To determine the safety of lamotrigine rechallenge after a first episode of skin rash in bipolar patients.
MethodAn open cases prospective study was conducted with patients, who developed a skin rash when first treated with lamotrigine, were refractory to other treatments, and were offered lamotrigine rechallenge using a different dose titration. Additionally a review was performed on previous skin rash management strategies and lamotrigine rechallenge reports.
ResultsEvery 3 out of 10 lamotrigine rechallenge patients required drug interruption due to persistent rash. One of them was potentially serious and resolved by stopping the lamotrigine. The review of available literature identified several lamotrigine rechallenge studies with rates of positive results varying between 70% and 87% depending on the study. No patient developed Stevens–Johnson syndrome or toxic epidermal necrolysis after rechallenge. The rate of rash was higher when rechallenge began between 4 weeks from initial rash (19% vs 7%, P=.001) and decreased when first rash showed no potentially serious signs (0% vs 19%, P=.01).
ConclusionsRechallenge is a viable option after a benign lamotrigine-induced rash, and can even be rechallenged after rash with greater precautions when there exists one or two potentially serious signs. In cases of more serious rash there are no reliable data available on rechallenge safety and it may pose a significant risk. In those cases rechallenge should better be avoided between 4 weeks from first rash.
Investigar la seguridad de la reintroducción de lamotrigina después de un episodio de rash inicial en pacientes con trastorno bipolar.
MétodoEstudio prospectivo de casos abiertos de pacientes que desarrollaron rash inicial al ser tratados con lamotrigina y que fueron refractarios a otros tratamientos, a los cuales se les ofreció reintroducción de lamotrigina usando distintas estrategias de escaladas de dosis. Además, se efectuó una revisión de reportes previos de estrategias de manejo de rash y reintroducción de lamotrigina.
ResultadosDe 10 pacientes retratados con lamotrigina 3 requirieron discontinuación de la droga debido a rash persistente. Uno de estos fue potencialmente serio y se resolvió con la discontinuación de lamotrigina. La revisión de la literatura identificó varios estudios de retratamiento con lamotrigina con tasas variables de resultados satisfactorios, entre 70 y 87% según los estudios. Ningún paciente desarrolló síndrome de Stevens-Johnson o necrólisis epidérmica tóxica después de la reintroducción. La tasa de rash fue elevada cuando el retratamiento comenzó dentro de las 4 semanas del rash inicial (19 vs 7%, p=0,001) y se redujo cuando el rash inicial no tuvo signos de seriedad potencial (0 vs 19%, p=0,01).
ConclusionesRetratamiento es una opción viable después de rash benigno por lamotrigina y se puede retratar con más precauciones después del rash con uno o 2 signos de seriedad potencial. Para los casos de rash con mas signos de gravedad no hay datos fiables sobre al seguridad del retratamiento y puede conllevar un riesgo significativo. En estos casos el retratamiento debería evitarse dentro de las 4 semanas del rash inicial.
Lamotrigine represents a valid option in treating bipolar disorder.1 Patients with bipolar disorder spend nearly 40% of their lives in a depressive phase and the most important effects of lamotrigine lie in its prevention of bipolar depression.2 Other advantages of lamotrigine include its good tolerance, favourable cognitive profile and patient adherence.3 This profile is particularly useful for bipolar patients, given their low rate of adherence to treatment.4 However, its use has been limited due to the risk of potentially serious dermatological reactions, mainly Stevens–Johnson syndrome and toxic epidermal necrolysis.5 The known risk factors for this type of reaction include rapid dosage increase, concurrent use of valproic acid, previous history of rash associated with anticonvulsants, being female and being younger than 13 years.6,7 When introduced with the standard gradual increase in dosage, the rate of severe skin reactions with lamotrigine has been decreasing from 1% to 0.1–0.01%.8 However, the rate of benign skin reactions has not changed, remaining between 8% and 11%.9 The appearance of a benign rash is a problem for clinicians, who have few therapeutic alternatives at their disposal that are effective with bipolar depression. To avoid this adverse reaction, a series of precautions have been identified for patients that start their treatment with lamotrigine,10 without having been able to reduce the appearance rate, as shown in a recent randomised study.11 Reinstating lamotrigine treatment with a slower dose titration is a strategy habitually used when a rash appears. This usually begins with 5mg/day, as long as its use is clinically justified. However, there are no large-scale studies that have identified specific and reliable markers, which would make it possible to predict the failure or success of this strategy in advance. In the present study, some markers were detailed that predict adverse events when reinstating lamotrigine treatment after the appearance of a rash. This was accomplished through a study of open cases and a review of existing literature.
MethodCase seriesAfter a washout period of 5 half-lives with any previous psychotropic medication taken by the participant, lamotrigine treatment was begun to assess its effectiveness and tolerability in treating type I and II bipolar disorder. Participants were aged between 18 and 57 years and complied with the DSM-IV criteria for type I and II bipolar disorder. In addition, they were in a depressive or mixed phase of their disorder. The disorder was confirmed using the DSM-IV clinical interview. Patients had a value ≥20 on the Hamilton Rating Scale for Depression (17 items)12 and a value ≤12 on the Young Mania Rating Scale.13 Exclusion criteria included having another axis I diagnosis, presence of a major depressive episode lasting 12 months, substance use or dependence disorder (DSM-IV) (except nicotine) or another clinically significant medical illness. The study was approved by the local ethics committee and carried out in accordance with the standards of good clinical practice. Informed consent was obtained from each subject before his or her inclusion in the study.
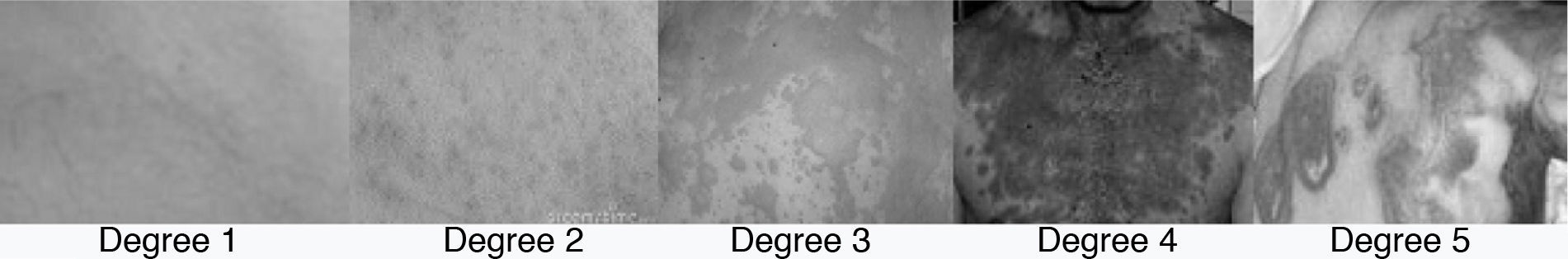
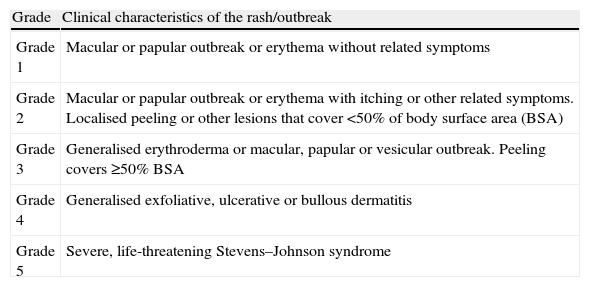
Ingesting benzodiazepines were not permitted during the washout period. Patients with bipolar disorder that developed a rash within 4 months after starting lamotrigine treatment were identified prospectively during 2010 in an outpatient psychiatric ward. To assess the intensity of the rash, a 5-point scale was used, based on the intensity and location of the outbreak (Table 1).14
Classification of the rash lesions.
| Grade | Clinical characteristics of the rash/outbreak |
| Grade 1 | Macular or papular outbreak or erythema without related symptoms |
| Grade 2 | Macular or papular outbreak or erythema with itching or other related symptoms. Localised peeling or other lesions that cover <50% of body surface area (BSA) |
| Grade 3 | Generalised erythroderma or macular, papular or vesicular outbreak. Peeling covers ≥50% BSA |
| Grade 4 | Generalised exfoliative, ulcerative or bullous dermatitis |
| Grade 5 | Severe, life-threatening Stevens–Johnson syndrome |
In Fig. 1, there are some examples of the rash lesions according to their severity. In each case, the severity of the outbreak was contrasted with the potential benefit of lamotrigine. Informed consent was obtained from each of the patients considered appropriate for lamotrigine reinstatement. Cases of grades 3–5 lesions were excluded due to the possibility of developing Stevens–Johnson syndrome or epidermal necrolysis. In the case of benign lesions (grade 1), an attempt was made to improve the rash by reducing the lamotrigine dosage. If there was no improvement, the drug was discontinued and the possibility of reinstating treatment later was suggested. At least 2 weeks had to pass after the improvement in the rash before reinstating treatment could be attempted. At this point, treatment began with a lamotrigine dosage of 5mg daily, or 5mg every other day for patients with concomitant valproic acid treatment. Daily dosage was increased by 5mg every 2 weeks until a 25mg/day dose was reached, at which point titration would continue according to the manufacturer's instructions.15 One patient did not follow the treatment reinstatement plan correctly (titrating more quickly than instructed); nonetheless, this patient was included in the analysis. To analyse improvement with lamotrigine, the Clinical Global Impression-Improvement (CGI-I) scale was used.16
Review of literature concerning lamotrigine rechallenge studies or trialsA search of Medline was performed using the keywords “lamotrigine” and “rash” (n=240). The abstracts were reviewed to identify those studies where lamotrigine was reinstated after a rash appeared, using a slower dose titration than in the initial plan (n=8). In addition, the references in the indicated studies were reviewed (n=4). Data extracted from the publications mentioned were evaluated and the outbreaks were classified according to the scale in Table 1. Data obtained from this literature review were combined with the current case series to form a meta-analysis. A post hoc analysis was carried out to assess whether the risk of a rash when reinstating lamotrigine treatment after an outbreak was associated with the severity of the outbreak or with the interval between the initial outbreak and the rechallenge.
ResultsCase seriesOf the 80 patients treated with lamotrigine for type I or II bipolar disorder, 15 (18.75%) developed a skin rash within the 2 months following treatment; 10 of these patients underwent a lamotrigine rechallenge. The rechallenge presented adverse results in 2 patients: 1 developed a severe grade 4 rash and the other developed a scaly grade 3 rash. No patients developed Stevens–Johnson syndrome or epidermal necrolysis during initial treatment or the lamotrigine rechallenge (Table 2).
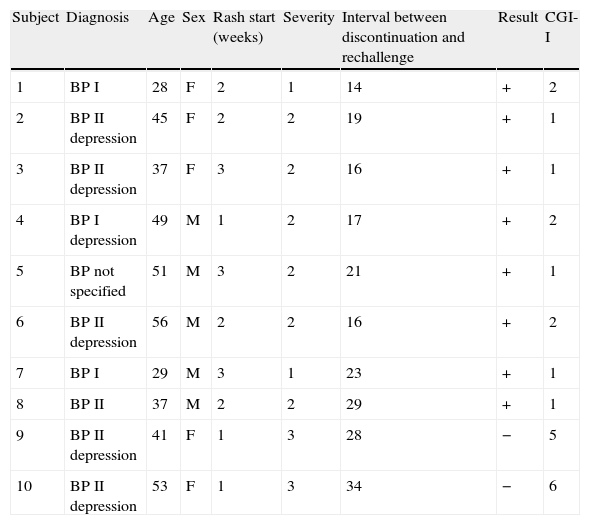
Characteristics of patients that underwent lamotrigine rechallenge.
| Subject | Diagnosis | Age | Sex | Rash start (weeks) | Severity | Interval between discontinuation and rechallenge | Result | CGI-I |
| 1 | BP I | 28 | F | 2 | 1 | 14 | + | 2 |
| 2 | BP II depression | 45 | F | 2 | 2 | 19 | + | 1 |
| 3 | BP II depression | 37 | F | 3 | 2 | 16 | + | 1 |
| 4 | BP I depression | 49 | M | 1 | 2 | 17 | + | 2 |
| 5 | BP not specified | 51 | M | 3 | 2 | 21 | + | 1 |
| 6 | BP II depression | 56 | M | 2 | 2 | 16 | + | 2 |
| 7 | BP I | 29 | M | 3 | 1 | 23 | + | 1 |
| 8 | BP II | 37 | M | 2 | 2 | 29 | + | 1 |
| 9 | BP II depression | 41 | F | 1 | 3 | 28 | − | 5 |
| 10 | BP II depression | 53 | F | 1 | 3 | 34 | − | 6 |
The 5 patients who did not undergo a lamotrigine rechallenge spontaneously recovered from the rash after their dosage was reduced (n=1), after they were switched to another drug (n=2) or after they refused to continue treatment (n=1). The rash severity in patients who underwent the rechallenge oscillated between grades 1 and 2 (mean=1.75). The 2 patients who underwent rechallenge with adverse results had a grade 3 rash. Furthermore, after the rechallenge, they again presented potentially serious blistering skin rashes on the back and face, in addition to fever, eosinophilia and mucous blisters in the oral cavity, which subsided after corticosteroid treatment and discontinuing lamotrigine. Of the patients that underwent rechallenge, 37.5% reported improvement afterwards. These patients also obtained results of 1 and 2 (improvement and much improvement) relative to the baseline after the lamotrigine rechallenge in the meta-analysis.
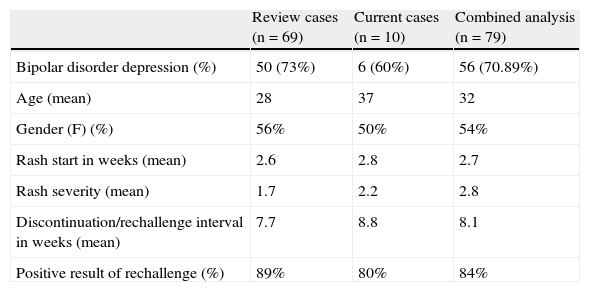
We recovered 69 cases extracted from the 12 publications in which a lamotrigine rechallenge was carried out following a rash (Table 3).17–22
Analysis of current cases and of the review of lamotrigine rechallenge after initial rash.
| Review cases (n=69) | Current cases (n=10) | Combined analysis (n=79) | |
| Bipolar disorder depression (%) | 50 (73%) | 6 (60%) | 56 (70.89%) |
| Age (mean) | 28 | 37 | 32 |
| Gender (F) (%) | 56% | 50% | 54% |
| Rash start in weeks (mean) | 2.6 | 2.8 | 2.7 |
| Rash severity (mean) | 1.7 | 2.2 | 2.8 |
| Discontinuation/rechallenge interval in weeks (mean) | 7.7 | 8.8 | 8.1 |
| Positive result of rechallenge (%) | 89% | 80% | 84% |
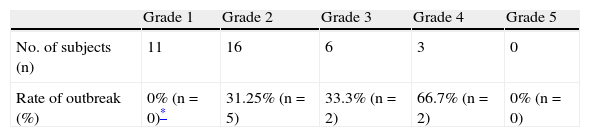
In 18% of the cases, a skin reaction appeared after treatment was reinstated. Discontinuation resolved these reactions, but no serious grade 4 or 5 reactions suggesting Stevens–Johnson syndrome or toxic epidermal necrolysis were reported. The majority were retrospective or prospective cases, while 4 of them were single-case studies.19,21,23,24 In 30% of the cases, lamotrigine was reinstated at a dose higher than 5mg/day, due to initially high values in starting doses of more than 200mg/day in the first week. The rate of positive results in these cases did not differ from that found in rechallenge with 5mg/day (83% vs 89% respectively). The relationship between the risk of a rash during the rechallenge and the waiting interval, as well as that between the risk and initial rash severity, was studied with a post hoc analysis. This included previously published cases that were appropriate for the analysis, obtained from the literature review (n=26 for severity, n=48 for interval). The rate of positive results for rechallenge following a benign rash (grade 1) was 100%. For grades 2 and 3 rashes, the rates of relapse were similar to each other (31.25% and 33.3% respectively). An initial grade 3 rash had a 66.7% rate of relapse (Table 4).
Rash rate in rechallenge according to initial severity (combined analysis).
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| No. of subjects (n) | 11 | 16 | 6 | 3 | 0 |
| Rate of outbreak (%) | 0% (n=0)* | 31.25% (n=5) | 33.3% (n=2) | 66.7% (n=2) | 0% (n=0) |
The 2 current cases who underwent rechallenge after a grade 3 rash did not show favourable evolution. Instead, a more severe rash appeared with blisters on the back, face and in the oral cavity, which subsided after the 2 patients discontinued treatment.
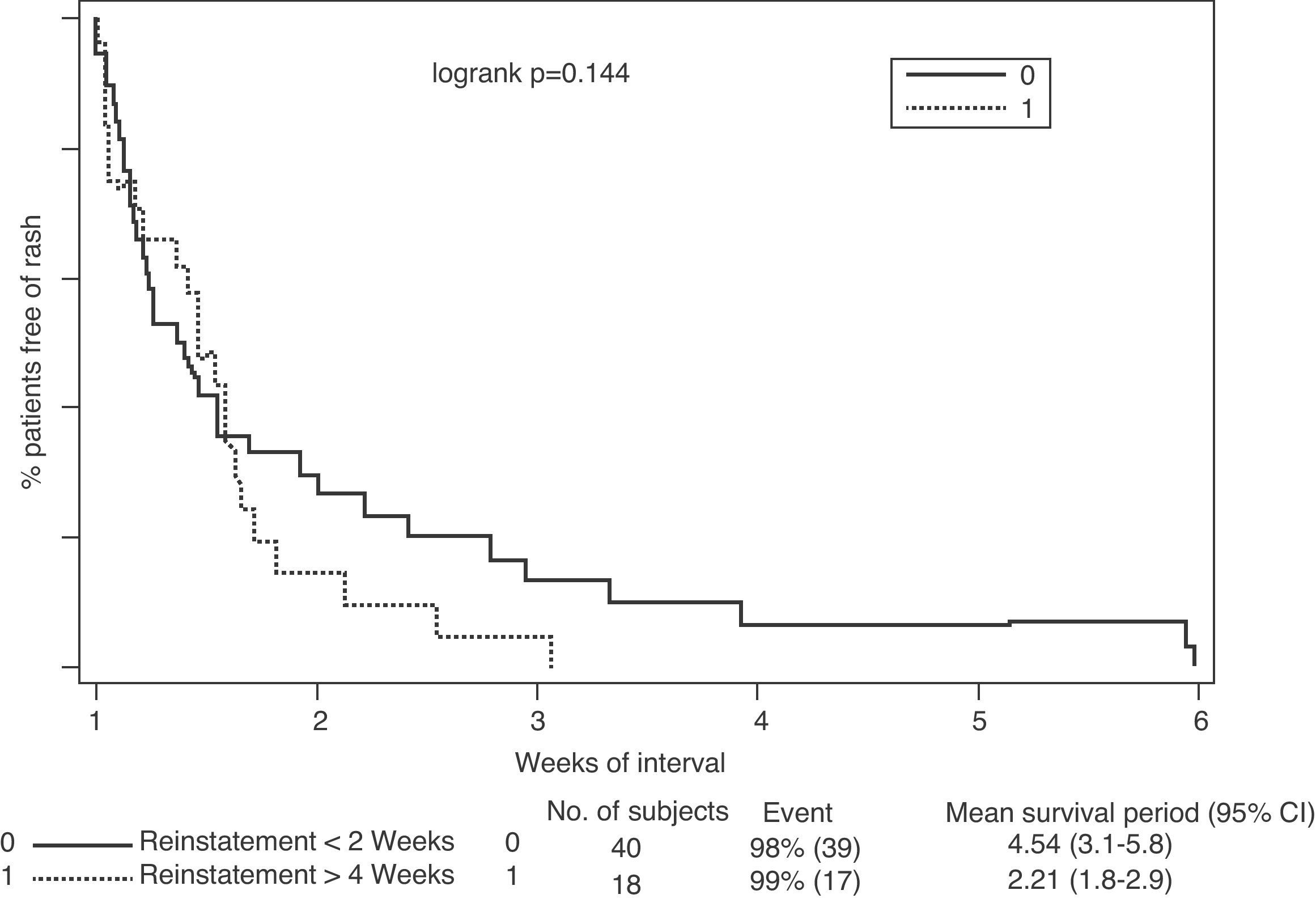
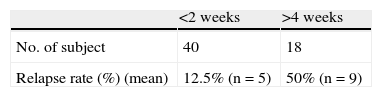
Regarding the association between rash relapse during rechallenge and the interval between discontinuing treatment and reinstating lamotrigine treatment, results indicated that the shorter the interval, the higher the risk of relapse (Fig. 2). This effect became especially significant when lamotrigine was reinstated in less than 2 weeks, compared to reinstatement after 4 weeks (46% vs 2%, P=.001) (Table 5).
DiscussionThe results of the present study supported the idea of titrating the lamotrigine rechallenge after a rash using lower doses. In the case of grade 1 skin reactions, the last dose could be reduced by 25–50mg, with monitoring until the rash subsided. Afterwards, titration could continue with increasing dosages until clinical stabilisation was reached. If the rash did not subside, you could discontinue lamotrigine and retitrate the dosage at a slower pace after an interval of 4–6 weeks without the appearance of skin rashes. In the case of grade 2 skin rashes, you could follow the previous plan with greater precautions concerning the persistence of a skin reaction. For more severe rashes (such as grade 3 or 4), rechallenge is not recommended, as was demonstrated in the cases of the 2 patients in the present study with poor development. Post hoc analyses suggested that waiting 4–6 weeks after the improvement of the rash, before reinstating lamotrigine treatment, is an option that offers more safety compared to less conservative reports.10 If continuing lamotrigine treatment is necessary despite the persistence of a rash, you could opt for combining lamotrigine with riluzole23 or with valproate.19,25 Riluzole and lamotrigine inhibit glutamate by blocking sodium channels. The former has been studied in resistant depression and as a substitute for lamotrigine after a severe rash. The combination of lamotrigine and an enzyme inhibitor makes it possible to reduce lamotrigine levels while conserving its therapeutic effectiveness. Another alternative that could be used before discontinuing lamotrigine in the case of a rash is to consult a dermatologist with experience in this type of outbreak, after a reasonable interval of 48–72h. However, if the consultation is delayed even more, the patient runs the risk of aggravating the rash lesions. At this point, it is recommended that the patient should resort to the other alternatives, like reinstating treatment at a lower dosage or discontinuing treatment and reinstating it 2–4 weeks later. Regarding the treatment of rashes with corticosteroids, they can aggravate the depressive or mixed state of bipolar disorder, thus making it advisable to avoid them as much as possible.26
Ethical disclosuresProtecting people and animals. The authors declare that the procedures followed conform to the ethical standards of the responsible human experimentation committee and are in accordance with the World Medical Association and the Declaration of Helsinki.Data confidentiality. The authors declare that they have followed the protocols of their work centre regarding the publication of patient data and that all patients included in the study received sufficient information and gave their informed consent in writing to participate in said study.Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Serrani Azcurra DJL. Retratamiento con lamotrigina después de reacción cutánea de rash. Estudio de casos abiertos y metaanálisis combinados. Rev Psiquiatr Salud Ment (Barc.). 2013;6:144–149.