The immune system is a key element in the organism's defence system and participates in the maintenance of homeostasis. There is growing interest in the aetiopathogenic and prognostic implications of the immune system in mental disorders, as previous studies suggest the existence of a dysregulation of the immune response and a pro-inflammatory state in patients with mental disorders, as well as an increased prevalence of neuropsychiatric symptoms in patients suffering from autoimmune diseases or receiving immune treatments. This study aims to conduct a narrative review of the scientific literature on the role of psychoneuroimmunology in mental disorders, with special focus on diagnostic, prognostic and therapeutic issues. The development of this body of knowledge may bring in the future important advances in the vulnerability, aetiopathogenic mechanisms, diagnosis and treatment of some mental disorders.
El sistema inmunitario es una pieza fundamental en la defensa del organismo y participa en el mantenimiento de la homeostasis. Existe un interés creciente en las implicaciones etiopatogénicas y pronósticas del sistema inmunitario en los trastornos mentales, avalado por estudios previos que sugieren la existencia de una disregulación de la respuesta inmune y un estado proinflamatorio en pacientes con una enfermedad mental, así como la elevada prevalencia de síntomas neuropsiquiátricos en pacientes con enfermedades autoinmunes o que reciben tratamientos inmunológicos. En el presente trabajo se realiza una revisión narrativa de la literatura científica sobre el papel de la psiconeuroinmunología en los trastornos mentales, especialmente en aspectos diagnósticos, pronósticos y terapéuticos. El desarrollo de este cuerpo de conocimiento puede aportar en el futuro importantes avances en la vulnerabilidad, mecanismos etiopatogénicos, diagnóstico y tratamiento de algunos trastornos psiquiátricos.
The first studies of mind–body interaction date from the first half of the 20th century, when the physiologist Walter Cannon coined the term homeostasis in his work “The wisdom of the body”.1 He described the physiological mechanisms that intervene in a physical–chemical balance that is essential, and proved that the emotional state of an animal (anxiety, stress or anger) may be accompanied by the stoppage of stomach movements. On the other hand, Hans Selye developed the concept of the general adaptation syndrome, a set of psychophysiological changes which rats suffered when exposed to different harmful agents in the laboratory2 as a reaction of the organism to new conditions, and which years later he termed “stress”.3 In 1975, with the works of the psychologist Robert Ader and the immunologist Nicholas Cohen the term “psychoneuroimmunology” was coined, based on studies which showed that an adverse signal channelled through the nervous system led to reactions in the immune system.4 Due to the fact that immunological factors are often associated with endocrinological factors, sometimes the term “psychoneuroendocrinoimmunology” is used. This field of scientific interest would be dedicated to the study of hormonal and immunological aspects of mental disorders. These would include the psychiatric manifestations of hormonal or immunological diseases and those associated with hormonal or immunological treatments.
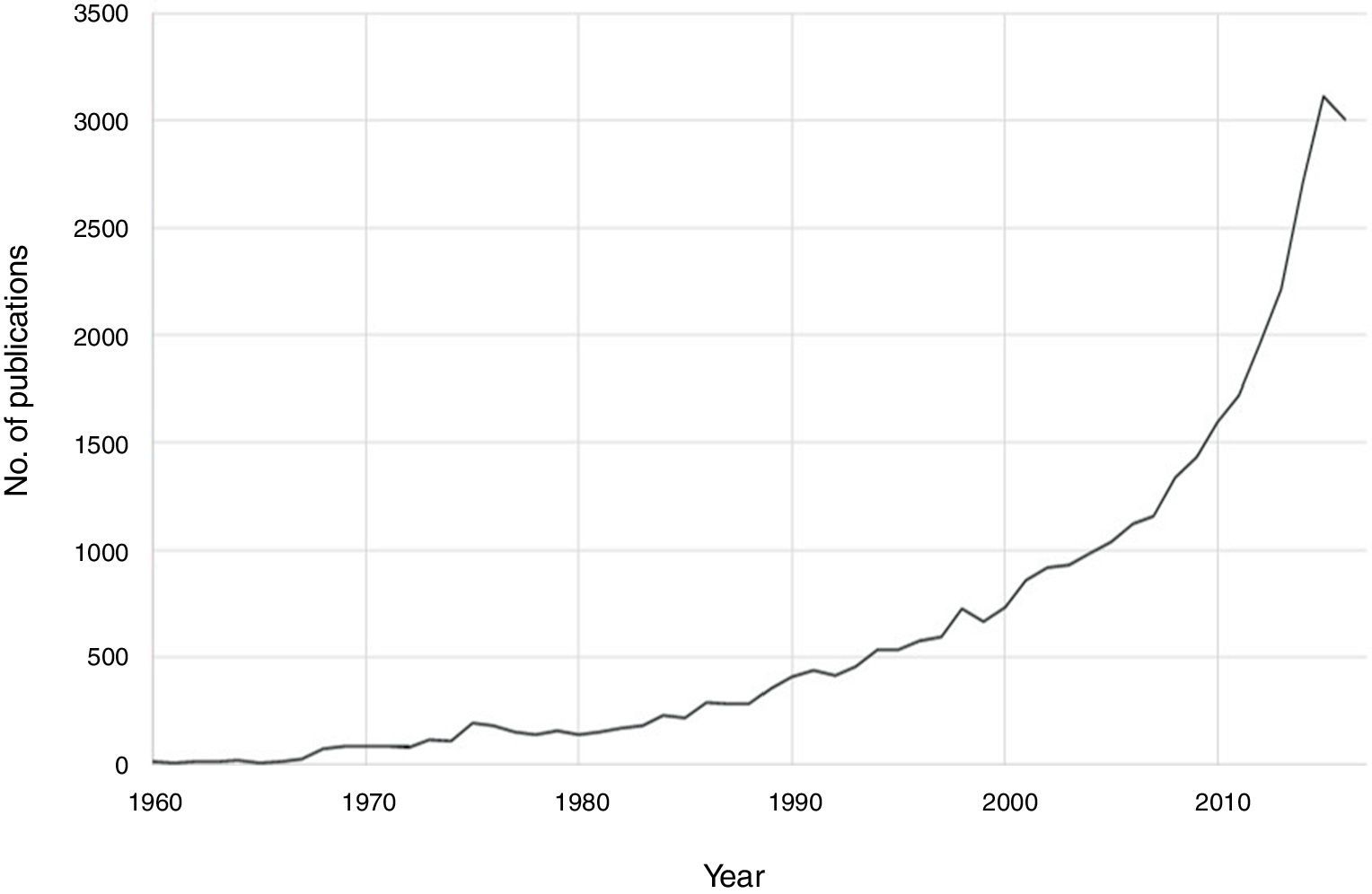
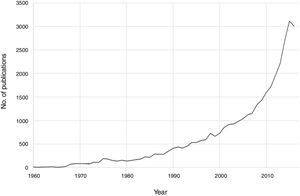
Notable progress has been made in recent decades in the field of psychoneuroimmunology. Therefore, if PubMed is searched for immunological aspects of mental disorders using the search strategy (immune OR inflammat*) AND (psychiatry OR mental disorder OR schizophrenia OR depression OR bipolar), then a total of 36,127 publications until 2016 are obtained, with an exponential increase in the last two decades (Fig. 1). Progress in this field of knowledge in connection with mental disorders runs from aetiopathological aspects to therapeutic ones. One advance involved the discovery of autoimmune markers that have helped in the diagnosis of some encephalitis symptoms that until recently lacked an exact diagnosis. On the other hand, it has been suggested that anti-inflammatory agents be used in the therapeutic approach for several mental disorders associated with conventional psychopharmacological treatments and psychotherapies.
This paper presents a narrative review of relevant aspects of the immune system in the pathogenesis, clinical expression and treatment opportunities of mental disorders. It also covers aspects in connection with the hormones that are involved in the response to stress, due to their important connection with the inflammatory system and their association with severe mental disorders, as well as some specific stress-related disorders. To undertake this review a search of papers was conducted using PubMed with a time limit commencing in the year 2000. It also occasionally includes earlier classical references and seminal works cited in recent papers or reviews of the same subject.
The immune systemGeneral considerationsThe immune system comprises those structures and biological process which defend the organism against aggressions. These may be external (such as pathogenic microorganisms) or internal (such as cancer cells), and the aim is to re-establish homeostasis (for a general review see Delves and Roitt, 20005). The immune system can be classified as “innate” (non-specific) and “acquired” (specific).
The innate system is the first line of defence of the organism. It includes physical barriers such as the skin and mucus membranes, together with other elements such as phagocytes, including macrophages (which in brain tissue form the microglia) and granulocytes (neutrophils). Acquired immunity is more sophisticated and has delayed action onset. It consists of the recognition and destruction of antigens. This involves the development of an “immune memory” and for this it has lymphocytes as basic units. This immunity includes, in turn, humoral immunity (antibodies produced by B lymphocytes and the complement system), and cellular immunity (mediated by T lymphocytes that are divided into other sub-populations such as cytotoxic T cells [CD8] and cooperative T cells [CD4]). The natural killer lymphocytes are considered to be a third lymphocyte population that, although they are differentiated from T lymphocytes after a common ancestor, they do not mature in the thymus and are components of innate immunity.
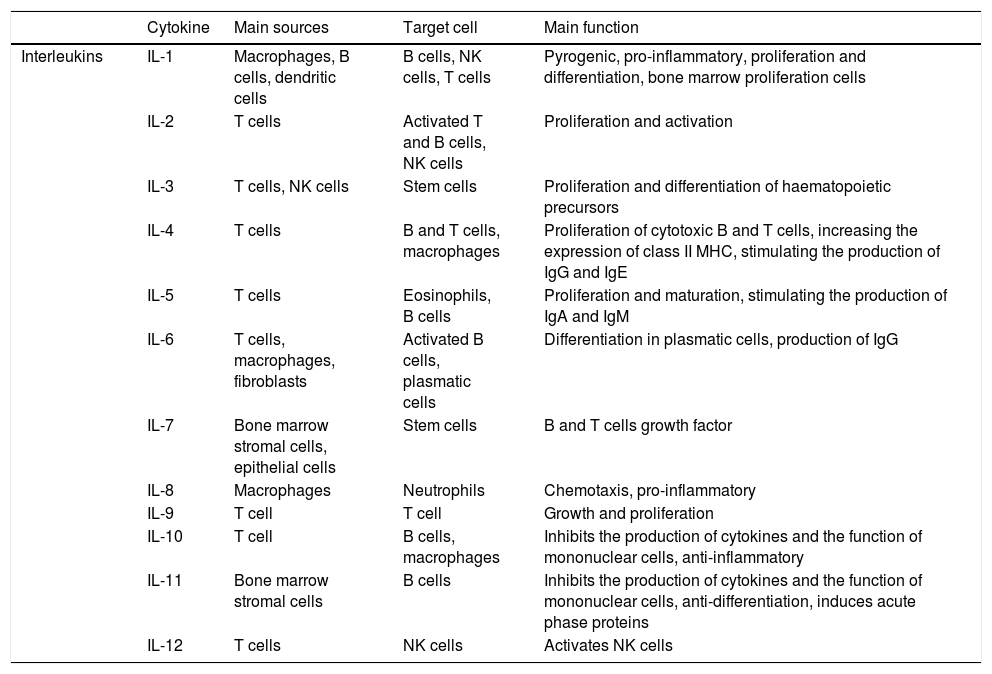
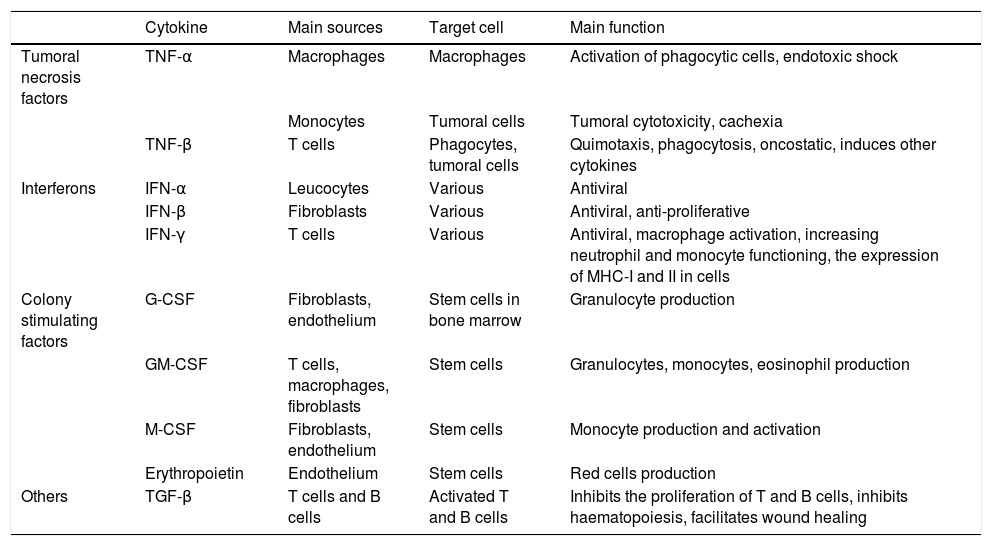
The acute phase inflammatory response is the first reaction of the organism to aggression, attacking pathogens with phagocytes and presenting antigens to T lymphocytes. Depending on the nature of the aggression the immune system will react by activating certain routes or others, and modulating the interaction of the different agents within the system, balancing elements that cause or inhibit inflammation (Tables 1 and 2, adapted from Turner et al., 20146).
The function of the main cytokines (I): interleukins.
| Cytokine | Main sources | Target cell | Main function | |
|---|---|---|---|---|
| Interleukins | IL-1 | Macrophages, B cells, dendritic cells | B cells, NK cells, T cells | Pyrogenic, pro-inflammatory, proliferation and differentiation, bone marrow proliferation cells |
| IL-2 | T cells | Activated T and B cells, NK cells | Proliferation and activation | |
| IL-3 | T cells, NK cells | Stem cells | Proliferation and differentiation of haematopoietic precursors | |
| IL-4 | T cells | B and T cells, macrophages | Proliferation of cytotoxic B and T cells, increasing the expression of class II MHC, stimulating the production of IgG and IgE | |
| IL-5 | T cells | Eosinophils, B cells | Proliferation and maturation, stimulating the production of IgA and IgM | |
| IL-6 | T cells, macrophages, fibroblasts | Activated B cells, plasmatic cells | Differentiation in plasmatic cells, production of IgG | |
| IL-7 | Bone marrow stromal cells, epithelial cells | Stem cells | B and T cells growth factor | |
| IL-8 | Macrophages | Neutrophils | Chemotaxis, pro-inflammatory | |
| IL-9 | T cell | T cell | Growth and proliferation | |
| IL-10 | T cell | B cells, macrophages | Inhibits the production of cytokines and the function of mononuclear cells, anti-inflammatory | |
| IL-11 | Bone marrow stromal cells | B cells | Inhibits the production of cytokines and the function of mononuclear cells, anti-differentiation, induces acute phase proteins | |
| IL-12 | T cells | NK cells | Activates NK cells |
Ig: immunoglobulin; IL: interleukin; MHC: main histocompatibility complex; NK: natural killer; Th: T cell helper.
The functions of the main cytokines (II): tumoral necrosis factors, interferons and other factors.
| Cytokine | Main sources | Target cell | Main function | |
|---|---|---|---|---|
| Tumoral necrosis factors | TNF-α | Macrophages | Macrophages | Activation of phagocytic cells, endotoxic shock |
| Monocytes | Tumoral cells | Tumoral cytotoxicity, cachexia | ||
| TNF-β | T cells | Phagocytes, tumoral cells | Quimotaxis, phagocytosis, oncostatic, induces other cytokines | |
| Interferons | IFN-α | Leucocytes | Various | Antiviral |
| IFN-β | Fibroblasts | Various | Antiviral, anti-proliferative | |
| IFN-γ | T cells | Various | Antiviral, macrophage activation, increasing neutrophil and monocyte functioning, the expression of MHC-I and II in cells | |
| Colony stimulating factors | G-CSF | Fibroblasts, endothelium | Stem cells in bone marrow | Granulocyte production |
| GM-CSF | T cells, macrophages, fibroblasts | Stem cells | Granulocytes, monocytes, eosinophil production | |
| M-CSF | Fibroblasts, endothelium | Stem cells | Monocyte production and activation | |
| Erythropoietin | Endothelium | Stem cells | Red cells production | |
| Others | TGF-β | T cells and B cells | Activated T and B cells | Inhibits the proliferation of T and B cells, inhibits haematopoiesis, facilitates wound healing |
G-CSF: granulocyte colony stimulating factor; GM-CSF: granulocyte and macrophage colony stimulating factor; IFN-α: interferonα; IFN-β: interferon β; IFN-γ: interferon γ; M-CSF: macrophage colony stimulating factor; MHC: main histocompatibility complex; TGF-β: transforming growth factor β; TNF-α: tumoral necrosis factor α; TNF-β: tumoral necrosis factor β.
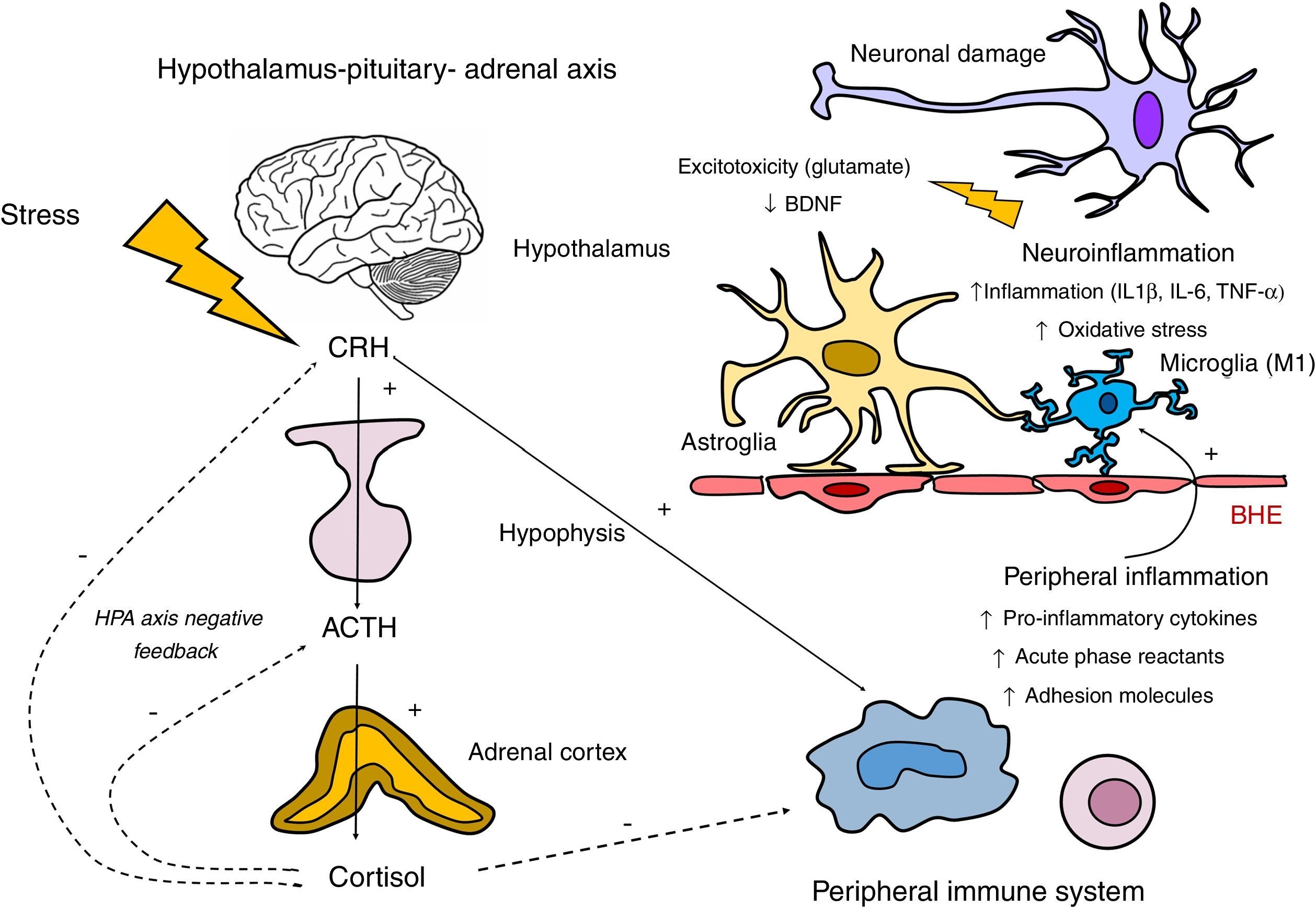
Communication between the different components of the immune system takes place by intercellular contact and the fundamental role of the cytokines, signalling proteins that act in cascade. The bidirectional communication routes between the immune system and the central nervous system include vagal innervation, the lymphatic system and its interaction with other neurohormonal axes, such as the hypothalamus–pituitary–adrenal (HPA) axis (Fig. 2).
Involvement of the hypothalamus–pituitary–adrenal axis (HPA) and the immune system in the neuroinflammatory response. The HPA axis is activated in response to exposure to stressful physical and psychological stimuli by means of the secretion of the corticotrophin-releasing hormone (CRH) by the hypothalamus. This hormone, in turn, stimulates the synthesis of corticotrophin (ACTH) in the hypophysis, which stimulates the secretion of cortisol by the adrenal glands. Regulation of the secretion of cortisol is subject to a negative feedback mechanism by means of which cortisol itself inhibits the synthesis of its precursors (CRH and ACTH). Hypothalamus and hypophysis glucocorticoid receptors take part in this inhibition, as do glucocorticoid and mineralocorticoid receptors present in the hippocampus. Regarding the relationship between the HPA axis and the peripheral inflammatory response, although cortisol inhibits this by exerting an immunosupressor effect, there is inflammatory stimulation by other hormones in the HPA axis such as the CRH. This relationship is bidirectional, as the activation of the peripheral inflammatory response may stimulate the HPA axis. The products of this peripheral stimulation, in which macrophages and lymphocytes participate, may cross the blood–brain barrier (BBB) and trigger a neuroinflammatory reaction by stimulating the microglia in M1 activated forms. This activation of the microglia will generate an inflammatory cascade by releasing cytokines and reactive forms of nitrogen and oxygen, inducing the activation of the astroglia, which in turn amplifies the inflammatory signals within the central nervous system. Three is also excessive release of glutamate by the astrocytes as well as oxidative stress mediators by the activated microglia (associated with the induction of the indolamine 2,3 dioxygenase [IDO] enzyme). These mechanisms negatively affect the production of neurotrophic factors such as the brain derived neurotrophic factor (BDNF), and neurogenesis.
The response of the cytokines may therefore be classified as pro-inflammatory (when the microglia promotes a type of inflammation that may be harmful for tissues, targeted against intracellular antigens) or anti-inflammatory (when the astroglia regulates humoral immunity targeting extracellular antigens).7 The neuronal brain cells and the non-neuronal brain cells express receptors for these mediators8 (Tables 1 and 2).
The microglia does not only modulate neuronal functioning during inflammatory response, as it is also involved in physiological phenomena of neuronal plasticity and pruning during brain synaptic development. It therefore controls the functional status of synapses, influencing neuroplastic changes by remodelling extracellular spaces and eliminating synaptic elements by phagocytosis. In response to harmful stimuli the microglia undergoes a series of changes (quantitative, functional and morphological). These have been identified in response to classical inflammatory stimuli such as infections, as well as in response to situations involving psychological stress.9
In spite of the immunological protection of the brain by the blood–brain barrier, an increase in its permeability has been described in patients with severe mental disorders. This means that pro- or anti-inflammatory factors may enter the periphery or escape from the brain into the system circulation under certain neurophathological situations.10
On the other hand, there is also the bidirectional neurohumoral system known as the intestine–brain axis. The intestinal microbiota consists of a bacterial community that largely resides in the small intestine, in symbiosis with the host individual. Recent studies suggest that the microbiota affect brain development and functioning, and that it may be relevant in the physiopathology of some neuropsychiatric disorders.11 In fact, manipulation of the composition of the intestinal microbiota has been observed to affect systemic concentrations of cytokines in animal models as well as in humans.12,13
Autoimmune diseases and psychiatric symptomsThe involvement of immunological factors in psychiatric disorders is based on different observations. On the one hand, certain autoimmune diseases (such as systemic lupus erythematosus [SLE], encephalitis caused by anti-NMDA antibodies) have a high prevalence of psychiatric symptoms. On the other hand, previous studies in which immune system modulators were administered to animals or humans have detected psychiatric symptoms. In animal models, injecting pro-inflammatory cytokines (IL-1β and TNF-α) produces behaviour similar to social isolation.8 In humans, the administration of endotoxins induces anhedonia and deactivates the ventral striatum nucleus, a region involved in the brain response to reward.14 Another recognised datum is that treating hepatitis C with IFN-α often induces depressive symptoms.15
The relationship between certain autoimmune diseases and psychiatric symptoms is described below.
Systemic lupus erythematosus (SLE)Up to 75% of SLE patients have involvement of the brain,16 and psychiatric symptoms typically appear in the first years of the disease, including anxiety, depression and psychosis. Although affective symptoms may have an adaptive component within the context of suffering a systemic disease and the resulting limitations, in other cases there are psychopathological manifestations associated with the disease, coinciding with the increase in immunological activity parameters (ANA and anti-DNA antibodies). Psychosis in association with lupus is considered to be a diagnostic criterion for SLE. It is often associated with positive antiribosomal P antibodies, although recent meta-analytical studies suggest that this is not specific to psychosis, as it is also associated with anxiety or depression.17 IF we consider the cognitive involvement associated with SLE, up to 80% of patients have slight to moderate cognitive symptoms, while these are severe in 3–5%.16 The most affected domains are attention, visual and verbal memory, executive functions and information processing speed. Neuroimaging studies using structural brain resonance have shown the existence of cortical atrophy, lesions in the subcortical white matter and diffuse changes in the grey matter.18,19
Autoimmune encephalitisCases of autoimmune encephalitis are characterised by an acute onset with epileptic crisis of the temporal lobe, behavioural symptoms of psychiatric manifestations and cognitive involvement. Antibodies against autoantigens have been implicated at a synaptic or intracellular level that may or may not be associated with a paraneoplastic origin.20 These antibodies may be directed against NMDA receptor subunits, voltage-dependent potassium channels, complexes and contact associated with 2 (CASPR2) protein, GluR1 and GluR2 subunits of the 3-hydroxi-5-methyl-l-4-isoxazolepropionic amino acid receptor (AMPAR) and B1 subunits of the B receptors of γ-aminobutyric acid (GABABR).7
Psychiatric manifestations may precede neurological symptoms or even dominate symptoms in the early stages. They include affective symptoms, schizophreniform disorder or even catatoniform symptoms.21 Thus up to two-thirds of patients with autoimmune encephalitis due to anti-NMDAR antibodies consult initially in mental health departments.
Paediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS)Paediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS) is a rare paediatric syndrome described in children who, after suffering an infection by group A β-haemolytic streptococcus, go on to develop tics, involuntary movements and obsessive–compulsive symptoms.22 The onset as well as exacerbations of obsessive–compulsive symptoms have been described following infections of this type. The existence of a crossed reactivity is suggested between antistreptococcus antibodies and proteins (autoantigens) of the basal brain ganglia. These include certain enzymes (aldolases and enolases) which are involved in neurotransmission, neuronal metabolism and cellular signalling, with a structure similar to that of streptococcus proteins. Being seropositive for antibodies against the basal ganglia in patients with obsessive–compulsive disorder (OCD) has been associated with high level of glycin in the cerebrospinal fluid, suggesting that these contribute to the increase in glutamatergic tone that has been described in patients with OCD.7 The improvement in obsessive–compulsive symptoms with immunological therapies supports the role of these antibodies in the pathogenesis of OCD, or at least in a subgroup of patients who associate the symptoms with PANDAS.23
The immune system and primary psychiatric disordersClinical evidence and laboratory data shows that alterations in cellular and humoral immunity are more prevalent in patients with mental disorders than they are in healthy individuals.
Stress and allostatic loadStress may be defined as a threat for the psychological or physiological integrity of an individual. When stress is acute catecholamine and cortisol are liberated from the spine and suprarenal cortex, respectively. This physiological response plays a protective short-term role, although if stress is maintained chronically or in case of hormonal secretion dysregulation it may be prejudicial for the organism.24 This is the aspect covered by the allostatic load model. The organism tends to seek a balance between regulatory physiological systems (homeostasis) by means of adaptation responses (allostasis) that involve the sympathetic nervous and neuroendocrine systems, especially the HPA axis.25 When there is chronic stress and the allostatic load surpasses a limit, chronic dysregulation of the allostasis mediators occurs together with a maladaptive response that has been associated with different medical conditions including mental disorders (unipolar depression,26 bipolar disorder27 and schizophrenia28), neurodegenerative diseases (cognitive deterioration29) or endocrine-metabolic disorders (obesity and metabolic syndrome.)30
Different factors play a role in the response to stress and the capacity to tolerate allostatic load. These factors include personal experiences, genetics and behaviour. When the brain perceives an experience as stressful, physiological and behavioural responses are triggered, including the participation of the immune system, which commence the process of allostasis and adaptation. The accumulation of allostasis, over-exposure to cellular stress, endocrinological and immunological mediators, will lead to the development of diseases. Allostatic load has been associated with different mental conditions. These include burn-out or chronic fatigue syndrome, as well as parameters associated with ageing such as cardiovascular risk, cognitive deterioration and mortality in elderly populations.29
Inflammation in depressionDepressive disorders, as well as their psychological symptoms, also have constellations of somatic or vegetative symptoms in their clinical expression which recall the non-specific symptoms of physical systemic diseases. These include asthenia, anergia, non-specific pain, appetite alterations, sleep anomalies and memory deficits. Additionally, major depression with melancholic symptoms or bipolar depressions have an episodic and recurrent course with periods of remission which recall the course of several autoimmune diseases. The administration of exogenous cytokines such as IFN-α may induce depressive symptoms,15,31 supporting the link between the immune system and depression.
In recent decades alterations have been described in the activation of the inflammatory response at several levels in patients with depression. This involves a reduction in B, T, helper T and suppressor lymphocytes,32 a fall in the activity of natural killer cells,33 and a fall in the proliferative response to non-specific mytogens. There is also an increase in neutrophils, in IL-6,32 IL-1,34 TNF-α,35 C-reactive protein34 and the activation of inflammatory cascade nuclear signalling factors.36 On the other hand, the levels of these factors have been correlated with the severity of depression37,38 and its response to treatment.
Alterations in oxidative stress have also been described.39 There is a double interaction between this and inflammation: oxidative molecules activate inflammatory mediators, while activation of the microglia produces oxidative stress metabolites. Under normal conditions the microglia controls the start and finish of the neuroinflammatory process, leading to its self-limitation. However, under exposure to stress hyperactivation of the microglia may occur, leading to an excess of inflammation that may cause neurotoxicity.40 Cognitive symptoms are considered to be a core dimension of major depression, and it may even persist after the remission of affective symptoms. It has been suggested that the cognitive symptoms of depression may arise due to the complex interaction of neuroinflammatory and neurohormonal factors associated with the HPA axis.15,41,42
Given the above considerations, the neuroinflammatory hypotheses for depression,43 together with the alterations in the neurohormonal and metabolic responses describes in these patients, supports the involvement of an alteration in the physiological mechanisms which respond to stress and several biological hazards in the aetiopathogenesis of depressive disorders. A subtype depressive disorder has even been described that is associated with cytokines, denominated inflammatory cytokine-associated depression (ICAD).44
Inflammation in schizophreniaA pro-inflammatory state has been proven to exist with an increase in the levels of the said cytokines in patients with schizophrenia when compared to healthy controls.45 Although the levels of inflammatory factors are relatively low in comparison with other inflammatory diseases, this state of low grade activation of inflammation has been implicated in a poorer prognosis for schizophrenia in connection with positive psychotic symptoms46 and negative ones,46,47 together with cognitive involvement48 and loss of brain volume.49 The association between inflammatory factors and poorer cognitive performance in the first psychotic episodes50 underlines the importance of inflammation in the worse prognosis of psychotic disorders in the early stages of the disease. High levels of pro-inflammatory cytokines45 have also been described in psychotic relapses, with a reduction in the levels of different pro-inflammatory cytokines45 after antipsychotic treatment and improved symptoms, as well as increases in some cytokines such as IL-6, even before the development of a psychosis in high-risk populations.51,52
As is the case in other disorders, it has been suggested that the harmful effects of inflammation in schizophrenia are caused by the participation of oxidative stress. Studies performed in recent years have shown abnormal oxidative stress metabolite levels in peripheral tissue53,54 as well as in nerve tissue.55,56 Synergies exist between inflammation, excitotoxicity mechanisms, mitochondrial dysfunction and abnormal protein aggregation to induce neurodegeneration. The activation or increase in the density of the microglia may involve the synthesis of prostaglandins, cytokines and reactive types of oxygen, causing cellular death.57 The role of the HPA axis in maintaining the secretion of cortisol has to be added to these effects, as this may contribute to brain neurotoxicity. In fact, an association has been described between high levels of cortisol and the reduction in the volume of certain regions of the brain such as the hippocampus.58 The latter region is highly important in cognitive processes and especially in the working memory, which is clearly affected in schizophrenia patients. This reduction has been linked to poorer social functioning59 and longer duration of the disease.60
One question in studies of patients with schizophrenia and the first psychotic episodes is whether inflammatory factors and oxidative stress markers may be considered to be state or characteristic markers. In terms of inflammation it has been suggested that some cytokines behave like state markers (IL-1β, IL-6 or TGF-β), given that they increase during acute imbalances and normalise with antipsychotic treatment.45 Other cytokines, on the other hand, may be considered to be characteristic markers (IL-12, IFN-γ, TNF-α, and sIL-2R), given that they increase in acute episodes and that this increase persists after starting antipsychotic treatment.45 Something similar occurs with oxidative stress markers, as some are considered to be state markers (total antioxidant state, catalase activity in red blood cells and plasmatic nitrite) while others are state markers (superoxide dismutase activity in red blood cells).54
Although more studies have been performed to date in schizophrenia patients than is the case for affective disorders, such as major depression or bipolar disorder, the scientific evidence suggests that the role of inflammation also plays a role in the pathogenesis of these diseases. For example, in a recent meta-analysis of studies that have analysed different cytokines in cerebrospinal fluid,61 the size of the effect for the increase of several cytokines (IL-1β, IL-6, and IL-8) was similar between diagnoses, and this was detected in patients with depression as well as those with schizophrenia. These three cytokines are modulated through the nuclear-kappa B (NF-kB signalling factor, which is commonly activated in inflammatory and autoimmune processes. This suggests that there may be common pathological routes in different primary psychiatric disorders.
Inflammation in other neuropsychiatric disordersBipolar disorder has been associated with a clinical profile of a pro-inflammatory state, including greater severity of mania,62 depressive63 and cognitive64,65 symptoms, a history of attempted suicide and longer duration of the disease.66 Studies of cohorts undertaken in populations of children have shown that some cytokines such as IL-667 are predictors of hypomania at an adult age. Other studies have compared levels of inflammatory factors in patients with bipolar disorder and unipolar depression. They detected higher levels in the former, which suggests the existence of greater inflammatory dysregulation in this disorder.68
In posttraumatic stress disorder (PTSD) the existence of a pro-inflammatory state has been described that has been replicated in different cohorts of war veterans.69 A meta-analysis of 20 studies which examined the relationship between inflammatory factors and the diagnosis of PTSD in comparison with healthy controls showed an increase in the levels of IL-6, IL-1β and IFN-γ in PTSD.70 Levels of IL-1β were associated with the duration of the disorder, and those of IL-6 were associated with the severity of symptoms.
High levels of cytokines have also been described in other anxiety disorders. These include generalised anxiety disorder, panic disorder, phobias or OCD, suggesting that findings are not specific to individual disorders.71,72 It is therefore proposed that activation of the response to stress induce the secretion of cytokines at peripheral and central levels, as well as the existence of an increased sympathetic tone and lower parasympathetic activity. This would contribute to increasing the degree of inflammation even further, leading to negative effect in critical regions of the brain for the regulation of fear and anxiety, such as the prefrontal cortex, the insular cortex, the amygdala and the hippocampus.
Increased levels of pro-inflammatory cytokines and oxidative stress markers have also been described in patients with eating behavioural disorders.73 A meta-analysis of anorexia nerviosa74 studies suggests that there is an increase in levels of TNF-α, IL-6, IL-1β, and TNF-R-II together with a fall in the levels of C-reactive protein and IL-6R in comparison with healthy controls.
Diagnostic and therapeutic implicationsIn an attempt to improve diagnostic power, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (DSM-V) initially considered including biomarkers together with clinical diagnostic criteria. However, this option was finally rejected and the DSM-V, like previous editions, is based solely on clinical criteria. Some authors75 stated that the initial expectations respecting the practical utility of biomarkers in psychiatry were hardly realistic, due to their low capacity to discriminate between defining signs and symptoms. The majority of laboratory tests are probabilistic disease markers rather than pathognomic ones. Therefore, although it is probable that in the future the use of biomarkers makes it possible to define subgroups or stages within diagnostic categories,76 no inflammatory parameter currently has sufficient sensitivity and specificity to be diagnostically useful in psychiatry.
Moreover, the control of distortions in research into inflammatory markers must be exhaustive and must consider numerous variables which may affect the characterisation of the immune function beyond study variables. These variables include the variability of laboratory techniques, the circadian rhythm of inflammatory parameters, the influence of modulating factors or physiological regulators of the marker studied, sleep quality, sex, age, weight, substance consumption and exposure to pharmacological agents, among others.
Another subject under discussion is the suggestion that anti-inflammatory drugs be used to treat mental disorders. Several meta-analyses analyse the benefit of using several anti-inflammatory drugs in unipolar depression,77 bipolar disorder64 and psychosis78 with favourable results. Nevertheless, there are important methodological distortions, the studies are highly heterogeneous with small samples, so that taken as a whole this hinders attributing benefits specifically to anti-inflammatory properties.
Nowadays prudence is still required when recommending the use of anti-inflammatory treatment for severe mental disorders, as the therapeutic implications are still a field that is open to research.
ConclusionsThe immune system is a fundamental part of the defence of an organism and it participates in maintaining homeostasis. Interaction between the endocrinological system and the autonomous nervous system would partially explain the reciprocal impact of the immune system in psychological functions and behaviour, as well as the impact of psychological stress on immune response.
Several alterations of the immune system have been linked to the presence of mental disorders, most especially dysregulation of the inflammatory response of the organism with the predominance of a pro-inflammatory state. Exposure to chronically stressful situations may lead to maladaptative responses by different hormonal, inflammatory and cardiovascular mediators which would be involved in the pathogenesis of different metabolic and neuropsychiatric disorders. On the other hand, there is a strong association between autoimmune diseases and psychiatric symptoms, including obsessive–compulsive, depressive or psychotic symptoms.
In the coming years the development of this body of knowledge may lead to important advances in the identification of risk populations, aetiopathological mechanisms and the diagnosis and treatment of certain psychiatric disorders. Nevertheless, to date no agreement has been reached on the use of immune system biomarkers that could make it possible to use them in diagnosis or to recommend immunomodulator treatments for primary mental disorders in everyday clinical practice.
Conflict of interestsThe authors have no conflict of interests to declare.
This work was supported by the Ministry of Economy, Industry and Competitiveness through the Carlos IIIHealth Institute (PI15/00662 and PI15/01386), the European Regional Development Fund (FEDER) “A way of making Europe” and the Mental Health Centre for Networked Biomedical Research (CIBERSAM).
Please cite this article as: Soria V, Uribe J, Salvat-Pujol N, Palao D, Menchón JM, Labad J. Psiconeuroinmunología de los trastornos mentales. Rev Psiquiatr Salud Ment (Barc). 2018;11:115–124.






![Involvement of the hypothalamus–pituitary–adrenal axis (HPA) and the immune system in the neuroinflammatory response. The HPA axis is activated in response to exposure to stressful physical and psychological stimuli by means of the secretion of the corticotrophin-releasing hormone (CRH) by the hypothalamus. This hormone, in turn, stimulates the synthesis of corticotrophin (ACTH) in the hypophysis, which stimulates the secretion of cortisol by the adrenal glands. Regulation of the secretion of cortisol is subject to a negative feedback mechanism by means of which cortisol itself inhibits the synthesis of its precursors (CRH and ACTH). Hypothalamus and hypophysis glucocorticoid receptors take part in this inhibition, as do glucocorticoid and mineralocorticoid receptors present in the hippocampus. Regarding the relationship between the HPA axis and the peripheral inflammatory response, although cortisol inhibits this by exerting an immunosupressor effect, there is inflammatory stimulation by other hormones in the HPA axis such as the CRH. This relationship is bidirectional, as the activation of the peripheral inflammatory response may stimulate the HPA axis. The products of this peripheral stimulation, in which macrophages and lymphocytes participate, may cross the blood–brain barrier (BBB) and trigger a neuroinflammatory reaction by stimulating the microglia in M1 activated forms. This activation of the microglia will generate an inflammatory cascade by releasing cytokines and reactive forms of nitrogen and oxygen, inducing the activation of the astroglia, which in turn amplifies the inflammatory signals within the central nervous system. Three is also excessive release of glutamate by the astrocytes as well as oxidative stress mediators by the activated microglia (associated with the induction of the indolamine 2,3 dioxygenase [IDO] enzyme). These mechanisms negatively affect the production of neurotrophic factors such as the brain derived neurotrophic factor (BDNF), and neurogenesis. Involvement of the hypothalamus–pituitary–adrenal axis (HPA) and the immune system in the neuroinflammatory response. The HPA axis is activated in response to exposure to stressful physical and psychological stimuli by means of the secretion of the corticotrophin-releasing hormone (CRH) by the hypothalamus. This hormone, in turn, stimulates the synthesis of corticotrophin (ACTH) in the hypophysis, which stimulates the secretion of cortisol by the adrenal glands. Regulation of the secretion of cortisol is subject to a negative feedback mechanism by means of which cortisol itself inhibits the synthesis of its precursors (CRH and ACTH). Hypothalamus and hypophysis glucocorticoid receptors take part in this inhibition, as do glucocorticoid and mineralocorticoid receptors present in the hippocampus. Regarding the relationship between the HPA axis and the peripheral inflammatory response, although cortisol inhibits this by exerting an immunosupressor effect, there is inflammatory stimulation by other hormones in the HPA axis such as the CRH. This relationship is bidirectional, as the activation of the peripheral inflammatory response may stimulate the HPA axis. The products of this peripheral stimulation, in which macrophages and lymphocytes participate, may cross the blood–brain barrier (BBB) and trigger a neuroinflammatory reaction by stimulating the microglia in M1 activated forms. This activation of the microglia will generate an inflammatory cascade by releasing cytokines and reactive forms of nitrogen and oxygen, inducing the activation of the astroglia, which in turn amplifies the inflammatory signals within the central nervous system. Three is also excessive release of glutamate by the astrocytes as well as oxidative stress mediators by the activated microglia (associated with the induction of the indolamine 2,3 dioxygenase [IDO] enzyme). These mechanisms negatively affect the production of neurotrophic factors such as the brain derived neurotrophic factor (BDNF), and neurogenesis.](https://static.elsevier.es/multimedia/21735050/0000001100000002/v1_201804270435/S2173505018300177/v1_201804270435/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)