Nonketotic hyperglycinemia (NKH) has a low incidence (1/76,000 newborns) and is an autosomal recessive inherited metabolic disorder. It leads to accumulation of high amounts of glycine in the brain and tissues; its prognosis depends on its severity.1 Glycine is an inhibitory amino acid but acts as excitatory at the brain glutamatergic NMDA receptors. This NMDA hyperactivity leads to seizures and other symptoms.
In its classic or neonatal form, NKH can manifest with a wide spectrum of symptoms such as low muscle tone, lethargy, coma and treatment-resistant seizures. High glycine levels in blood and cerebral spinal fluid lead to suspicion; and genetic testing, to confirmation of diagnosis. In the absence of curative treatment, the standard treatment is an appropriate diet and seizure stabilization. Among multiple treatments tried, sodium benzoate offers special interest due to its ability to scavenge the excess of glycine in the blood.2 NMDA antagonists can be associated with symptomatic benefits, but not with changes in the disease course.
We analyze the case of a child with severe behavioral problems associated with NKH who received clozapine with a satisfactory result despite prior treatment resistance. When clozapine was started, the patient was an 11-year-old girl (BMI 23.7kg/m2), the youngest among three children of unrelated Spanish Caucasian parents. Just as her oldest brother, she had NKH in its neonatal form and was diagnosed during her first year of life through the identification of two mutations of the GLDC gene,3 one of the genes encoding the glycine decarboxylase complex enzyme (chr9:6592219:c.1406G>A;p.Gly469Asp and chr9:6602138:c.1126A>G;p.Lys376Glu). At age 4 months, she developed hypotonia and seizures, followed by a marked neurodevelopmental delay with progressive spasticity and autistic symptoms. At 6 years of age, despite the absence of seizures, her behavior worsened with hyperactivity and severe self-harm. She showed poor response to multiple treatments and at age 12 she was admitted into our child and adolescent psychiatric unit (considered day 1). She was on a glycine-restricted diet, supplemented with tryptophan 150mg/day, carnitine 100mg/day and folate 1.25mg/day, and treated with dextromethorphan 180mg/day, sodium benzoate 400mg/kg/day and clobazam 20mg/day. However, glycine levels continued elevated until the last exam (1236 days after initiation: 4.71mg/dL; reference values 1.25–2.20).
Prior mood stabilizer (topiramate and valproic acid) and antipsychotic trials (risperidone, 0.7mg/day for four months; quetiapine, 150mg/day for two weeks; aripiprazole, 5mg/day for two months; and different typical antipsychotics such us piperazine, levomopromazine and haloperidol with low doses for a maximum of six months) had been tried since she was 6 or 7 years old, before being admitted into the Child and Adolescent Psychiatric Unit. Those trials were unsuccessful due to lack of efficacy or to the presence of adverse or paradoxical effects (data extracted through manual medical chart revision). With parent's agreement, which was registered in the medical chart, after informing them about the potential risks for the patient, clozapine was started on day 8 at a dose of 12.5mg/day and titrated to 175mg/day on day 749. In all measurements, clozapine levels were below ≤150ng/mL. As there is no information on clozapine dosing for NKH, dosing was based on clinical response.4
Since the first week of clozapine treatment, we observed a reduction in self-aggressive behavior (hand biting), hyperactivity and normalization of sleep patterns and the dose was adjusted upward progressively until maximum clinical efficacy was achieved according to the opinion of family relatives, teachers, and other physicians treating the patient. Severity was blindly evaluated with the Clinical Global Impression (CGI) severity scale by two clinical psychiatrists (M.G. and L.G.-R.) based on the chart notes. They were blind to date of the note, clozapine dose and plasma clozapine concentrations. The inter-rater reliability of these CGI assessments was high, with intra-class correlation coefficient of 0.81 (95% confidence interval, 0.55–0.92; P<0.001). Their ratings were averaged when there was only a 1-point difference (in 63% of the assessments); otherwise, a rating was obtained by consensus including the advice of the treating psychiatrist (S.J.-F.).
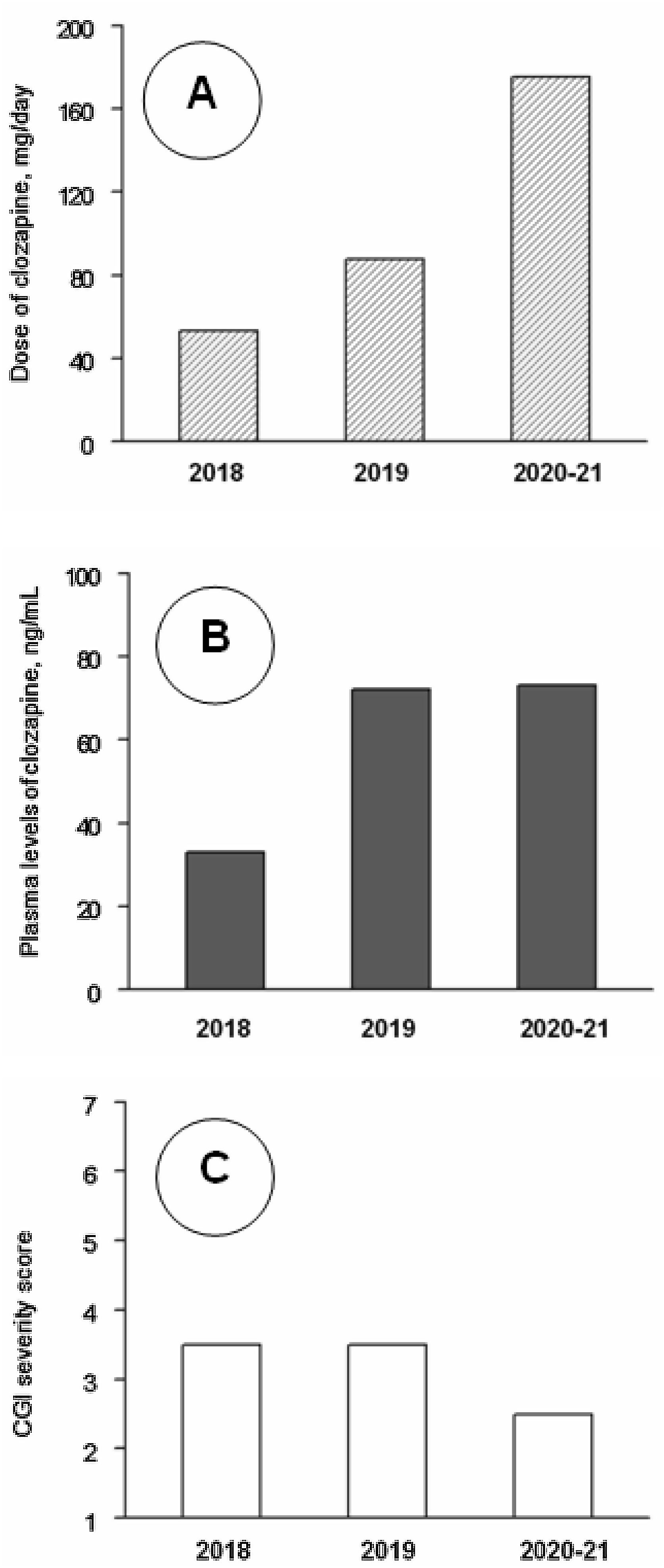
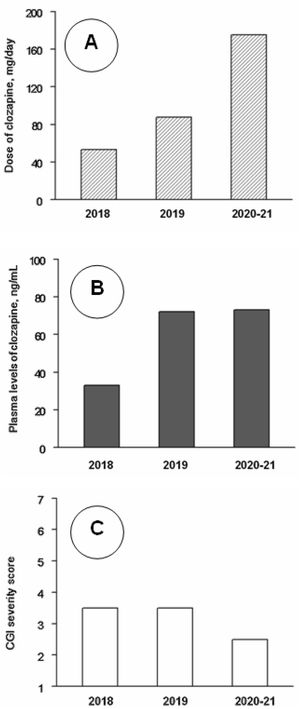
Clinical follow-up, from day 1 to day 1206 (June 2018–August 2021) was divided into three periods. Analytical controls were performed periodically, weekly during the first three months and progressively more spaced up to a blood test every month and a half. At no time were found alterations in the hemogram or in the metabolic or hepatic profile. Clozapine plasma values were also measured. The mean±standard deviation of clozapine doses (mg/day) and plasma concentrations (ng/ml) were 48.5±14.0 and 32.5±13.4 during 2018 (8 assessments), 94.6±27.8 and 78.3±41.8 during 2019 (7 assessments), and 171.4±9.4 and 74.1±17.6 during 2021–22 (7 assessments). The CGI severity scores were respectively 3.62±1.16, 3.21±0.86 and 2.86±0.99. The changes in clozapine doses and concentrations were statistically significant (P<0.01), but the changes in CGI scores did not reach statistical significance in the Kruskal–Wallis test (Fig. 1). CGI severity scores were inversely and significantly correlated with clozapine concentrations (rs=−0.49; P=0.021); clozapine concentrations were positively correlated with clozapine doses (rs=0.67; P=0.001) and with weight (r=0.49; P=0.032).
Median values of clozapine doses (A), clozapine plasma levels (B) and Clinical Global Impression (CGI) severity scale scores (C) throughout the follow-up. Note: Across 2018 (8 assessments), 2019 (7 assessments) and 2020–21 (7 assessments), as shown by Kruskal–Wallis tests, there were significant changes in clozapine doses (P<0.001) and levels (P=0.005), but not in CGI scores (P=0.33). Post hoc Mann–Whitney tests showed that clozapine doses increased significantly from 2018 to 2019 (P<0.001) and 2020–21 (P<0.001) and from 2019 to 2020–21 (P=0.001); clozapine plasma levels were lower in 2018 than in 2019 (P=0.014) and 2020–21 (P=0.001), but the differences between 2019 and 2020–21 were not significant (P=0.95).
In the treatment of aggression and self-injury in intellectual disability, psychiatrists agree that non-pharmacological interventions are the first choice of treatment.5 In this case it consisted of proper follow-up of dietary treatment and close coordination with family and school environment for behavior management. The use of antipsychotics in children with intellectual disability is controversial because it carries a major risk of occurrence of adverse side effects.6,7 However, they are necessary when aggressiveness is severe and other psychotropic drugs are ineffective.8 Thus, they could play a crucial role in the treatment of aggression.9 With regard to clozapine, it has showed efficacy in treating aggressiveness among patients with intellectual disabilities10 and, in case of children, its use is reserved for organic syndromes, severe conduct disorders or early-onset psychosis.9 As with other antipsychotics, clozapine is a D2 antagonist, but has other less-understood actions. According to animal studies, clozapine prevents thalamo-cortical hyperglutamatergic transmission which may explain clozapine's efficacy in NKH.11 There is no consensus on clozapine dosing for children and for adults with indications other than schizophrenia, but they can respond to lower clozapine doses without reaching the minimum therapeutic concentrations of 350ng/mL.12 The low incidence of side effects in young populations requires careful dose titration with close monitoring of clozapine.9 We found a significant inverse correlation of clozapine levels with severity despite low serum concentrations. The correlation of -0.49 is a reasonable value for biological variables and indicates a large effect size.13
The parents were informed and gave consent to the publication of this clinical experience, which was registered in the medical record.
Author's disclosureS. Jiménez-Fernández, M. Gurpegui and J. de Leon declare no conflict of interest.
L. Gutiérrez-Rojas has received consultancy and/or lecture honoraria from Lundbeck, Pfizer, Novartis, Janssen, Neuraxpharm, and Otsuka in the last 5-years, none of them with direct relation to this work.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors thank Lorraine Maw, MA, at the Mental Health Research Center at Eastern State Hospital, Lexington, KY, who helped in editing this article.