Breast carcinoma is the most common malignancy in women worldwide. It is also one of the most frequent causes of brain metastasis (BM). Studies have identified BM as one of the worst prognostic signs.
MethodsWe retrospectively analyzed data from 71 patients with BM from BC with the aim of clarifying the epidemiological criteria and management in our setting. We also aimed to identify predictors of survival and factors affecting the length of the BM-free interval in our group of patients.
ResultsAll the patients were female with a mean age at diagnosis of primary cancer of 41.6 years. The most common site of BM was the parietal lobe. The BM-free interval was longer with N1 disease (in comparison to N2 and 3) and in luminal B breast cancer subtype. Survival was shorter in older patients, those with hormone receptor negative and/or HER2-neu positive disease, synchronous BM, primary tumour not removed, soft tissue/non-regional nodes concomitant metastasis, and those who did not receive palliative chemotherapy. Survival tended to be longer in patients with temporal lobe metastasis, but this result was not statistically significant.
ConclusionBM is a bad prognostic sign. Large scale prospective studies are needed to further delineate its nature.
El carcinoma de mama es la neoplasia maligna más común en las mujeres del mundo. Además, es una de las causas más comunes de metástasis cerebral (MC). Los estudios detectan MC como uno de los peores signos pronósticos.
MétodosAnalizamos retrospectivamente los datos de 71 pacientes con MC de origen mamario con el objetivo de clarificar los criterios epidemiológicos y el esquema de manejo en nuestra localidad de esta enfermedad, además de detectar predictores de supervivencia y factores que afectan la longitud del intervalo libre de MC en nuestro grupo de pacientes.
ResultadosTodos los pacientes fueron mujeres con una edad media de diagnóstico de cáncer primario de 41,6 años. El sitio más común de MC fue el lóbulo parietal. El intervalo libre de propagación cerebral fue más largo con la enfermedad N1 (en comparación con N2 y 3) y en el subtipo de cáncer de mama luminal B. La supervivencia fue menor en pacientes mayores, aquellos con receptores hormonales negativos y/o enfermedad HER2-neu positiva, MC sincrónica, tumor primario no extirpado, metástasis concomitantes de tejido blando nodos no regionales y aquellos que no recibieron quimioterapia paliativa. Además, aquellos con metástasis del lóbulo temporal tienden a tener una mejor supervivencia, aunque no alcanzaron significación estadística.
ConclusiónLa MC es un mal signo pronóstico. Se necesitan estudios prospectivos a gran escala para delinear aún más su naturaleza.
Brain metastasis (BM) is considered one of the commonest causes of cancer deaths worldwide. It is widely known that its main origin is bronchogenic and breast malignancies.1–3 Approximately, 10–30% of breast cancer cases will develop BM in their disease course.4 Therefore, a lot of research work is directed towards better evaluation of management and prognosis of these cases.5–7
While a lot of solid data are available about early breast cancer cases, there is a paucity of information that is available about the treatment and outcome of BM cases especially the prognostic value of breast cancer molecular sub types.8
Factors that have been reported as being of good prognostic value include good general condition, solitary metastasis in bone or soft tissue, few numbers of metastatic sites and long metastatic-free interval.9–11
The objectives of this study are to analyze the clinic-pathological factors of breast cancer patients with BM and to illustrate the prognostic value of molecular subtypes based on HR and HER2 status.
Patients and methodsThis is a retrospective study, where the institutional registry at oncology centre Mansoura University (OCMU) was thoroughly revised for breast cancer cases with brain metastasis that attended the hospital from July 2005 to March 2018.
The data of these patients were analyzed and statistical values were obtained using SPSS version 22 (Inc., Chicago, IL). Continuous variables are presented as mean when symmetrical or median and range when asymmetrical. Categorical variables are presented as proportions. Bivariate analysis was done using Chi-Square test and Kruskal–Wallis test. Survival analysis was done using Kaplan–Meier curve and significance determined by log rank test.
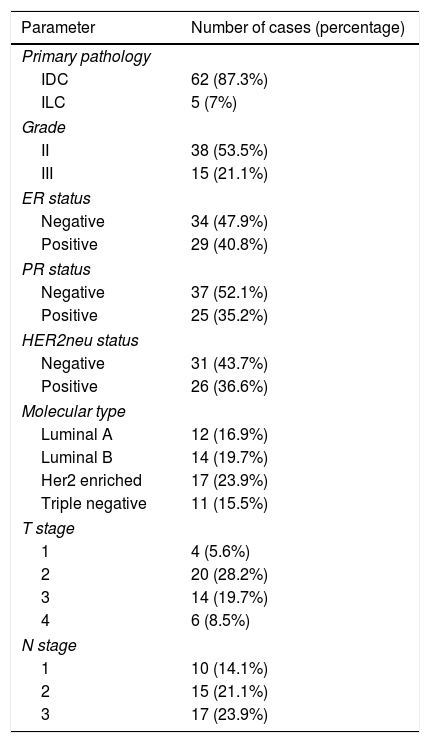
ResultsEpidemiologySeventy one cases were enrolled in the study. Mean age at diagnosis was 41.6 (SD=11.2) years. All were females. Distribution of pathologic T and N stage is displayed in Table 1.
Basic epidemiologic criteria of the patients.
| Parameter | Number of cases (percentage) |
|---|---|
| Primary pathology | |
| IDC | 62 (87.3%) |
| ILC | 5 (7%) |
| Grade | |
| II | 38 (53.5%) |
| III | 15 (21.1%) |
| ER status | |
| Negative | 34 (47.9%) |
| Positive | 29 (40.8%) |
| PR status | |
| Negative | 37 (52.1%) |
| Positive | 25 (35.2%) |
| HER2neu status | |
| Negative | 31 (43.7%) |
| Positive | 26 (36.6%) |
| Molecular type | |
| Luminal A | 12 (16.9%) |
| Luminal B | 14 (19.7%) |
| Her2 enriched | 17 (23.9%) |
| Triple negative | 11 (15.5%) |
| T stage | |
| 1 | 4 (5.6%) |
| 2 | 20 (28.2%) |
| 3 | 14 (19.7%) |
| 4 | 6 (8.5%) |
| N stage | |
| 1 | 10 (14.1%) |
| 2 | 15 (21.1%) |
| 3 | 17 (23.9%) |
Mastectomy was done in 49 (69%) of the patients and CBS in only 4 (5.6%), while 18 (25.3%) of the patients did not undergo surgical treatment for the primary. 62% of the enrolled patients received chemotherapy (neoadjuvant/adjuvant) before diagnosis of BM.
Brain metastasis patternMedian interval between breast cancer initial diagnosis till brain metastasis was21 (ranging from 0 to 135) months. The interval time between appearance of the BM and the diagnosis of the primary breast cancer was significantly correlated to resecting the primary tumour (P-value=.050) (median 16 to 24 months, respectively), PR status (P-value=.026) (median 22.4 to 32.6, for PR-ve versus PR+ve respectively), molecular type (P-value=.039) (Luminal A 38, Luminal B 21.5, Her2-enriched 20 and triple negative 13 months), nodal stage of the primary (P-value=.019), i.e. the higher the nodal stage the shorter interval to BM (median 34, 29, 13 months for N1, N2 and N3, respectively) and also to the adjuvant use of endocrine therapy (P-value=.010), i.e. the patient who did not receive endocrine therapy develop BM earlier (median 20 versus 28 months).
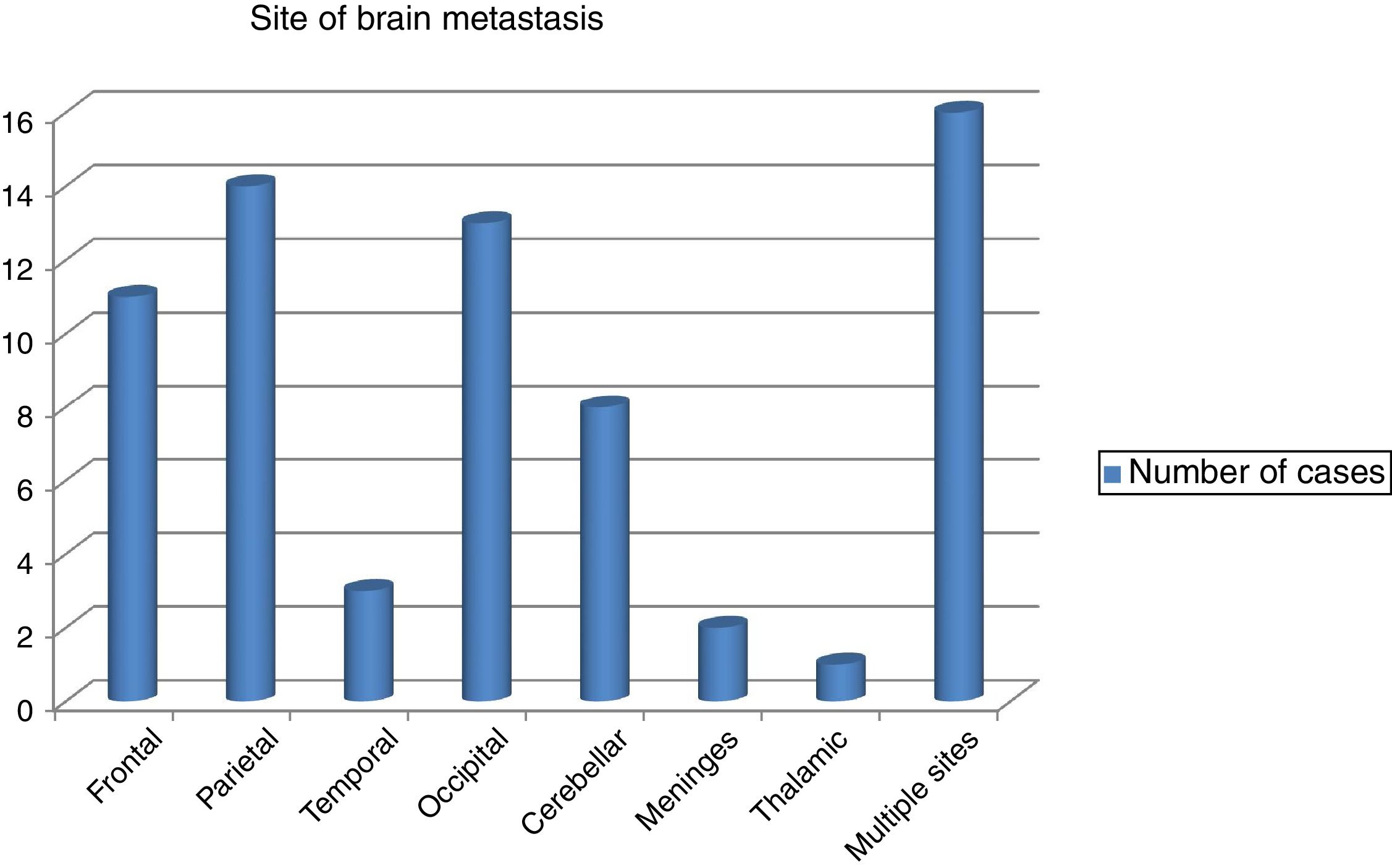
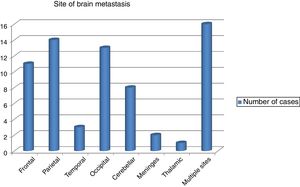
The commonest site of BM was the parietal lobe in 14 cases followed by occipital lobe in 13 and the frontal lobe in 11 patients. However, in 8 patients the cerebellum was affected and in rare instances the meninges and the thalamus were affected in 2 and 1 patients, respectively. Scattered BM was detected in 16 patients (Fig. 1). The brain metastasis was almost always (91.5%) in association with other metastasis. Concomitant distant spread was commonly bony in 34 (47.9%) patients, followed by visceral in23 (32.4%), then other sites in 8 (11.3%) patients. BM was metachronous in 59 (83.1%), while synchronous in 9 (12.7%) of patients.
Brain metastasis management40 (56.3%) of patients received palliative chemotherapy for BM, while 43 (60.6%) received whole brain radiotherapy. On the other side, about 43% received endocrine therapy either in the context of adjuvant therapy for the primary breast cancer or after development of BM. The endocrine therapy lines used was Tamoxifen in 19 (26.8%), while aromatase inhibitors and oophrectomy were used in 9.9 and 7%, respectively.
A great variability of chemotherapeutic treatment of BM were used, including Paclitaxel, Gemcitabine in combination with Platinum, 5FU in combination with Vinorelbine or single agent Capcitabine. In addition, 8 (11.3%) patients of HER2 positive patients received second line HER2 target therapy (Lapitinib) in combination with Capcitabine.
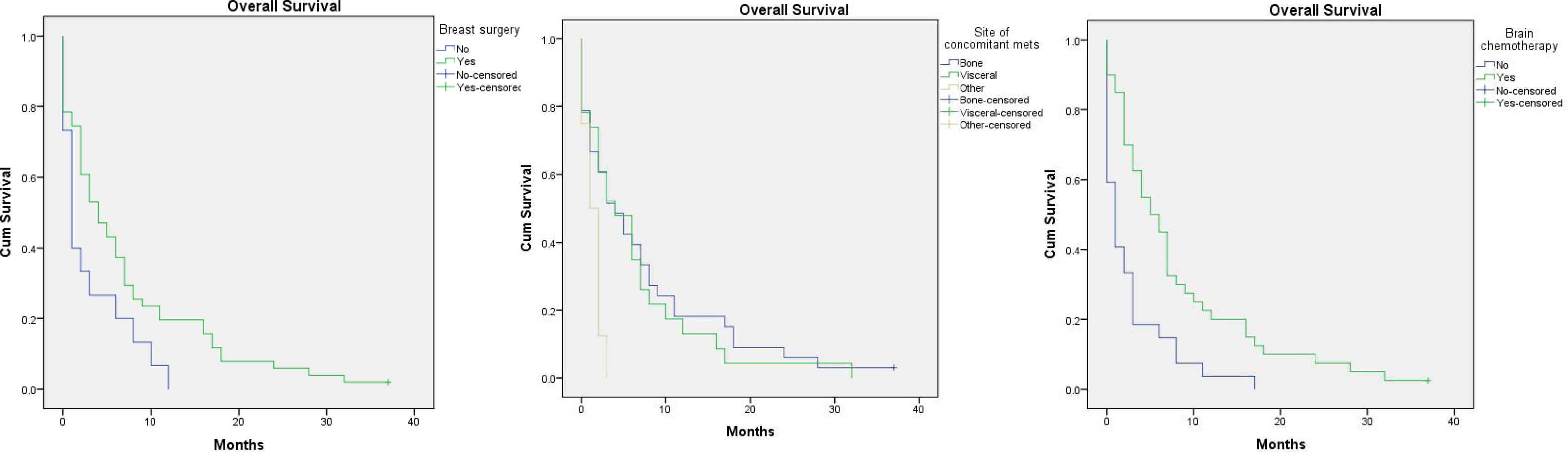
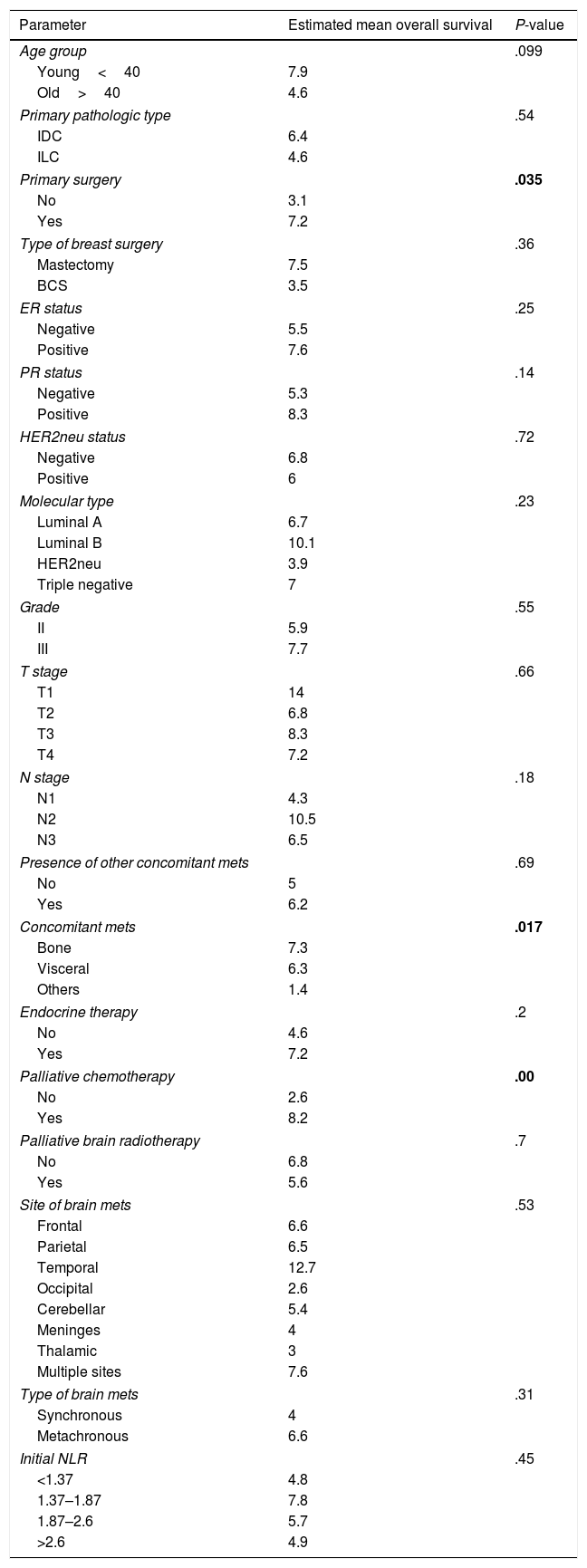
Factors affecting overall survival (OAS) (Table 2)One year survival was 14%, and two year survival was 4%. The overall survival was improved in younger age, those with invasive ductal carcinoma, patients who had removed the primary breast tumours especially if mastectomy was done, those who had ER positive PR positive especially luminal B tumours, and those who received endocrine and/or palliative chemotherapy for their brain metastasis. On the other hand, the OS was shortened in those with synchronous brain metastasis and those with concomitant metastasis to soft tissue or to non-regional lymph nodes.
Statistical analysis of factors predicting overall survival.
| Parameter | Estimated mean overall survival | P-value |
|---|---|---|
| Age group | .099 | |
| Young<40 | 7.9 | |
| Old>40 | 4.6 | |
| Primary pathologic type | .54 | |
| IDC | 6.4 | |
| ILC | 4.6 | |
| Primary surgery | .035 | |
| No | 3.1 | |
| Yes | 7.2 | |
| Type of breast surgery | .36 | |
| Mastectomy | 7.5 | |
| BCS | 3.5 | |
| ER status | .25 | |
| Negative | 5.5 | |
| Positive | 7.6 | |
| PR status | .14 | |
| Negative | 5.3 | |
| Positive | 8.3 | |
| HER2neu status | .72 | |
| Negative | 6.8 | |
| Positive | 6 | |
| Molecular type | .23 | |
| Luminal A | 6.7 | |
| Luminal B | 10.1 | |
| HER2neu | 3.9 | |
| Triple negative | 7 | |
| Grade | .55 | |
| II | 5.9 | |
| III | 7.7 | |
| T stage | .66 | |
| T1 | 14 | |
| T2 | 6.8 | |
| T3 | 8.3 | |
| T4 | 7.2 | |
| N stage | .18 | |
| N1 | 4.3 | |
| N2 | 10.5 | |
| N3 | 6.5 | |
| Presence of other concomitant mets | .69 | |
| No | 5 | |
| Yes | 6.2 | |
| Concomitant mets | .017 | |
| Bone | 7.3 | |
| Visceral | 6.3 | |
| Others | 1.4 | |
| Endocrine therapy | .2 | |
| No | 4.6 | |
| Yes | 7.2 | |
| Palliative chemotherapy | .00 | |
| No | 2.6 | |
| Yes | 8.2 | |
| Palliative brain radiotherapy | .7 | |
| No | 6.8 | |
| Yes | 5.6 | |
| Site of brain mets | .53 | |
| Frontal | 6.6 | |
| Parietal | 6.5 | |
| Temporal | 12.7 | |
| Occipital | 2.6 | |
| Cerebellar | 5.4 | |
| Meninges | 4 | |
| Thalamic | 3 | |
| Multiple sites | 7.6 | |
| Type of brain mets | .31 | |
| Synchronous | 4 | |
| Metachronous | 6.6 | |
| Initial NLR | .45 | |
| <1.37 | 4.8 | |
| 1.37–1.87 | 7.8 | |
| 1.87–2.6 | 5.7 | |
| >2.6 | 4.9 | |
However, the only significant determinants statistically where primary breast tumour resection, soft tissue/non-regional nodes concomitant metastasis and treatment with chemotherapy, P-value=.035, .017 and .00 respectively (Fig. 2).
DiscussionPrognosis of metastatic breast cancer cases and long term outcome of its molecular subtypes are not well studied, although this knowledge is very beneficial in guiding the management.12
In addition, prognosis of breast cancer cases with brain metastasis is considered to be worse than patients free from metastasis,13 and median period of survival after appearance of the metastasis is said to be around 6 to 9 months.6,14–16
The incidence of breast cancer cases with brain metastasis has been increasing in the last years. This may be attributed to the increase in patient cancer survival and advances in brain imaging.17,18
It was illustrated that HER2-positive cases with four or more brain metastasis at the time of diagnosis had a worse outcome regardless of ER positivity.19
Many other articles listed cerebellum as the most common site for BM in breast cancer.20–22 Sunghyon and his team discovered BM spreads widely in different sites of the brain in triple negative breast cancer cases. On the contrary, Luminal types and HER2-positive cases showed BM lesions more in the occipital lobe and cerebellum.23
On the same way, data from the German brain metastasis breast cancer registry showed that BM was most common with HER2 positive cases, while leptomeningeal affection with metastasis was accompanied with triple negative tumours. They also linked site of BM with prognosis where they found cases with leptomeningeal affection had shorter survival compared with patients without signs of leptomeningeal metastasis (median survival 3 vs. 5 months, P=0.025). A shorter survival was also linked to occipital lobe metastasis.24 Referring to our cohort, we noticed that the parietal lobe was the commonest site for metastasis in our series with temporal lobe metastasis having the best overall survival.
Several articles have illustrated factors that are correlated with occurrence of brain metastasis (BM) such as young age, nodal affection, size and grade of the tumour and biological behaviour of the tumour.25,26
Over expression of HER2 had been widely considered as a main factor for the occurrence of BM.27,28 Brufsky et al. reported brain metastasis in 377 (37.3%) of 1012 patients with confirmed HER2-positive.29 Also, patients with triple-negative tumours are at an increased risk of occurrence of BM compared with the other molecular subtypes.30,31
Disease course and prognosis of HER2-positive cases with brain metastasis are not well studied due to the small number of cases. In our analysis, HER2-positive and especially luminal HER2 patients had the most favourable prognosis after BM development, compared to other subtypes.
BM screening is not considered a routine test yet, since its early detection was not found to improve survival of those patients.6
Treatment of these cases is considered a challenge and it varies using either surgery, whole-brain irradiation, radiosurgery, systemic therapy or a combined approach.32 Surgery or radiosurgery is considered an ideal option for patients with one to three metastases. While in case of multiple BM, whole brain radiotherapy (WBRT) usually is the first line of treatment. However, in selected cases, systemic treatment may be offered initially in order to avoid neurocognitive toxicities and as a potential option evaluated in clinical trials.33
In a recent research on 308 patients, more than one third of the patients lived more than one year, of whom only one fifth required whole brain radiotherapy in a late stage. The increased use of radiosurgical techniques has shown a potential impact on survival.34 In another cohort of 50 patients, median survival was around 3 years after receiving radiosurgery for treatment of brain metastasis secondary to breast cancer.35
Regarding the use of target therapy, Naoki and his colleagues showed that HER2 positive patients who were treated using either Trastuzumab or Lapatinib or a combination of both after developing brain metastases had significantly longer survival.19
Moreover, multiple clinical trials are ongoing to use novel agents like; HER2-directed therapies (Ado-trastuzumab emtansine (T-DM1), Neratinib and Tucatinib), poly-ADP ribose polymerase (PARP) inhibitors (Olaparib, Veliparib), cyclin dependent kinase 4/6 (CDK4/6) inhibitors (Palbociclib, Abemaciclib) and taxane derivatives (e.g. ANG1005 and TPI-287).36,37
It was observed that metastatic breast cancer patients with a good general condition, metastasis limited to bone and soft tissue, fewer number of metastatic sites, and long metastatic free interval have a better prognosis.9,38
In our cohort, we observed a better prognosis with younger patients, invasive ductal carcinoma pathology, patients who underwent mastectomy, ER and PR positive patients specially luminal B, and those who received endocrine adjuvant therapy. In contrast to this cohort, the ECOG-ACRIN Research Group trial (E2108) found that early resection of the primary breast tumour did not improve survival in patients with de novo metastatic breast cancer, although they encountered a 2.5-fold higher risk of local disease progression without local therapy for the breast primary.39
The limitations of our study may be due to its retrospective nature that made management of brain metastasis not standardized and also the paucity of radiotherapy machines.
ConclusionDevelopment of brain metastasis in breast cancer patients entails bad prognosis with shorter overall survival. Resection of the primary breast tumour and chemotherapy seems to improve survival. The time lag between diagnosis of breast cancer and the development of brain metastasis are longer in those who had their primary tumour removed, those with PR receptor positive primary, luminal tumours, lower N stage and those who received adjuvant endocrine therapy.
Authors’ contributionsSA conceptualization, data validation and draft approval. IHM did the formal analysis, investigation, methodology and share writing-original draft. MZ & OH revised data and manuscript editing. LAM, ZFA & HME data collection and revision. RE & ZE shared in draft writing and editing. WN supervision and draft approval. KA wrote the original draft.
FundingNo specific funding has been received for this work.
Confidentiality of dataThe authors declare that they have followed the protocols of their Institution regarding the publication of patient data. The study has been approved by Mansoura Faculty of Medicine Institutional Research Board (MFM-IRB) with approval code (R/20.10.1039).
Conflict of interestThe authors declare that they have no conflict of interest.