Several studies have described increased oxidative stress parameters in patients with schizophrenia. The objectives of the current study were to identify potential oxidative stress biomarkers in stable patients during first 10 years of schizophrenia and determine if they are associated with specific clinical dimensions.
Material and methodsSeventy-three clinically stable outpatients with schizophrenia and 73 sex and age-matched healthy controls were recruited. Sociodemographic, clinical and biological data were collected at enrollment. Blood biomarkers included homocysteine, the percentage of hemolysis, lipid peroxidation subproducts, and as an antioxidant biomarker, catalase activity in erythrocytes.
ResultsComparative analyses after controlling for smoking and metabolic syndrome evidenced a significant increase in catalase activity in patients. Also, lower lipid peroxidation levels showed an association with negative symptoms.
ConclusionsIn conclusion, compensatory antioxidant mechanisms might be increased in stable patients with schizophrenia at early stages. Furthermore, there may be an inverse relationship between oxidative stress and negative dimension.
Diversos estudios han encontrado un aumento de los parámetros de estrés oxidativo en pacientes con esquizofrenia. Los objetivos de este estudio han sido identificar potenciales biomarcadores de estrés oxidativo en pacientes con esquizofrenia estables, durante los primeros 10 años de enfermedad, y determinar si se asocian con dimensiones clínicas específicas.
Material y métodosSe evaluaron 73 pacientes clínicamente estables y 73 controles sanos pareados por edad y sexo. Se recogieron datos sociodemográficos, clínicos y parámetros biológicos. Los biomarcadores sanguíneos incluyeron homocisteína, porcentaje de hemólisis, subproductos de peroxidación lipídica y, como biomarcador antioxidante, actividad de la catalasa en eritrocitos.
ResultadosLos análisis comparativos tras controlar por tabaquismo y síndrome metabólico evidenciaron un aumento significativo en la actividad de la catalasa en pacientes. Asimismo, niveles inferiores de peroxidación lipídica se asociaron de manera significativa con la sintomatología negativa.
ConclusionesComo conclusión, los mecanismos compensatorios antioxidantes podrían estar aumentados en pacientes con esquizofrenia estables durante las fases iniciales. Además, podría existir una relación inversa entre el estrés oxidativo y la dimensión negativa.
Schizophrenia is a chronic and severe mental disorder characterized by heterogeneous symptoms and a long-term debilitating course. The diagnostic criteria are based on descriptive phenomenology of clinical symptoms and clinical course due to the lack of reliable and specific biomarkers.1 However, in recent decades, several biological parameters such as inflammatory, metabolic, and neuroimaging biomarkers have been described in this population toward the goal of personalized, precision psychiatry.2–6
At present, the classical concept of schizophrenia has been reformulated,7 and some authors suggest this disease has a multisystem impact from the early stages.8 Indeed, blood biomarker studies have shown evidence of abnormalities in metabolic and immune response functions in subjects with schizophrenia.9–11 Furthermore, oxidative imbalance has been involved in the pathophysiology of this disorder and some authors suggest a potential link between the oxidative stress and the increased risk of metabolic abnormalities in these patients.12
Several studies have documented changes in oxidative parameters (lipid peroxidation products, nitric oxide) and antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase), although these results are not consistent, as increases or decreases in these parameters have been reported in patients.13–16 More ambitious studies have attempted to determine a relationship between peripheral biomarkers and the severity of different clinical dimensions. Garcia-Alvarez et al. (2016) recently published a review of this issue.17 Regarding inflammation, several cytokines and CRP, have been associated with positive, negative, and cognitive symptoms in several studies.18–21 However, most studies have not identified a significant association between oxidative stress biomarkers and clinical severity in chronic schizophrenia patients or patients with first-episode psychosis.22–25
One of the probable reasons for these inconsistent results is the heterogeneity of schizophrenia and the difficulty of accurate categorization. Another underlying obstacle to studying peripheral markers is that different clinical disease stages may be associated with distinctive biomarkers, and they could fluctuate depending on whether patients are in their first-episode, an acute relapse, or a stable phase. Also, potential confounders as smoking, obesity or other metabolic disturbances were not considered in all the studies.26
Therefore, the main objective of the present study was to identify if peripheral levels of oxidative stress parameters are different in stable outpatients in the first 10 years of schizophrenia from those in matched healthy controls (HC). Secondly, the ultimate objective was to explore whether oxidative stress biomarkers are associated with different clinical dimensions in schizophrenia.
Material and methodsThis was a multicenter, longitudinal, one-year follow-up study of patients with schizophrenia and HC, whose objective was to determine differential biomarkers of the negative dimension. In this paper, we have employed only data collected at baseline. This study was approved by the local ethics committee, “Comité Ético de Investigación Clínica Regional del Principado de Asturias (Ref. 25/2014)”.
ParticipantsSeventy-three outpatients with schizophrenia (SZ) and 73 sex and age-matched HC from Asturias (Spain) participated in this study. The sample characteristics are provided in Table 1. All patients were in the first 10 years of illness, aged 18–45, and were on stable maintenance treatment for at least three months. Diagnosis of schizophrenia was made by a psychiatrist and confirmed with a SCID Clinical Interview (according to DSM-5 criteria). Exclusion criteria for both groups were: (1) somatic comorbidities–both acute (acute infection, fever, allergic or inflammatory processes) and chronic (cancer, autoimmunity disorder, chronic infections)–that could interfere with the inflammatory parameters, (2) treatment with immunosuppressants or vaccines during the 6 months prior to enrollment, and (3) treatment with anti-inflammatory drugs two days before blood collection. Exclusion criteria for HC also included a past history of mental health disorders. A 94.5% of both patients and control subjects were Caucasian while 4 patients and 4 HC were not. All participants received information about the purposes and protocol of the study, and signed informed consents before any study procedures were performed.
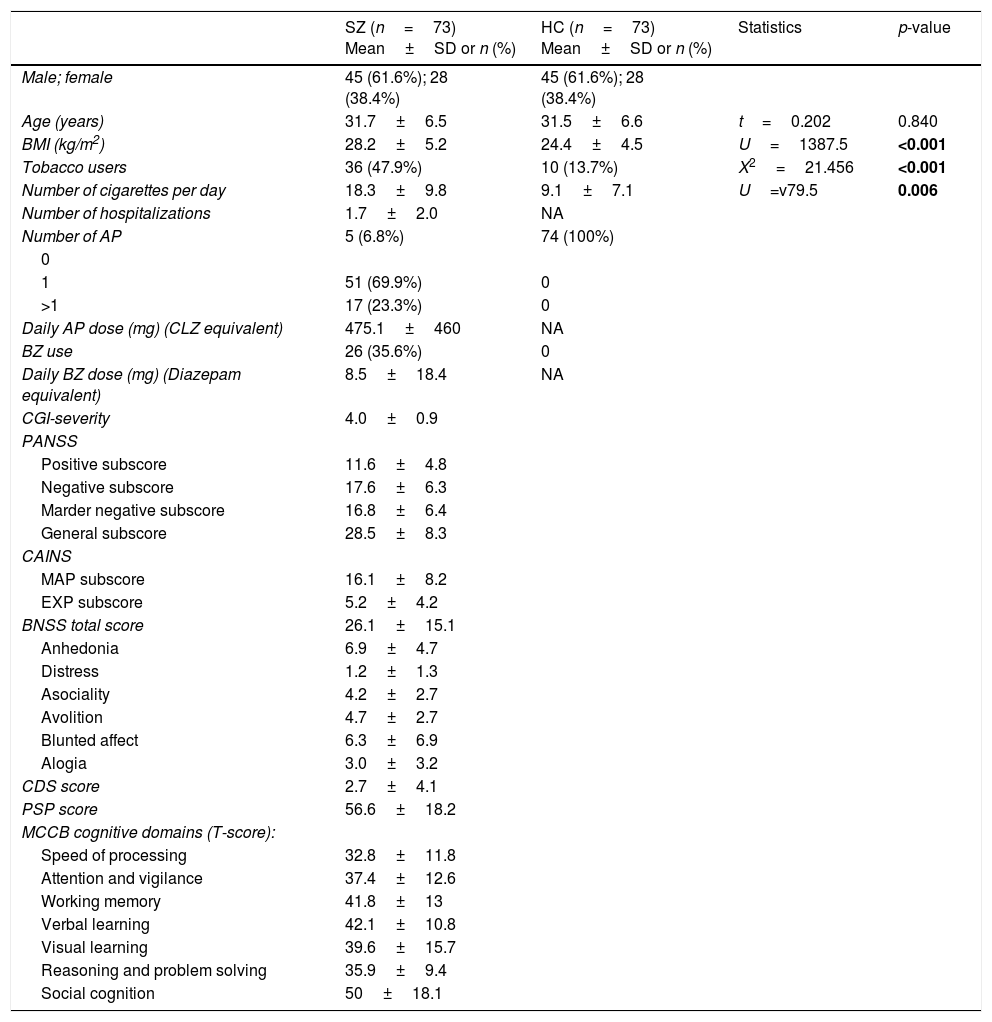
Demographic and clinical characteristics of patients with schizophrenia (SZ) and healthy controls (HC).
| SZ (n=73) Mean±SD or n (%) | HC (n=73) Mean±SD or n (%) | Statistics | p-value | |
|---|---|---|---|---|
| Male; female | 45 (61.6%); 28 (38.4%) | 45 (61.6%); 28 (38.4%) | ||
| Age (years) | 31.7±6.5 | 31.5±6.6 | t=0.202 | 0.840 |
| BMI (kg/m2) | 28.2±5.2 | 24.4±4.5 | U=1387.5 | <0.001 |
| Tobacco users | 36 (47.9%) | 10 (13.7%) | X2=21.456 | <0.001 |
| Number of cigarettes per day | 18.3±9.8 | 9.1±7.1 | U=v79.5 | 0.006 |
| Number of hospitalizations | 1.7±2.0 | NA | ||
| Number of AP | 5 (6.8%) | 74 (100%) | ||
| 0 | ||||
| 1 | 51 (69.9%) | 0 | ||
| >1 | 17 (23.3%) | 0 | ||
| Daily AP dose (mg) (CLZ equivalent) | 475.1±460 | NA | ||
| BZ use | 26 (35.6%) | 0 | ||
| Daily BZ dose (mg) (Diazepam equivalent) | 8.5±18.4 | NA | ||
| CGI-severity | 4.0±0.9 | |||
| PANSS | ||||
| Positive subscore | 11.6±4.8 | |||
| Negative subscore | 17.6±6.3 | |||
| Marder negative subscore | 16.8±6.4 | |||
| General subscore | 28.5±8.3 | |||
| CAINS | ||||
| MAP subscore | 16.1±8.2 | |||
| EXP subscore | 5.2±4.2 | |||
| BNSS total score | 26.1±15.1 | |||
| Anhedonia | 6.9±4.7 | |||
| Distress | 1.2±1.3 | |||
| Asociality | 4.2±2.7 | |||
| Avolition | 4.7±2.7 | |||
| Blunted affect | 6.3±6.9 | |||
| Alogia | 3.0±3.2 | |||
| CDS score | 2.7±4.1 | |||
| PSP score | 56.6±18.2 | |||
| MCCB cognitive domains (T-score): | ||||
| Speed of processing | 32.8±11.8 | |||
| Attention and vigilance | 37.4±12.6 | |||
| Working memory | 41.8±13 | |||
| Verbal learning | 42.1±10.8 | |||
| Visual learning | 39.6±15.7 | |||
| Reasoning and problem solving | 35.9±9.4 | |||
| Social cognition | 50±18.1 |
SD, standard deviation; BMI, body mass index; NA, not applicable; AP, antipsychotics; CLZ, chlorpromazine; BZ, benzodiazepines; CGI, Clinical Global Impression; PANSS, Positive and Negative Syndrome Scale; CAINS, Clinical Assessment Interview for Negative Syndrome; MAP, motivation and pleasure; EXP, expression; BNSS, Brief Negative Syndrome Scale; CDS, Calgary Depression Scale; PSP, Personal and Social Performance Scale; MCCB, MATRICS Consensus Cognitive Battery.
Sociodemographic and clinical variables related to schizophrenia were assessed by semi-structured interview, including: duration of illness, psychopharmacological treatment, history of psychiatry hospitalizations and tobacco use (measured as cigarettes per day in both groups). Each antipsychotic dose was converted to chlorpromazine equivalents in mg/day.27 Benzodiazepine treatment was recorded in diazepam equivalent doses.28
The anthropometric data included weight (kg), height (cm), and waist circumference (cm), measured in both groups. The body mass index (BMI – kg/m2) was calculated as the individual's weight divided by the square of their height.
Metabolic syndrome (MetS) prevalence was estimated using criteria of the statement from the American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI).29 Thus, it was defined by the presence of three or more of the following components: hypertension (systolic and diastolic blood pressure ≥130/85mmHg), hypertriglyceridemia (fasting triglyceride concentration ≥150mg/dL), dyslipidemia (fasting HDL cholesterol <40mg/dL in males and <50mg/dL in females), hyperglycemia (fasting glucose concentration ≥100mg/dL), and abdominal obesity (waist circumference >102cm in males and >88cm in females).
Clinical assessmentPsychopathology and global functioningAll subjects in the SZ group were assessed with the Spanish versions of the Positive and Negative Syndrome Scale (PANSS),30 Clinical Assessment Interview for Negative Symptoms (CAINS),31 Brief Negative Symptom Scale (BNSS),32 Calgary Depression Scale (CDS),33 the Clinical Global Impression (CGI) scale,34 and Personal and Social Performance Scale (PSP).35 Due to methodological problems of the PANSS for assessing negative symptoms,36 we employed the CAINS, which is made up of two subscales covering “motivation/pleasure” (CAINS-MAP, whose items include expected pleasure and motivation from recreation, social, work and school activities) and “expression” (CAINS-EXP, whose items include facial and vocal expression, expressive gestures and speech), and the BNSS, organized into six subscales (anhedonia, distress, asociality, avolition, blunted affect and alogia).
CognitionThe Spanish version of the MATRICS Consensus Cognitive Battery (MCCB) was administered to explore neuropsychological functioning. The MCCB includes 10 standardized neuropsychological tests to measure cognitive performance in 7 cognitive domains: processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition.37
Blood collectionAll blood samples were obtained in the morning between 8:00 and 10:00a.m. by venipuncture after a confirmed overnight fast, on the same day as the clinical assessment. Blood counts and routine biochemistry tests including lipid profile, fasting glucose, and homocysteine were performed in the laboratory of Hospital Universitario Central de Asturias.
The remaining blood samples were processed in the laboratories of Psychiatry and Cellular Response to Oxidative Stress (Department of Morphology and Cellular Biology) Research Groups of the University of Oviedo. Blood tubes were centrifuged (3000rpm for 15min, 4°C). The resultant plasma was divided into aliquots and stored at −20°C. Erythrocytes were washed two times with ice-cold isotonic NaCl solution (0.9%) followed by centrifugation (4000rpm for 5min, 4°C). The prepared hemolysates were stored at −20°C pending analysis. Erythrocyte membranes were prepared according to the method developed by Dodge et al. (1963)38 and stored at −80°C.
Oxidative stress parametersTo study in vitro resistance of erythrocytes to reactive oxygen species (ROS), we performed the erythrocyte hemolysis test (HT) using a modification of the technique described by Farrel et al. (1977) and de Gonzalo-Calvo et al. (2011).39,40 The extent of hemolysis was determined spectrophotometrically by measuring the absorbance of the hemolysate at 540nm in a microplate reader (Thermo Scientific, Thermo Plate, USA).
Lipid peroxidation (LPO) of erythrocyte membranes was assessed by determining the levels of the reactive aldehyde malondialdehyde (MDA), an end product of the lipid peroxidation cascade.41 The amounts of MDA were determined in the erythrocytes using a LPO Assay Kit (SIGMA, 108383, 1,1,3,3-Tetramethoxypropane) based on the condensation reaction of the chromogen N-methyl-2-phenylindole with MDA. Data are expressed as nmoles of MDA/gram of hemoglobin (Hb).
Catalase activity in erythrocytes (CAT) (EC 1.11.1.6) was determined by the method of Lubinsky and Bewley42 using hydrogen peroxide (H2O2) as the substrate. This method measures the rate of reduction of H2O2 to water and molecular oxygen by CAT spectrophotometrically at 240nm at 25°C. Measures were recorded every minute for 4min. One enzyme unit of CAT is defined as the necessary quantity of enzyme to reduce 1μmol of H2O2 per minute under the assay conditions. Data are expressed as μmoles of H2O2/milligram of Hb per minute.
Statistical analysesThe statistical software package SPSS 23.0 for Windows was used for statistical analyses. Extreme outliers of biomarkers were removed from the database, and the normality of the data was analyzed using the Kolmogorov–Smirnov test. Categorical variables in the HC and SZ groups were compared using the Chi-squared test while continuous variables were compared using Student's t-test for independent samples and the non-parametric Mann–Whitney U-test for non-normally distributed variables. Oxidative stress continuous parameters were compared using an analysis of covariance (ANCOVA) or the non-parametric ANCOVA (Quade's test) adjusted for the presence of MetS and cigarettes per day. Differences were considered statistically significant when p<0.05.
Associations between oxidative stress biomarkers and clinical variables in the group of patients were identified through Pearson correlations. Once confounding factors associated with any of these biomarkers were determined (gender, MetS, tobacco use, chlorpromazine and diazepam equivalent doses), they were included as covariates in Stepwise multiple regression analyses to explore the effect of biomarkers on clinical dimensions scores. Due to literature and expert criteria, we also included age, duration of illness, years of education and BMI as potential confounders.
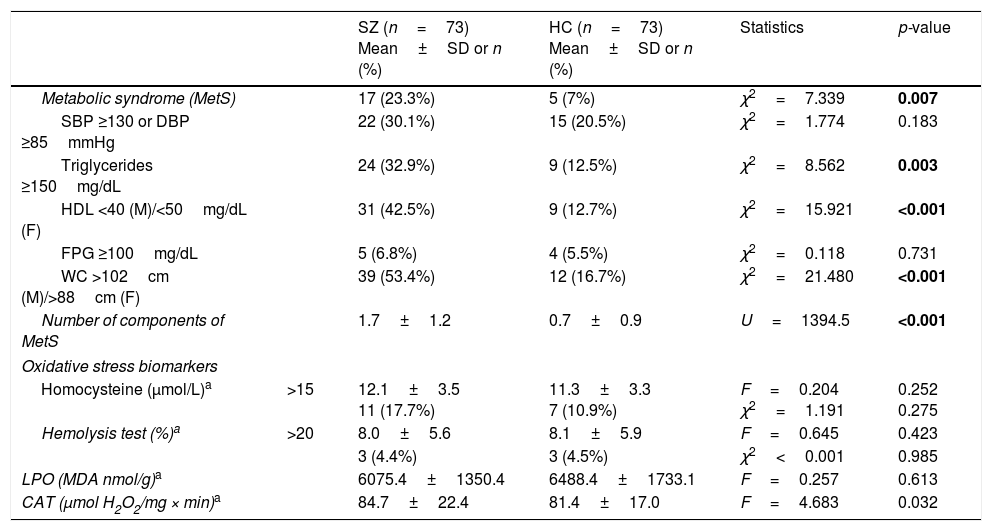
ResultsSociodemographic and clinical dataTable 1 summarizes characteristics of the study sample, including psychopathological scores and cognitive domains (T-scores) in the SZ group. As expected, both groups did not differ in age and sex. The average length of illness of patients at enrollment in the study was 4.6±3.4 years. Only 17 patients (23.3%) were receiving antipsychotic polytherapy, and 5 (6.8%) were not taking any antipsychotic. Most of them were atypical, except one patient who was receiving haloperidol in combination. As shown in Table 2, metabolic syndrome was more prevalent in the group of patients (23.3% vs 7%) with a higher prevalence of hypertriglyceridemia and abdominal obesity, and lower levels of HDL.
Comparison of metabolic syndrome and oxidative stress biomarkers between patients with schizophrenia (SZ) and healthy controls (HC).
| SZ (n=73) Mean±SD or n (%) | HC (n=73) Mean±SD or n (%) | Statistics | p-value | ||
|---|---|---|---|---|---|
| Metabolic syndrome (MetS) | 17 (23.3%) | 5 (7%) | χ2=7.339 | 0.007 | |
| SBP ≥130 or DBP ≥85mmHg | 22 (30.1%) | 15 (20.5%) | χ2=1.774 | 0.183 | |
| Triglycerides ≥150mg/dL | 24 (32.9%) | 9 (12.5%) | χ2=8.562 | 0.003 | |
| HDL <40 (M)/<50mg/dL (F) | 31 (42.5%) | 9 (12.7%) | χ2=15.921 | <0.001 | |
| FPG ≥100mg/dL | 5 (6.8%) | 4 (5.5%) | χ2=0.118 | 0.731 | |
| WC >102cm (M)/>88cm (F) | 39 (53.4%) | 12 (16.7%) | χ2=21.480 | <0.001 | |
| Number of components of MetS | 1.7±1.2 | 0.7±0.9 | U=1394.5 | <0.001 | |
| Oxidative stress biomarkers | |||||
| Homocysteine (μmol/L)a | >15 | 12.1±3.5 11 (17.7%) | 11.3±3.3 7 (10.9%) | F=0.204 χ2=1.191 | 0.252 0.275 |
| Hemolysis test (%)a | >20 | 8.0±5.6 | 8.1±5.9 | F=0.645 | 0.423 |
| 3 (4.4%) | 3 (4.5%) | χ2<0.001 | 0.985 | ||
| LPO (MDA nmol/g)a | 6075.4±1350.4 | 6488.4±1733.1 | F=0.257 | 0.613 | |
| CAT (μmol H2O2/mg × min)a | 84.7±22.4 | 81.4±17.0 | F=4.683 | 0.032 | |
Differences in metabolic parameters were assessed using a Chi-squared test for categorical variables, and Student's t-test for independent samples, or the non-parametric Mann–Whitney U-test for continuous and non-normally distributed data. Inflammatory and oxidative stress continuous variables were compared using an analysis of covariance (ANCOVA) or the non-parametric ANCOVA (Quade's test) adjusted for the presence of metabolic syndrome and cigarettes per day. SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; M, males; F, females; FPG, fasting plasma glucose; WC, waist circumference; LPO, lipid peroxidation, CAT, erythrocyte catalase activity.
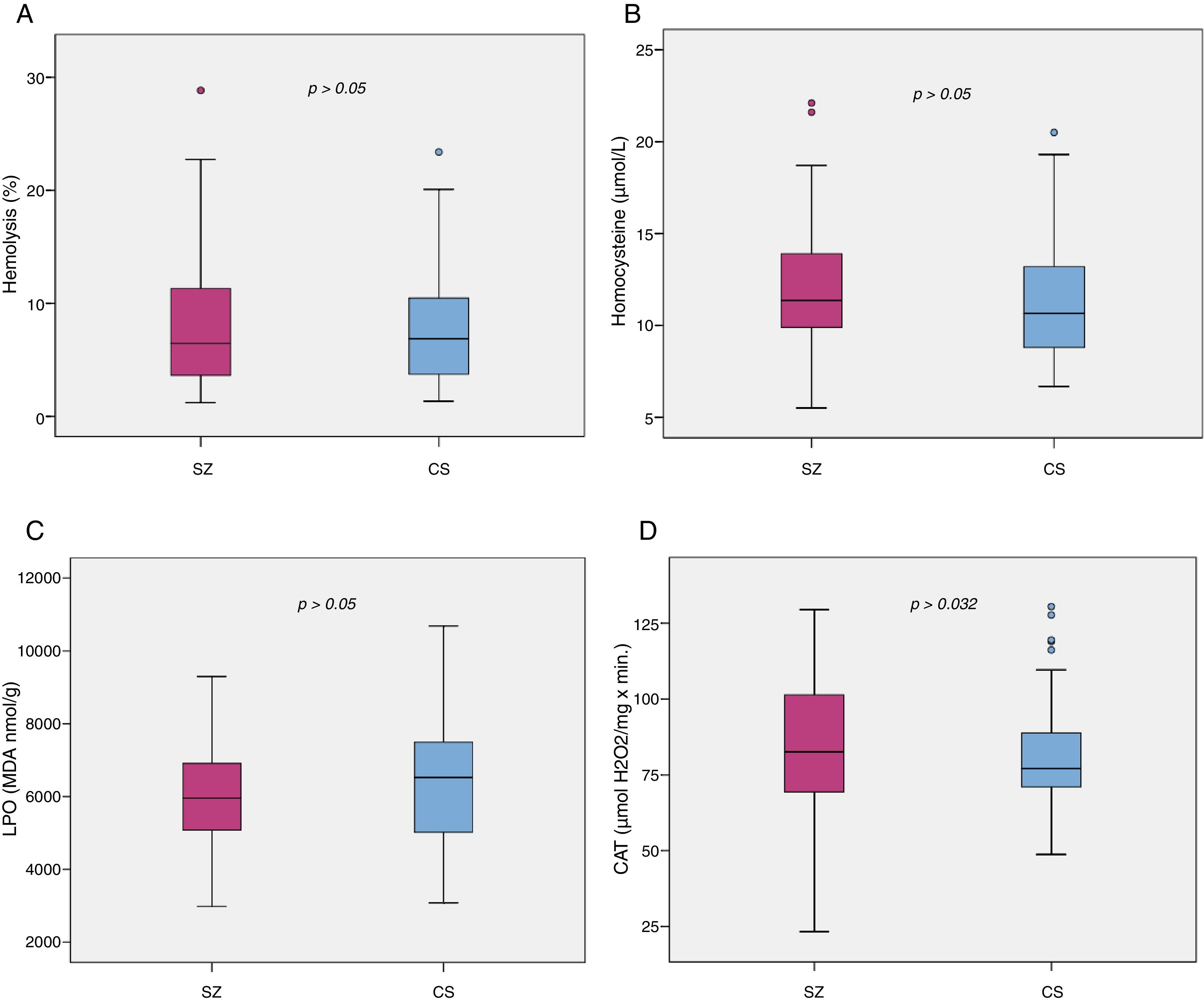
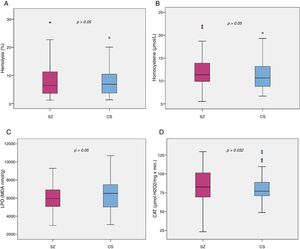
Biomarker comparisons between the HC and SZ groups are shown in Table 2. After controlling for the presence of MetS and cigarettes per day, only CAT was significantly higher in patients compared to HC subjects. Boxplots of oxidative stress parameters are shown in Fig. 1.
Boxplot of the oxidative stress biomarkers: (A) percent of hemolysis (erythrocyte fragility), (B) homocysteine, (C) lipid peroxidation subproducts, (D) erythrocyte catalase activity (CAT); with the p-values from analysis of covariance between schizophrenia patients (SZ) and healthy control subjects (HC), after adjusting for body mass index and cigarettes per day, shown above. *, statistically significant; MDA, malondialdehyde.
Correlation analyses demonstrated that homocysteine levels are positively correlated with scores on the PANSS-Positive (r=0.295; p=0.02), PANSS-General (r=0.301; p=0.017), CGI-Severity (r=0.253; p=0.049), Expression domain of the CAINS (r=0.268; p=0.035), and negatively correlated with PSP scores (r=−0.253; p=0.047). On the other hand, LPO is negatively correlated with scores on the PANSS-Negative (r=−0.330; p=0.005) and Negative Marder Factor subscales (r=−0.345; p=0.003), BNSS-Total (r=−0.290; p=0.015), and specifically with avolition (r=−0.277; p=0.020), alogia (r=−0.237; p=0.049) and blunted affect subscales of the BNSS (r=−0.325; p=0.006), and Expression domain of the CAINS (r=−0.282; p=0.018). Lipid peroxidation was also associated with PSP (r=0.246; p=0.04). No associations were found between HT, CAT, and psychopathology or functioning. Finally, no biomarker had any correlation with depressive symptoms.
Final models of regression analyses obtained to assess the effect of homocysteine and LPO on psychopathology or functioning are shown in Table 3, including only variables that explained an effect on specific clinical dimensions.
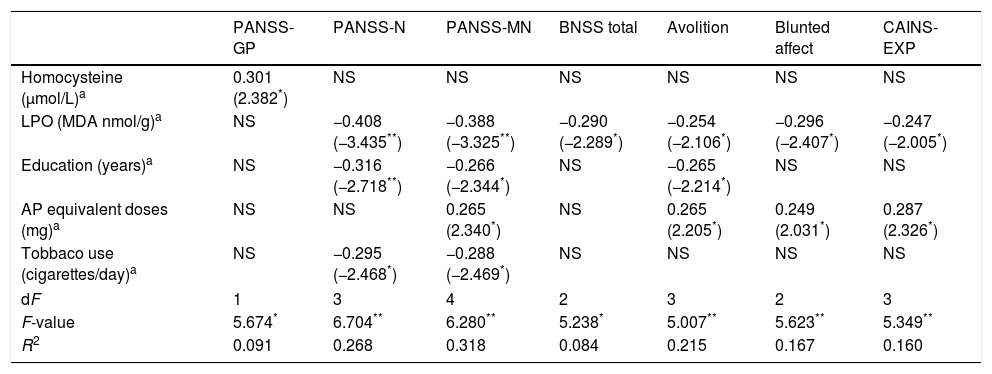
Summary of linear regression models on the association between oxidative stress biomarkers and clinical scores in patients.
| PANSS-GP | PANSS-N | PANSS-MN | BNSS total | Avolition | Blunted affect | CAINS-EXP | |
|---|---|---|---|---|---|---|---|
| Homocysteine (μmol/L)a | 0.301 (2.382*) | NS | NS | NS | NS | NS | NS |
| LPO (MDA nmol/g)a | NS | −0.408 (−3.435**) | −0.388 (−3.325**) | −0.290 (−2.289*) | −0.254 (−2.106*) | −0.296 (−2.407*) | −0.247 (−2.005*) |
| Education (years)a | NS | −0.316 (−2.718**) | −0.266 (−2.344*) | NS | −0.265 (−2.214*) | NS | NS |
| AP equivalent doses (mg)a | NS | NS | 0.265 (2.340*) | NS | 0.265 (2.205*) | 0.249 (2.031*) | 0.287 (2.326*) |
| Tobbaco use (cigarettes/day)a | NS | −0.295 (−2.468*) | −0.288 (−2.469*) | NS | NS | NS | NS |
| dF | 1 | 3 | 4 | 2 | 3 | 2 | 3 |
| F-value | 5.674* | 6.704** | 6.280** | 5.238* | 5.007** | 5.623** | 5.349** |
| R2 | 0.091 | 0.268 | 0.318 | 0.084 | 0.215 | 0.167 | 0.160 |
NS=variables excluded in the final model; AP, antipsychotics; PANSS, Positive and Negative Syndrome Scale; -GP, general psychopathology; -N, negative; -NM, negative Marder factor; BNSS, Brief Negative Syndrome Scale; CAINS, Clinical Assessment Interview for Negative Syndrome; -EXP, expression.
Higher levels of homocysteine showed a predicting effect on general psychopathology measured by the PANSS, while lower concentrations of LPO were a predictor of greater scores on the PANSS-Negative, PANSS-Negative Marder Factor, CAINS-EXP and BNSS, and specifically on avolition and blunted affect subscales.
In the case of PANSS-Positive only the variable antipsychotic equivalent doses, but not any oxidative stress parameter, was included in the explaining model (R2=0.124; β=0.330, p=0.004, R2=0.100, respectively). Furthermore, scores on alogia subscale of the BNSS were only predicted by shorter duration of illness (β=−0.320, p=0.014, R2=0.102) and both global severity, measured by CGI, and functioning, measured by PSP, were significantly predicted by antipsychotic equivalent doses and years of education (R2=0.181 and R2=0.188 respectively).
CognitionIn relation to cognitive function, only CAT showed a significant positive correlation with a specific domain of the MCCB: verbal learning (r=0.239; p=0.046). However, multiple regression analysis revealed that only education level (β=0.330, p=0.004) and antipsychotic equivalent doses (β=−0.239, p=0.036) were predictors of verbal learning T-scores (model dF=2, F=6.561, p=0.002).
DiscussionAmong the oxidative stress biomarkers studied, we found that only CAT is increased in stable patients with schizophrenia during the first 10 years of illness compared to matched HC when MetS and smoking habit are controlled.
Few previous studies in stable patients with schizophrenia reported higher CAT in erythrocytes43,44 while few others detected lower levels.45,46 Nevertheless, our findings are consistent with a Flatow et al. (2013) meta-analysis reporting that this antioxidant enzyme seemed to be a state-related marker, as levels were significantly lower in first-episode psychosis, increased in stable patients, and subsequently decreased in chronic patients.47
It is likely that, in patients stabilized after an acute episode, increased CAT activity, within the antioxidant defense system, will neutralize free radicals, preventing potential damage from maintained oxidative stress. In this line, the finding of normal levels of LPO and erythrocyte hemolysis in our sample might be the result of this efficient response. Also, in contrast to previous findings,48 we have not detected increased levels of homocysteine, an amino acid that produce oxidative stress in cells by interacting with NMDA receptor and which has been involved in the pathogenesis of schizophrenia.49
A further finding of our work is the significant relationship between oxidative stress parameters and severity of clinical dimensions. When confounding factors were considered, associations between these biomarkers and general and negative symptomatology remained significant, while positive and cognitive dimension did not.
On the one hand, the severity of general psychopathology was related to higher homocysteine levels, as the previous study reported.50 However, we did not replicate previous findings of a positive correlation between homocysteine and severity of negative symptoms.50–52 On the other hand, LPO levels seemed to be lower in patients with greater severity of negative symptoms, measured by both the PANSS and BNSS scales. A single publication reported this significant negative association in a multiple linear regression,53 while the majority of studies failed to replicate any correlation between this parameter and any clinical features.47 In contrast, the previous report found increased oxidative stress parameters in patients with deficit schizophrenia.54 To our knowledge, we are the first to report that lower LPO was specifically related to avolition and blunted affect but not related to anhedonia, asociality or alogia, in stable outpatients in their first ten years of illness. It should be mentioned that Garcia-Portilla et al. (2015) proposed a three-component structure of BNSS within which avolition and blunted affect both constitute the “inner world” component of negative dimension.55 Different antioxidant status in patients during an early stage of schizophrenia might be responsible for the discrepancy. We hypothesized that young patients with an excess of antioxidant activity manage to compensate for oxidative stress, even reaching lower levels, although in the long term these mechanisms are depleted. The mechanisms underlying this association need further investigation in longitudinal studies. Finally, for neurocognitive functioning, despite homocysteine has been related to cognitive performance in healthy elderly subjects,56 we cannot conclude any significant relationship with any of the oxidative stress parameters in SZ.
Several limitations of our study should be mentioned. First, the patient group differed from the control group not only in their illness but also in their psychopharmacological treatment, which may have contributed to differences in the study parameters. However, no significant correlation between CAT concentrations and chlorpromazine equivalent-doses were detected in our sample (data not shown). Secondly, other factors such as exercise, diet and vitamin levels, which are known to affect oxidative biomarkers were not considered in the present study. Another limitation is that we had only one healthy control group but not another group of patients with another severe mental disorder, such as bipolar disorder, for comparison. Thus, we can detect only biomarkers that differentiate between patients with schizophrenia and healthy subjects, but we cannot conclude that they are specific to this disorder. Regarding associations with clinical dimensions, although we controlled for antipsychotic and benzodiazepine doses, the potential differential effect on biomarkers of each type of antipsychotic was not considered nor was the effect of other medications like mood stabilizers (2 patients required valproate and 1 lithium) or anticholinergic drugs (2 patients used biperiden). Finally, the cross-sectional nature of the data presented in this paper does not allow us to infer causality. Further studies with a longitudinal design are needed to elucidate the causal relationships among oxidative stress biomarkers, clinical symptoms, and cognitive impairment.
Despite these limitations, some key strengths of the current study are noteworthy. We had an age and sex-matched control group in our study sample, and a large number of confounding factors were considered in multiple regression analyses. Furthermore, adequate psychometric instruments were used for a detailed clinical assessment in the group of patients, especially for negative symptoms, cognition, and global functioning. To our knowledge, no previous oxidative stress biomarker studies in schizophrenia have employed the BNSS, CAINS, or PSP for a more accurate assessment of the negative dimension and global functioning, and a small number of them have used reliable and valid cognitive tools such as the MCCB for examining cognitive performance in this population. Finally, our sample was quite homogeneous, including clinically stable outpatients in their first 10 years of schizophrenia, and mostly treated with antipsychotic monotherapy.
In conclusion, these findings connecting biological pathways to clinical features in patients with schizophrenia are especially relevant for translational psychiatry. Although we are still far from determining valid and specific biomarkers of this heterogeneous illness, a biological approach to this type of research is leading us into promising new horizons in the field of diagnostic, prognostic, and therapeutic methods of clinical practice.
FundingThis study is funded by a grant from the Ministerio de Economía y Competitividad, Instituto de Salud CarlosIII (PI13/02263) and the Fondo Europeo de Desarrollo Regional (FEDER).
Conflict of interestLGB received a grant from the Fundación de Psiquiatría y Salud Mental (Psychiatry and Mental Health Foundation). In addition, the author has received fees as a speaker and for logistical support to attend Janssen-Cilag, Otsuka, Lundbeck, and Pfizer conferences. MPGPG has been a consultant and has received fees/grants from the Otsuka-Lundbeck Alliance, CIBERSAM, the European Commission, Carlos III Health Institute, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Servier, Roche, and Rovi. LGA has received fees from the 7th Framework Program, European Union. PAMS has been a consultant and has received fees/grants from Adamed, AstraZeneca, Brainpharma, Bristol-Myers Squibb, CIBERSAM, Esteve, European Commission, Ferrer inCode, GlaxoSmithKline, Carlos III Health Institute, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, National Drugs Plan, Rovi, and Servier. JBG has received research grants and has been a consultant/speaker in the last 5 years for: AB-Biotics, Adamed, Almirall, AstraZeneca, Bristol-Myers Squibb, Ferrer, Glaxo-Smith-Kline, Hoffman La Roche, Janssen-Cilag, Indivior, Lilly, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Reckitt-Benckiser, Sanofi-Aventis, Servier, Shering-Plough and Shire, a research fund from the Ministry of Economy and Finance–Mental Health Network at the Biomedical Research Centre (CIBERSAM) and the Carlos III Health Institute, Spanish Ministry of Health, Social Services and Equality–National Drugs Plan- and the 7th Framework Program of the European Union. LFT, CIG, SRG, and ACM have no conflicts of interest to declare.
Please cite this article as: González-Blanco L, García-Portilla MP, García-Álvarez L, de la Fuente-Tomás L, Iglesias García C, Sáiz PA, et al. Biomarcadores de estrés oxidativo y dimensiones clínicas en los 10 primeros años de esquizofrenia. Rev Psiquiatr Salud Ment (Barc.). 2018;11:130–140.