS),124 which can help assess the level of cognitive impairment of the patient, and are being used in clinical trials for the assessment of interventions directed to this. However, more pragmatic tools are needed to allow screening of cognitive disorders in patients with schizophrenia.
The study of the variables that can mediate cognition, both in general cognitive functioning and social cognition, has been very limited, with small sample size in studies and with limitations when measuring the desired variables. So far it has only been possible to demonstrate that clinical factors and psychological treatments are mediators which are moderately related to cognition.70,125 Thus, patients with greater severity of symptoms present poorer neurocognitive performance, this being related to poorer social functioning. This last is influenced by the duration of unmasked psychosis126. Both cognitive therapy in the case of neurocognition, and intervention programmes on social cognition are the programmes that have yielded the most promising results to date.127
As with primary negative symptoms, most studies evaluating the efficacy of antipsychotic drugs to improve cognition in schizophrenia, when taking into account improvement, secondary to a reduction in positive symptomatology, do not find positive results in this domain.128 Moreover, in both animal models and healthy individuals, dopaminergic receptor blockers appear to worsen cognitive performance.129 Given the limitations of pharmacological treatments, neuropsychological rehabilitation therapies encompassed within the term “cognitive rehabilitation techniques” (CRT)70 have been proposed as an adjunctive treatment for cognitive disorders. They consist of applying specific techniques directed at exercising cognitive abilities and learning compensatory strategies, directed and supervised by a therapist.
There are different formats and programmes that can be presented in traditional pen and paper format or through computer programmes.130 The objective of the CRTs is to alter the level of individual functioning by improving the deficient cognitive area. For a CRT to be considered effective, it should be generalised to daily life. Overall, we can say that cognitive rehabilitation can become the critical factor for treatment success in at least one group of patients. It is important to note, in this regard, that cognitive impairment is present in the early stages of the disease and that cognitive rehabilitation shows moderate to major changes in these stages.131 The use of new technologies for cognitive training has succeeded in influencing the facilitation of mechanisms of cerebral plasticity132 and at the same time promoting improvements in functioning in the real world.133
In summary, clinicians should be aware that cognition is affected in most patients, even before the onset of the disease, and is therefore an important part of the clinical assessment of patients. It should be noted that alterations in cognition are associated with a greater degree of functional and social disability. This association has made cognition a relevant treatment target. The investigation of treatment strategies aimed at improving cognition is still at a very early stage, and there is still no suitable pharmacological treatment. Although this is an area that needs further development, cognitive rehabilitation is the most interesting option.
Quality of life, autonomy and functional capacityThe approach to treatment of people with schizophrenia requires a paradigm shift, which would lead to a greater degree of personal recovery, attempting to restore the premorbid situation and resume daily life.134 This implies focussing on three fundamental aspects: quality of life, personal autonomy and social functioning. Quality of life, as a subjective assessment of the person's life circumstances; personal autonomy, such as the capacity and right to make decisions that affect their personal life; and social functioning, such as the ability to function and develop in different areas of life.
Until now this dimension of recovery has been little considered in studies that assessed the efficacy and effectiveness of different treatments, especially psychopharmacological. There is more data on the efficacy, effectiveness and efficiency of psychosocial interventions and the organisation of services on outcomes in quality of life, personal autonomy and social functioning. In order to achieve this recovery, in addition to acute treatment of symptoms and maintenance of treatment to avoid relapses, all those affected require to be empowered: introducing the family as a fundamental element and ensuring better organisation in the care of persons with schizophrenia. Empowerment has to take place simultaneously at the patient population and individual level, as it consists of a multidimensional social process through which individuals and groups achieve better knowledge and control over their lives; As a consequence, they can transform their social and political environment to improve their circumstances of daily life which are related to health.135
The family dimension has begun to be considered in recent years, in the sense that the family and the patient form an indissoluble unit for treatment action with the aim of improving the progression and prognosis of the disease. It is necessary to detect homogenous groups of relatives and patients with schizophrenia to be able to intervene at family level with interventions based on the criteria of efficiency and effectiveness applied to the Spanish population and in our environment i.e. this would bear in mind our high-pressure healthcare situation; resource constraints, over-medicalisation, and lack of community resources. It is therefore necessary to provide healthcare and economic policies which are tailor-made for these circumstances.136
Improving the organisation of care for people with schizophrenia implies136: providing the required integrated, evidence-based care that will meet the needs of physical and mental healthcare; providing support for people with schizophrenia to live in their usual environment, and developing mechanisms to help them navigate complex employment and social benefits systems; providing specific support, information, and educational programmes for family members and caregivers in order that they can provide care for individuals with schizophrenia in a manner that minimises disruption to their lives; regularly reviewing and improving care procedures for people with schizophrenia, with all interested parties involved, including organisations that support people with schizophrenia; providing support which is equivalent to the impact of the disease, researching and seeking new treatments, and running adequately and regularly funded awareness campaigns that would form a permanent part of the action plans.
Regarding pharmacological treatment, in general terms, patients receiving this treatment have a better quality of life than those who do not receive it.137 Second-generation antipsychotics would have advantages in this regard compared to first-generation antipsychotics, 138 and long-term injectable antipsychotics appear to have advantages over oral antipsychotics in terms of better functioning, quality of life and patient satisfaction.139 However, some studies do not show any benefit for long-term injectable antipsychotics versus oral antipsychotics, in terms of reducing relapse.140
A related aspect that is increasingly relevant is the concept of “recovery from the patient's perspective”. This concept goes beyond the improvement in quality of life or subjective experience, and implies the patient's overall perception of feeling recovered as a person, in all senses. That is, it places the subject-patient and his own subjective perception at the centre. This aspect has been gaining importance in recent years, to become an essential aspect in the planning of mental healthcare services in many countries. In a recent qualitative study with Delphi groups on 381 patients diagnosed with schizophrenia,94 items were recorded which were related to the concept of recovery from the perspective of the patient. The first two most frequently cited items were: 1) to achieve an acceptable quality of life and 2) having a positive feeling towards oneself.141
In summary, this dimension needs to be incorporated as a fundamental measure of outcome, as regards both the preparation of individual care plans, and the treatments made available to those affected and their care provider organisation. To that end, their monitoring in usual clinical practice, and the use of this in research are key elements which require a prior critical and consensus-based assessment of the different scales existing.
InclusionAccording to biopsychosocial models, stigma is revealed in three aspects of social behaviour142: stereotypes, understood as general agreement as to what characterises a group of people; social prejudices, that is, the application or emotional experience of these stereotypes; and effective discrimination, that is, the behaviour of rejection towards those groups. The person with mental illness must face a threefold difficulty in recovering: the illness itself, the prejudices and discriminations he receives as a result of it (social stigma) and self-stigma (or internalised stigma), and the anticipated discrimination that the patient believes will occur before they have experienced it. The prevalence of internalised stigma is 41.7%,143 and 40–79% of the relatives of patients with severe mental disorders are considered stigmatised.144 The consequences of stigma are severe, ranging from inhibition when seeking help or treatment for their illness to a negative impact on the quality of life of the patient and their caregivers. The stigma assessment has so far focussed on internalised stigma, using scales such as the Internalised Stigma Scale (ISS)145 or the Internalised Stigma Inventory of Mental Illness (ISMI).146 However, it is also necessary to assess stigma within the general population with questionnaires such as the Mental Health Knowledge Scale (MAKS),147 the Community Attitudes towards the Mentally Ill (CAMI) scale148 or the Informed Behaviour Scale and Intended (RIBS).149
The fight against stigma should be a key element in mental healthcare plans in the coming years. This fight against stigma should include interventions on: 1) the patient: with strategies aimed at optimising pharmacological treatment, psychoeducational strategies to improve knowledge of the disease, managing stress, improving cognitive deficits, preventing relapses and consumption of substances, strategies to improve personal skills and promote autonomy, involving the patient in identifying discriminatory practices, and promoting access to and use of new technologies; 2) the family: with psychoeducational strategies aimed at improving knowledge and management of the disease, involving families in the treatment process; 3) health professionals: with training for social action as a model of tolerance and acceptance of people with mental disorders; 4) health authorities: promoting legislative and policy initiatives that favour the inclusion of mentally ill patients and developing management plans which would meet the expectations and needs of users; and 5) the media: through activities that promote the dissemination and implementation of existing guidelines for the treatment of mental health information and, among others, seeking to avoid transmitting a negative impression of mental illness.
ConclusionsWhat is required is a change in the paradigm of schizophrenia treatment, where the different dimensions reviewed in this article are considered, not only in terms of parameters of efficaciousness, but also of effectiveness and efficiency. There are numerous needs which are not covered in the healthcare received by persons with schizophrenia, but that they do require. The individual, family and social costs are very high. Studies are needed that would include key outcome variables which are ecological, pragmatic and relevant to the quality of life and functioning of persons with schizophrenia, their families and society.
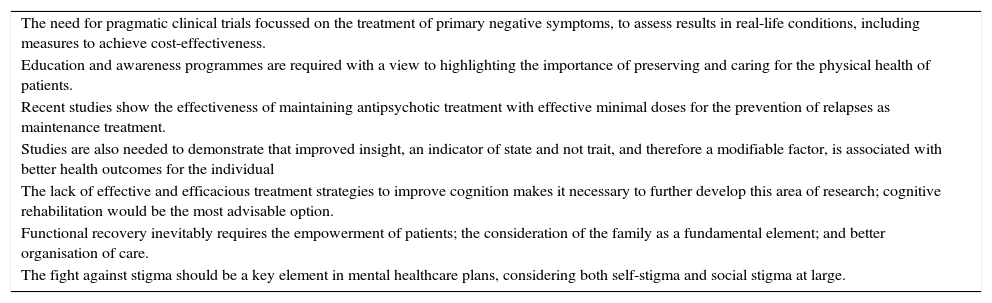
The Rethinking working group, on an interdisciplinary basis, has contributed, through the collaboration of all parties involved (psychiatrists, clinical psychologists, nursing personnel, health economics specialists, health managers, representatives of scientific societies and patient associations, as well as family members and caregivers), a consideration and set of proposals to prioritise lines of action, including parameters of effectiveness and efficiency, that would address the unmet needs identified. Table 1 summarises the main conclusions of each of the dimensions analysed.
Relevant conclusions.
| The need for pragmatic clinical trials focussed on the treatment of primary negative symptoms, to assess results in real-life conditions, including measures to achieve cost-effectiveness. |
| Education and awareness programmes are required with a view to highlighting the importance of preserving and caring for the physical health of patients. |
| Recent studies show the effectiveness of maintaining antipsychotic treatment with effective minimal doses for the prevention of relapses as maintenance treatment. |
| Studies are also needed to demonstrate that improved insight, an indicator of state and not trait, and therefore a modifiable factor, is associated with better health outcomes for the individual |
| The lack of effective and efficacious treatment strategies to improve cognition makes it necessary to further develop this area of research; cognitive rehabilitation would be the most advisable option. |
| Functional recovery inevitably requires the empowerment of patients; the consideration of the family as a fundamental element; and better organisation of care. |
| The fight against stigma should be a key element in mental healthcare plans, considering both self-stigma and social stigma at large. |
One relevant conclusion is highlighted for each of the 7 dimensions.
The growing scientific advances in the field of schizophrenia, improvements in treatment and care, and the greater information available to those affected and their caregivers, are leading us to a new stage characterised by growing optimism about the future of those who suffer from this condition.150 Our responsibility as experts must be to lead the movement to respond to the needs identified, effectively and efficiently. It is the desire of the Rethinking movement to implement concrete action plans over the next few years to continue to improve the lives of people with schizophrenia.
Ethical responsibilitiesProtection of people and animalsThe authors state that no experiments have been performed on humans or animals for this research.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
FinancingOtsuka and Lundbeck have provided financial support to hold working meetings but have not participated in any way in the manuscript or in its design, content or conclusions.
Conflicts of interestBenedicto Crespo-Facorro has received consulting and lecture fees from Janssen Johnson & Johnson, Lundbeck, Roche and Otsuka Pharmaceuticals.
Miguel Bernardo has received consulting fees or research grants from ABBiotics, Adamed, Almirall, Amgen, Boehringer, Eli Lilly, Ferrer, Forum Pharmaceuticals, Gedeon, Hersill, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Roche and Servier. He has obtained research grants from the Carlos III Health Institute, the Spanish Ministry of Science and Innovation, the Ministry of Economy and Competitiveness, the Spanish Centre for Biomedical Research in the Mental Health Network (CIBERSAM), the Government of Catalonia, the Department of Universities and Research Department D’Economia i Coneixement (2014SGR441), and the 7th Framework Programme of the European Union.
Josep Maria Argimon does not have any conflicts of interest.
Manuel Arrojo has been a consultant and/or has received fees/fees from the Otsuka-Lundbeck Alliance, Janssen-Cilag, Lilly, Lundbeck, Otsuka and Adamed.
Maria Fe Bravo has been a consultant and/or has received fees or grants from the Carlos III Health Institute, IdiPaz, AstraZeneca, Roche, Janssen-Cilag, Lundbeck, Otsuka and the Spanish Ministry of Health, Social Policy and Equality.
Ana Cabrera Cifuentes has been a consultant to and/or received fees/grants from Otsuka-Lundbeck Alliance, CIBERSAM, Otsuka and Janssen-Cilag.
Julián Carretero Román has been a consultant and/or has received fees or grants from Ferrer, Formannova, Lundbeck, Otsuka and the Spanish Ministry of Health, Social Policy and Equality.
Manuel A. Franco has received consulting fees or grants from Roche, Lilly, Lundbeck, Otsuka, Ferrer, Servier, Pfizer, Janssen, Astra-Zeneca, Castilla y León (health and education departments), FIS (Ministry of Health, Spain), Ministry of Economy and Competitiveness (CDTI), European Commission (Horizon2020/AAL/6th Framework Programme of the European Union).
Paz García-Portilla has been a consultant and/or has received fees/grants from Otsuka-Lundbeck Alliance, CIBERSAM, European Commission, the Spanish Health Institute Carlos III, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Servier, Roche and Rovi.
Josep Maria Haro has received consultancy fees or grants from AstraZeneca, Caja Navarra, CIBERSAM, European Commission, GSK, Carlos III Health Institute, Lilly, Lundbeck, Ministry of Science and Innovation, Ministry of Health, Ministry of Economy and Competitiveness, Otsuka, Pfizer, Roche, and Takeda.
José Manuel Olivares has received consultancy fees or grants from Roche, Lilly, Lundbeck, Otsuka, Janssen, FIS (Spanish Ministry of Health), Pfizer, Glaxo, Xunta de Galicia (regional govt.) and the Biocaps Project.
Rafael Penadés has received aid for research and travel from Otsuka-Lundbeck.
Javier del Pino-Montes has received consulting fees from the Otsuka-Lundbeck Alliance.
Julio Sanjuán has participated in clinical trials on antipsychotics sponsored by Amgen and Roche laboratories. He has been an advisor at Lundbeck and Osuka Laboratories.
Celso Arango has received consultancy/advisory fees or grants from Abbot, Amgen, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Forum, Alicia Koplowitz Foundation, Carlos III Health Institute, Janssen-Cilag, Lundbeck, Merck, Ministry of Science and Innovation, Ministry of Health, Ministry of Economy and Competitiveness, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Schering Plow and Takeda.
María José Acuña Oliva (USMC Dos Hermanas, UGC de Salud Mental del Hospital de Valme, Sevilla, España).
Luis Agüera Ortiz (Servicio de Psiquiatría, Hospital Universitario 12 de Octubre, Madrid, España).
Eduardo J. Aguilar (Universidad de Valencia, INCLIVA, CIBERSAM, Valencia, España).
Cristina del Álamo Jiménez (Sección de Psiquiatría, Hospital Universitario Infanta Cristina, Madrid, España).
Benedikt L Amann (FIDMAG Research Foundation Germanes Hospitalàries, CIBERSAM, Barcelona, España).
Nel Anxelu (Confederación de Salud Mental, Madrid, España).
Adolfo Benito Ruiz (Unidad de Hospitalización Breve, Hospital Provincial de Toledo, España).
Miquel Bioque (Hospital Clínic de Barcelona, CIBERSAM, Barcelona, España).
Pilar Caminero Luna (Oficina Regional de Salud Mental, Dirección General de Coordinación de la Asistencia Sanitaria, Servicio Madrileño de Salud, Madrid, España).
Mateo Campillo Agustí (Hospital Morales Meseguer, Murcia, España).
Ricardo Campos Ródenas (Sección de Psiquiatría, Hospital Clínico Universitario de Zaragoza, España).
Raquel Carmona Jurado (Hospital Comarcal Valle de los Pedroches, Pozoblanco, Córdoba, España).
Jorge Cervilla (Universidad de Granada, Servicio de Salud Mental, Complejo Hospitalario Universitario de Granada, España).
Jordi Cid (Programa de Salud Mental, Instituto de Asistencia Sanitaria, Girona, España).
Eugenio Ramón Chinea Cabello (Centro de Terapias Integradas para la Salud [CENTIS], Santa Cruz de Tenerife, Tenerife, España).
Iluminada Corripio Collado (Servicio de Psiquiatría, Hospital de Sant Pau, CIBERSAM, Barcelona, España).
Olga Delgado (Servicio de Medicina Preventiva, Hospital Universitario Son Dureta, Palma de Mallorca, España).
Olimpia Diaz-Mandado (Centro de Rehabilitación Psicosocial y Laboral de Toledo, Fundación Sociosanitaria de Castilla-La Mancha, Toledo, España).
Marina Díaz Marsá (Hospital Clínico San Carlos, Programa de Intervención Precoz en Psicosis de Inicio Reciente, Universidad Complutense, CIBERSAM, Madrid, España).
María Blanca Fernández-Abascal-Puente (Hospital Universitario Marqués de Valdecilla, Santander, España).
Juan José Fernández Miranda (Área de Gestión Clínica de Salud Mental-V, Servicio de Salud del Principado de Asturias [SESPA], Oviedo, España).
Alejandro Fernández Pellicer (Complejo Hospitalario Universitario de Pontevedra, España).
Julia Fraga (Servicio Gallego de Salud, Vigo, España).
David Fraguas (Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón [IiSGM], CIBERSAM, Madrid, España).
Jesús de la Gándara (Jefe del Servicio de Psiquiatría, Complejo Asistencial de Burgos, España).
Juan Carlos García Álvarez (Unidad de Salud Mental de Adultos de Los Ángeles, Hospital General Universitario de Alicante, España).
Ignacio García Cabeza (Hospital General Universitario Gregorio Marañón, Madrid, España).
Paz García López (Hospital Comarcal Vega Baja de Orihuela, Alicante, España).
Josep Gascón Barrachina (Servicio de Psiquiatría, Hospital Universitari de la Mutua de Terrassa, Barcelona, España).
Cristina Gisbert Aguilar (Servicio de Rehabilitación Psiquiátrica Hospitalaria, IAS, Girona, España).
José Carlos González (Universidad de Valencia, España).
Ana González-Pinto (Servicio de Psiquiatría, Hospital Universitario Araba, CIBERSAM, Universidad del País Vasco [UPV/EHU], Vitoria, España).
Delio Guerro Prado (Servicio de Psiquiatría, Complejo Asistencial de Ávila [SACYL], Ávila, España).
Rosa María Hernández Cifuentes (Hospital de Día de Psiquiatría, Hospital Clínico de Valladolid, Facultad de Enfermería, Universidad de Valladolid, España).
Álvaro Hidalgo (Área de Fundamentos de Análisis Económicos, Universidad de Castilla-La Mancha, España).
Javier Labad Arias (Corporació Sanitària Parc Taulí, Sabadell, Barcelona, España).
Fernando Lana (Hospital de Día de Salud Mental, Instituto de Neuropsiquiatría y Adicciones [INAD], Hospital del Mar, Barcelona, España).
Anna Manné (Centro de Investigación en Red de Salud Mental [CIBERSAM], Hospital del Mar-Parc de Salut MAR, Barcelona, España).
Jesús Marín (Hospital La Paz, Madrid, España).
Demetrio Mármol Pérez (Unidad de Salud Comunitaria, Mairena del Aljarafe, Sevilla, España).
Manuel Martín Carrasco (Consejo Europeo de Sociedades Psiquiátricas [European Psychiatric Association], Clínica Padre Menni, Pamplona, España).
Jose Martinez-Raga (Unidad Docente de Psiquiatría y Psicología Médica, Hospital Universitario Dr. Peset, Valencia, España).
María Mayoral (Servicio de Psiquiatría del Niño y del Adolescente, Hospital General Universitario Gregorio Marañón, Madrid, España).
Fermín Mayoral Cleries (Hospital Regional de Málaga, Instituto de Investigación Médica de Málaga [IBIMA], España).
Gema Medina Ojeda (SACYL, Hospital Clínico Universitario de Valladolid, España).
Javier Min (Complejo Asistencial Universitario de León, España).
Salvador Miret Fallada (Hospital Universitari Santa Maria-Lleida, CIBERSAM, Lleida, España).
Fabiola Modrego Aznar (Unidad de Salud Mental-Consultas, Hospital Clínico Universitario, Zaragoza, España).
Juan D. Molina (Universidad Camilo José Cela, Hospital R. Lafora, Madrid, España).
Rosa Molina Ramos (Servicio de Psiquiatría, Área de Salud Mental de Llevant, Hospital de Manacor, Mallorca, España).
Fernando Mora (Hospital Universitario Infanta Leonor, Fundación Psiformación, Madrid, España).
Teresa Moreno-Calle (Programa de Primeros Episodios de Psicosis, Red de Salud Mental Bizkaia [RSMB], Osakidetza-Servicio Vasco de Salud, España).
Carlos Mur de Víu Bernad (Hospital Universitario de Fuenlabrada, Estrategia en Salud Mental del SNS, Madrid, España).
Santiago Navarro (Fundación Canaria de Investigación y Salud, Servicio de Evaluación del Servicio Canario de la Salud, España).
Mercedes Navio Acosta (Oficina Regional de Coordinación de Salud Mental, Consejería de Sanidad, Madrid, España).
Araceli Oltra Ponzoda (Conselleria de Sanitat Universal i Salut Púbica, València, España).
Miguel Angel Ortega Esteban (Departamento de Salud Mental, Servicio Riojano de Salud, España).
Mario Páramo Fernández (Hospital de Conxo, Universidad de Santiago, Santiago de Compostela, España).
Juan Manuel Pascual Paño (Universidad de Cádiz, Unidad Hospitalaria de Salud Mental, Jerez de la Frontera, España).
Salvador Peiró (Conselleria de Sanitat, Generalitat Valenciana, España).
José Pereira Miragaia (Servicio de Salud Mental, Servicio Canario de la Salud, Las Palmas de Gran Canaria, España).
Piedad Pérez Marín (Hospital Universitario Gregorio Marañón, Madrid, España).
Alfonso Pérez Poza (Universidad de Zaragoza, Hospital de Día, Hospital Universitario Miguel Servet [HUMS], Zaragoza, España).
Rafael del Pino López (Unidad de Gestión Clínica de Salud Mental, Hospital Universitario Virgen de la Victoria, Málaga, España).
Asunción Pino Pino (Centro Médico Asistencial La Vall d’Uixó, Castellón, España).
A.J. Ramírez-García.
Marta Rapado-Castro Romero (Departamento de Psiquiatría Infantil y Adolescencia, Hospital Universitario Gregorio Marañón, LiSGM, CIBERSAM, Madrid, España).
Carmen Rodríguez del Toro (Servicio de Psiquiatría, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, España).
Gabriel Rubio (Universidad Complutense, Servicio de Psiquiatría, Hospital Universitario 12 de Octubre, Madrid, España).
Samuel Leopoldo Romero Guillena (UGC Salud Mental, Área Hospitalaria Virgen Macarena, Sevilla, España).
Alfredo de la Rubia Martínez (Hospital Psiquiátrico, Mérida, Badajoz, España).
Gemma Safont (Hospital Universitari Mútua de Terrassa, Barcelona, España).
Estrella Salvador Vadillo (Hospital Universitario Ramón y Cajal, Madrid, España).
Manuel Serrano Vázquez (Servicio de Psiquiatría, Xerencia de Xestion Integrada de A Coruña, España).
Raúl Vázquez-Noguerol Méndez (Unidad de Rehabilitación Psiquiátrica, Hospital Nicolás Peña, Servizo Galego de Saúde, EOXI de Vigo, España).
Eulalio Valmisa (UGC Salud Mental, Hospital Universitario de Puerto Real, Cádiz, España).
Miguel Vega (Hospital Ramón y Cajal, Universidad de Alcalá de Henares, Madrid, España).
Diego de la Vega Sánchez (Área Psiquiátrica, Servicio Andaluz de Salud, Sevilla, España).
David Villavicencio (Consorcio Hospital General Universitario de Valencia, España).
José Luis Villegas Martínez (FEA Psiquiatría, Hospital Universitario de Salamanca, España).
Zafra Villena (Servicio de Psiquiatría, Hospital Arnau de Vilanova, Valencia, España).
M. Luisa Zamarro Arranz (Sección de Salud Mental-Alcobendas-San Sebastián de los Reyes, Hospital Infanta Sofía, Madrid, España).
Francisco Javier Zamora Rodríguez (Salud Mental, Área de Zafra-Llerena, Badajoz, España).
Conflicts of interest: Rethinking Group
María José Acuña Oliva has no conflicts of interest to declare.
Luis Agüera Ortiz has no conflicts of interests to declare.
Eduardo J. Aguilar has no conflicts of interest to declare.
Cristina del Álamo Jiménez has no conflicts of interests to declare.
Benedikt L Amann has been a rapporteur for Janssen, Lundbeck and Otsuka. He has received support through a stabilisation contract (CES12/024) and grants for research projects PI07/1278, PI10/02622 and PI/15/02242 from the Carlos III Health Institute - General Subdirectorate for Assessment and Promotion of Research, National Plan 2008–2011 and 2013–2016, and the European Regional Development Fund (ERDF).
Nel Anxelu has no conflicts of interest to declare.
Adolfo Benito Ruiz received payment from Janssen, Johnson & Jonhson and Otsuka in 2015.
Miquel Bioque has been a consultant for, received fees from and/or been rapporteur for Adamed, Ferrer, Janssen-Cilag, Lundbeck, Otsuka, and Pfizer.
Pilar Caminero Luna has no conflicts of interest to declare.
Mateo Campillo Agustí has received fees for scientific collaborations from Otsuka, Servier and Pfizer.
Ricardo Campos Ródenas has no conflicts of interest to declare.
Raquel Carmona Jurado has no conflicts of interest to declare.
Jorge Cervilla has no conflicts of interests to declare.
Jordi Cid has no conflicts of interest to declare.
Eugenio Ramón Chinea Cabello has received fees from the following pharmaceutical laboratories: Pfizer GEP, Pfizer, Lundbeck, Servier, Rovi, Janssen-Cilag, Janssen, Esteve, Lilly, Sanofi, Astrazeneca, Glaxo-Smithkline, Boehinger-Ingelheim and Bristol Myers Squibb.
Illuminada Corripio Collado has received consultancy fees or grants from Ferrer, Lilly, Otsuka and FIS (Ministry of Health, Spain).
Olga Delgado has no conflicts of interest to declare.
Olimpia Diaz-Mandado has received consulting fees from the Otsuka-Lundbeck Alliance.
Marina Díaz Marsá has received consulting fees from Janssen, as rapporteur for Servier and Esteve and collaborated with Alter and Ferrer projects.
Blanca Fernandez-Abascal Puente has received fees as a speaker from Lundbeck-Otsuka.
Juan José Fernández Miranda has received fees or grants from Janssen, Lundbeck, Otsuka and Pfizer, and the Government of the Principality of Asturias and the European Commission (G-V. European Union). He has received fees for lectures or grants from Janssen, Lundbeck, Otsuka, Pfizer Pharmaceuticals, and the regional government of Asturias and the European Commission.
Alejandro Fernández Pellicer has received fees for lectures from laboratories Jannssen, Pfizer, and Lilly. He has received fees from Lumbeck for participating in Rethinking project meetings.
Julia Fraga presents no conflicts of interest to declare.
David Fraguas has received consulting fees from Janssen, Lundbeck, and Otsuka; he has received fees for submissions from AstraZeneca; Bristol-Myers Squibb; Eisai; Janssen; Lundbeck; Otsuka; Pfizer, and has received research grants funded by the Carlos III Health Institute (Ministry of Economy and Competitiveness).
Jesus de la Gándara presents no conflicts of interest to declare.
Juan Carlos García Álvarez presents no conflicts of interests to declare.
Ignacio García Cabeza has been a consultant and has received fees or scholarships from Otsuka-Lundbeck, Janssen-Cilag, Eli Lilly, and Pfizer.
Paz García López presents no conflicts of interest to declare.
Dr. Josep Gascón Barrachina presents no conflicts of interest to declare.
Cristina Gisbert presents no conflicts of interest to declare.
José Carlos González presents no conflicts of interest to declare.
Ana González-Pinto has received consultancy fees from AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Janssen-Cilag, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Rovi, Roche, Ferrer, Ministry of Science and Innovation CIBERSAM), the Spanish Ministry of Science (Carlos III Health Institute), the Basque Government, the Stanley Medical Research Institute and the Wyeth Symposium: the Spanish Ministry of Science and Innovation (CIBERSAM), Ferrer, Lundbeck, Janssen-Cilag, Rovi, Roche, Astra Zeneca.
Delio Guerro Prado has no conflicts of interest to declare.
Rosa María Hernández has no conflicts of interest to declare.
Álvaro Hidalgo has no conflicts of interest to declare.
Javier Labad Arias has no conflicts of interest to declare.
Fernando Lana Moliner has received fees for attendance at Janssen-Cilag and Otsuka conferences, Janssen-Cilag and Otsuka travel grants, and fees for papers written by him from Janssen-Cilag.
Anna Manné has received lecture fees from Otsuka Pharmaceuticals and Janssen-Cilag.
Jesús Marín has no conflicts of interest to declare.
Demetrio Mármol Pérez has received lecture fees from Otsuka-Lundbeck, Janssen-Cylag, Pfizer and Esteve.
Manuel Martín Carrasco has no conflicts of interests to declare.
Jose Martinez-Raga has received consulting/consulting fees from Otsuka, Lundbeck, Janssen, Lilly and Astra Zeneca.
María Mayoral has no conflicts of interest to declare.
Fermin Mayoral Cleries has participated in training activities and scientific collaborations for the following companies: Jansen-Cilag, Astra Zeneca, Lilly, Ludbeck, Pfizer, and Roche.
Gema Medina Ojeda has no conflicts of interest to declare.
Javier Min has no conflicts of interest to declare.
Salvador Miret Fallada has no conflicts of interest to declare.
Fabiola Modrego Aznar has no conflicts of interest to declare.
Juan D. Molina has no conflicts of interests to declare.
Rosa Molina Ramos has no conflicts of interest to declare.
Fernando Mora has received fees for scientific collaborations from Janssen, GSK, Otsuka-Lundbeck, and Pfizer.
Teresa Moreno-Calle has received consulting/consultancy fees or research grants from Janssen-Cilag, Otsuka, Lundbeck and the Basque Government.
Carlos Mur de Víu Bernad has no conflicts of interest to declare.
Santiago Navarro has no conflicts of interest to declare.
Mercedes Ship Acosta has no conflicts of interest to declare.
Araceli Oltra Ponzoda has no conflicts of interest to declare.
Miguel Angel Ortega Esteban has no conflicts of interest to declare.
Mario Páramo Fernández has no conflicts of interest to declare.
Juan Manuel Pascual Paño has received fees for participation in conferences, clinical trials, as well as conference attendance coverage, from Otsuka, Janssen, Pfizer, Ferrer, Lilly, and Almirall.
Salvador Peiró has no conflicts of interest to declare.
José Pereira Miragaia has received fees from Lundbeck.
Piedad Pérez Marín has received fees as a speaker and adviser for Janssen-Cilag and Otsuka-Lundbeck.
Alfonso Pérez Poza has no conflicts of interest to declare.
Rafael del Pino López has no conflicts of interest to declare.
Asunción Pino has no conflicts of interest to declare.
Marta Rapado-Castro has no conflicts of interest to declare.
A.J. Ramírez-García has received consulting fees or grants from AstraZeneca, Janssen-Cilag, Lundbeck, and Otsuka.
Carmen Rodriguez del Toro has received fees from Otsuka-Lundbeck.
Gabriel Rubio has no conflicts of interest to declare.
Samuel Leopoldo Romero Guillena has received consulting fees or grants from: Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Servier, Sanofi and Rovi.
Alfredo de la Rubia Martínez has no conflicts of interest to declare.
Gemma Safont has received consultancy fees from the Otsuka-Lundbeck Alliance.
Estrella Salvador Vadillo has no conflicts of interest to declare.
Manuel Serrano Vázquez has no conflicts of interest to declare.
Raúl Vázquez-Noguerol Méndez has no conflicts of interest to declare.
Eulalio Valmisa has received consulting fees from Otsuka, and fees for submissions from Otsuka, Janssen, and Lundbeck.
Miguel Vega has received fees or grants from AB-Biotics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Juste, Lundbeck, Otsuka, Pfizer, and Sanofi.
Diego de la Vega Sánchez has received funding from the Jannsen and Lundbeck laboratories.
David Villavicencio has no conflicts of interest to declare.
José Luis Villegas Martínez has no conflicts of interest to declare.
Zafra Villena has no conflicts of interest to declare.
M. Luisa Zamarro Arranz has no conflicts of interest to declare.
Francisco Javier Zamora has received fees from Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer and Servier.
Please cite this article as: Crespo-Facorro B, Bernardo M, Argimon JM, Arrojo M, Bravo-Ortiz MF, Cabrera-Cifuentes A, et al. Eficacia, eficiencia y efectividad en el tratamiento multidimensional de la esquizofrenia: proyecto Rethinking. Rev Psiquiatr Salud Ment (Barc). 2017;10:4–20.