Complications and readmissions derived from surgical treatment of breast cancer have been less evaluated than recurrence and mortality. The aim of this study was to analyze the results of surgical treatment and prognosis in a screening population with known high surgical variability.
MethodsThis multicenter study included 1086 women diagnosed with breast cancer from the CaMISS cohort study of women aged between 50 and 69years participating in four breast cancer screening programs in Spain between 2000 and 2009 with a follow-up until 2014. Multivariate models were used to estimate the adjusted odds ratio of breast surgery (mastectomy vs conservative treatment) for complications and readmissions and hazard ratios for recurrences and mortality.
ResultsPrimary breast surgical treatment consisted of conservative treatment in 821 women (80.1%) and mastectomy in 204 (19.9%). Mastectomy was associated with readmissions, recurrences and mortality but this association was not statistically significant on multivariate adjusted analysis (ORa=1.51 [95%CI 0.89–2.57], HRa=1.37 [95%CI 0.85–2.19] and HRa=1.52 [95%CI 0.95–2.43] respectively). In our sample, the variables with greatest impact on complications, recurrences and mortality were stages III and IV (ORa=4.4[95%CI 1.22–16.16], HRa=7.96 [95%CI 3.32–19.06] and HRa=3.92[95%CI 1.77–8.67]).
ConclusionComplications, readmissions, recurrence and mortality were similar in both surgical techniques. These results support that surgical treatment for breast cancer can be adapted to professional and health system circumstances, and to the surgical needs and desires of each patient. At a time when screening programs are being questioned the variable with the greatest impact on mortality was stage III and IV.
Las complicaciones y los reingresos derivados del tratamiento quirúrgico del cáncer de mama han sido menos evaluados que la recidiva y la mortalidad. El objetivo de este estudio ha sido analizar los resultados y el pronóstico del cáncer de mama en función del tipo de cirugía recibida en una población con elevada variabilidad quirúrgica.
MétodosEn este estudio multicéntrico se incluyeron 1086 mujeres diagnosticadas de cáncer de mama de la cohorte CaMISS, con mujeres de entre 50 y 69 años participantes en 4 programas de cribado Españoles entre 2000 y 2009, con seguimiento hasta 2014. Se utilizó la regresión logística multivariada para estimar la odds ratio de complicaciones y reingresos. También modelos Cox para estimar hazard ratios de recidivas y mortalidad.
ResultadosSe realizó cirugía conservadora en 821 mujeres (80,1%) y mastectomía en 204 (19,9%). La mastectomía se asoció con reingresos, recidivas y mortalidad, pero esta asociación no fue estadísticamente significativa en el análisis multivariado ajustado (ORa=1,51[IC95% 0,89-2,57], HRa=1,37[IC95% 0,85-2,19] y HRa=1,52[IC95% 0,95-2,43] respectivamente). La variable con mayor impacto sobre complicaciones, recidivas y mortalidad fue el estadio III/IV (ORa=4,4[IC 95%: 1,22-16,16], HRa=7,96[IC 95%: 3,32-19,06] y HRa=3,92[IC 95%: 1,77-8,67]).
ConclusiónLas complicaciones, reingresos, recidiva y mortalidad fueron estadísticamente equivalentes en ambas técnicas quirúrgicas. El tratamiento quirúrgico del cáncer de mama puede adaptarse a las circunstancias profesionales, del sistema sanitario además de necesidades y deseos quirúrgicos de cada paciente. En un momento en que se cuestionan los programas de cribado, la variable con mayor impacto en mortalidad fue el estadio.
Survival in breast cancer patients has greatly improved due to all advances in multidisciplinary treatments1 and the early detection through screening2. However, breast cancer is still a potentially serious disease and is the most frequent form of cancer in women, with 1,671,149 new cases estimated per year worldwide, with an annual mortality of 521,907 patients3 Surgery plays a fundamental role in the cure of breast cancer and both mastectomy and breast conservative surgery are accepted as validated techniques. After the initial randomized trials for breast conservation surgery were published, several studies have compared mastectomy with conservative surgery for the treatment of breast cancer. In some of them, no difference in mortality has been observed, although in others, the results on mortality and recurrence are discordant in some aspects. Furthermore, there are fewer studies on the impact of surgical variability on complications and readmissions.
When the evidence on the effectiveness and safety of a given treatment is high, low variability in medical practice is expected.4 However, variability in surgical practice may be a problem to face today.5 A high surgical variability (mastectomy vs conservative surgery) was observed in women with the same characteristics and type of tumor in different hospitals from the CaMISS cohort study performed in Spain among women participating in breast cancer screening program,6 in agreement with other studies with moderate-to-high variability in the performance of surgical treatment of breast cancer.4
On this basis, with patients of the same age and tumor characteristics who received different surgical treatments, the aim of this study has been to analyze complications, readmissions, recurrence and mortality according to the surgical treatment received in women participating in a homogenous cohort from screening breast cancer program population in our environment.
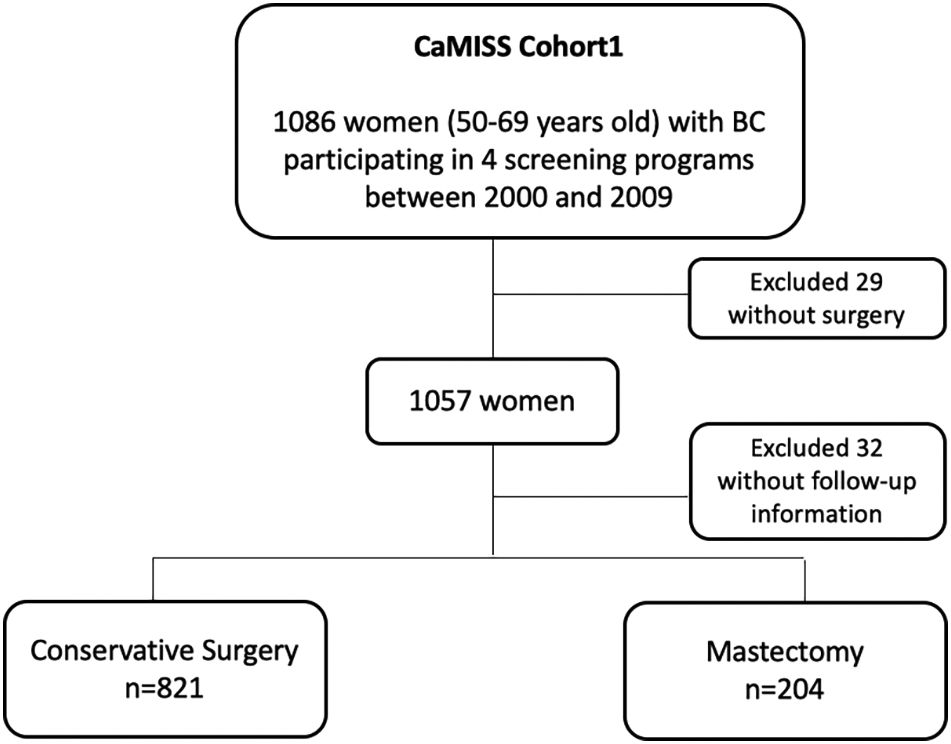
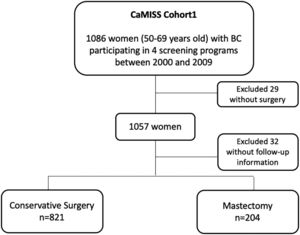
MethodsStudy populationThis study included 1086 women diagnosed with breast cancer included in the CaMISS cohort. All women were aged between 50 and 69years participating in breast cancer screening programs from Barcelona, Girona, Sabadell and Canary islands between 2000 and 2009 with a follow-up until 2014.7
The total target population included in the final analysis was 1025 patients (94.4%) (Fig. 1).
DiagnosisBreast cancer was detected through screening mammography or emerged as an interval cancer. In Spain, women aged between 50 and 69years are invited to participate in a population-based screening program every 2years to undergo a screening mammogram following the European guidelines for Quality Assurance in Mammographic Screening Recommendations.8
The final diagnosis was obtained by biopsy of the lesion detected through an imaging test and successive histopathological study in all cases. After diagnosis, each woman was treated at the referral hospital of their screening program.
Variables and data sourcesInformation on patient age and detection method (screening or interval) was obtained from the databases of the population and hospital-based screening programs. Information on tumor characteristics (TNM, histology, phenotype by immunohistochemical staining) and treatment was obtained from medical records and from hospital-based cancer registries.
Treatment was decided in a multidisciplinary committee in each hospital. Surgical treatment was classified in two categories: conservative or mastectomy. Almost all patients who undergo conservative surgery receive radiotherapy. Regarding mastectomy, it consists of complete surgical resection of the breast tissue and includes all the non-conservative surgeries.
In axillary surgery, the performance of the sentinel node biopsy (SNB) in N0 or the performance of axillary lymph node dissection (ALND) in N1/N2/N3 was recorded.
Outcome variables consisted of complications after surgical treatment, hospital readmissions, cancer recurrences and mortality. Data were obtained from clinical records cataloged on specific annual time points from hospital-based cancer registries since the date of surgery until the end of follow-up in June 2014.
Complications included were systemic complications, surgery-related (seroma, wound infection), pain9 and psychological events (anxiety and/or depression).
Readmissions were included from the surgical intervention until the end of the follow-up. The causes of readmission included surgical causes, complications of the surgical site requiring admission and complications related to systemic causes.
Cancer recurrence was classified in three sections: local recurrence when there was a reappearance of cancer in the ipsilateral breast, regional recurrence when the tumor involved the ipsilateral regional lymph nodes and metastatic when the recurrence was remote. All-cause mortality was also included.
Data were collected through a protocol approved by the clinical research ethics committee of Parc de Salut Mar (Barcelona), and the rest of the participating institutions.
Statistical analysisA descriptive analysis including all the study variables was performed. Women's and tumor characteristics were compared by surgical treatment (conservative surgery or mastectomy) through the chi-squared test as all the study variables were categorical.
Multivariate logistic regression models were used to estimate crude and adjusted odds ratios (OR) according to complications and readmissions. Cox models were used for cancer recurrence and mortality after surgical treatment to take into account the time between the treatment and these outcomes.
The adjusted analysis included the following variables: age, diagnostic method, screening program, TNM stage, histology, phenotype, surgical treatment and performance of ALND.
Statistical significance was set at p<0.05. Statistical analyses were performed through the SPSS statistical package (version 23.0).
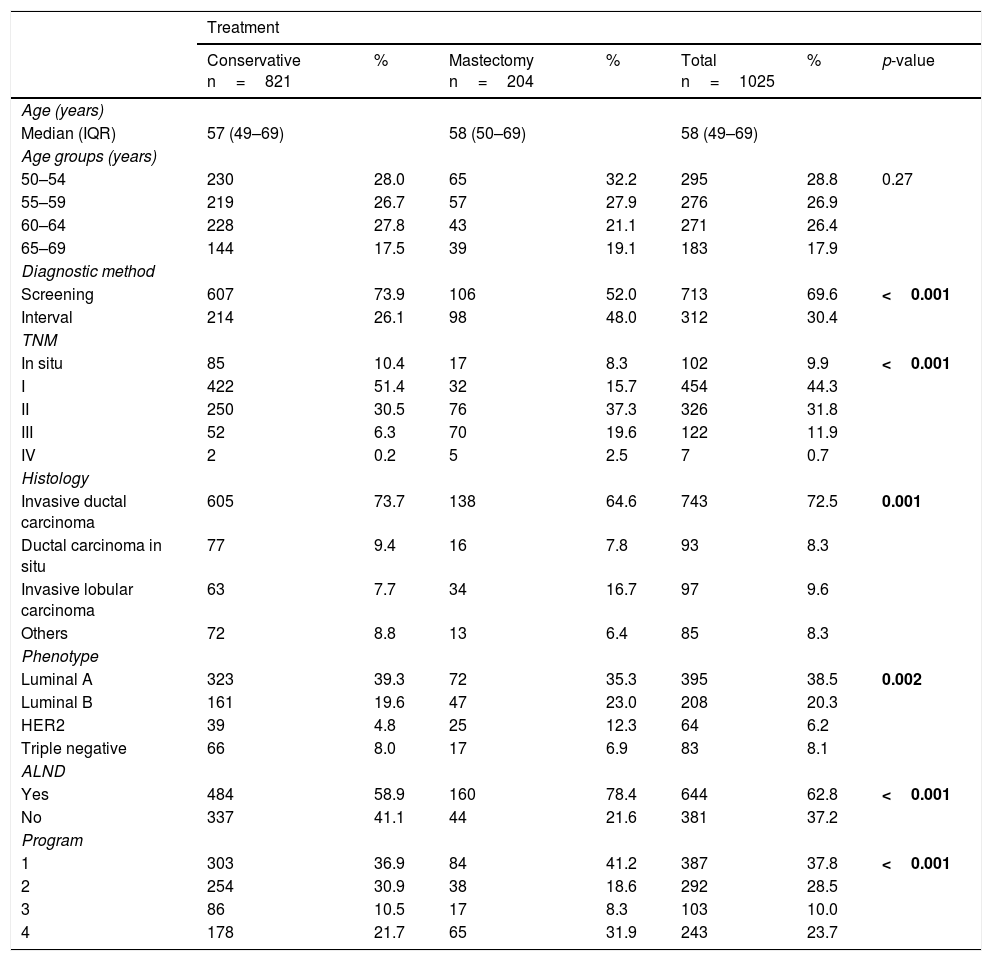
ResultsDescriptive analysis of the CaMISS cohort according to the received surgical treatmentThe results of the descriptive analysis are shown in Table 1.
CAMISS cohort descriptive analysis according to the surgical treatment received.
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Conservative n=821 | % | Mastectomy n=204 | % | Total n=1025 | % | p-value | |
| Age (years) | |||||||
| Median (IQR) | 57 (49–69) | 58 (50–69) | 58 (49–69) | ||||
| Age groups (years) | |||||||
| 50–54 | 230 | 28.0 | 65 | 32.2 | 295 | 28.8 | 0.27 |
| 55–59 | 219 | 26.7 | 57 | 27.9 | 276 | 26.9 | |
| 60–64 | 228 | 27.8 | 43 | 21.1 | 271 | 26.4 | |
| 65–69 | 144 | 17.5 | 39 | 19.1 | 183 | 17.9 | |
| Diagnostic method | |||||||
| Screening | 607 | 73.9 | 106 | 52.0 | 713 | 69.6 | <0.001 |
| Interval | 214 | 26.1 | 98 | 48.0 | 312 | 30.4 | |
| TNM | |||||||
| In situ | 85 | 10.4 | 17 | 8.3 | 102 | 9.9 | <0.001 |
| I | 422 | 51.4 | 32 | 15.7 | 454 | 44.3 | |
| II | 250 | 30.5 | 76 | 37.3 | 326 | 31.8 | |
| III | 52 | 6.3 | 70 | 19.6 | 122 | 11.9 | |
| IV | 2 | 0.2 | 5 | 2.5 | 7 | 0.7 | |
| Histology | |||||||
| Invasive ductal carcinoma | 605 | 73.7 | 138 | 64.6 | 743 | 72.5 | 0.001 |
| Ductal carcinoma in situ | 77 | 9.4 | 16 | 7.8 | 93 | 8.3 | |
| Invasive lobular carcinoma | 63 | 7.7 | 34 | 16.7 | 97 | 9.6 | |
| Others | 72 | 8.8 | 13 | 6.4 | 85 | 8.3 | |
| Phenotype | |||||||
| Luminal A | 323 | 39.3 | 72 | 35.3 | 395 | 38.5 | 0.002 |
| Luminal B | 161 | 19.6 | 47 | 23.0 | 208 | 20.3 | |
| HER2 | 39 | 4.8 | 25 | 12.3 | 64 | 6.2 | |
| Triple negative | 66 | 8.0 | 17 | 6.9 | 83 | 8.1 | |
| ALND | |||||||
| Yes | 484 | 58.9 | 160 | 78.4 | 644 | 62.8 | <0.001 |
| No | 337 | 41.1 | 44 | 21.6 | 381 | 37.2 | |
| Program | |||||||
| 1 | 303 | 36.9 | 84 | 41.2 | 387 | 37.8 | <0.001 |
| 2 | 254 | 30.9 | 38 | 18.6 | 292 | 28.5 | |
| 3 | 86 | 10.5 | 17 | 8.3 | 103 | 10.0 | |
| 4 | 178 | 21.7 | 65 | 31.9 | 243 | 23.7 | |
ALND: Axillary lymph node dissection.
Primary breast surgical treatment included conservative treatment in 821 women (80.1%) and mastectomy in 204 (19.9%). Regarding the diagnostic method, breast cancer was detected through screening mammograms in 713 women (69.6%). While in the group of mastectomized patients the percentage of diagnosis by screening or as an interval neoplasia is around 50% in both methods, within the group of patients treated by conservative surgery, it is much more frequent that they have been diagnosed by screening than as interval neoplasia (73.9 and 26.1%, respectively) (p<0.001).
Regarding the tumor characteristics, mastectomy was more frequent in women with advanced stage tumors than conservative surgery (stage III: 19.6% vs 6.3%, p<0.001). The percentages of B luminal and HER2 were higher in the mastectomy group than in the conservative surgery group (luminal B: 23% vs 19.6%, HER2: 12.3% vs 4.8%, p=0.002).
ALND was more frequent in the mastectomy group than in the conservative surgery group (78.4% vs 58.9%, p<0.001). There were differences in the surgical treatment received according to the screening program (p<0.001).6
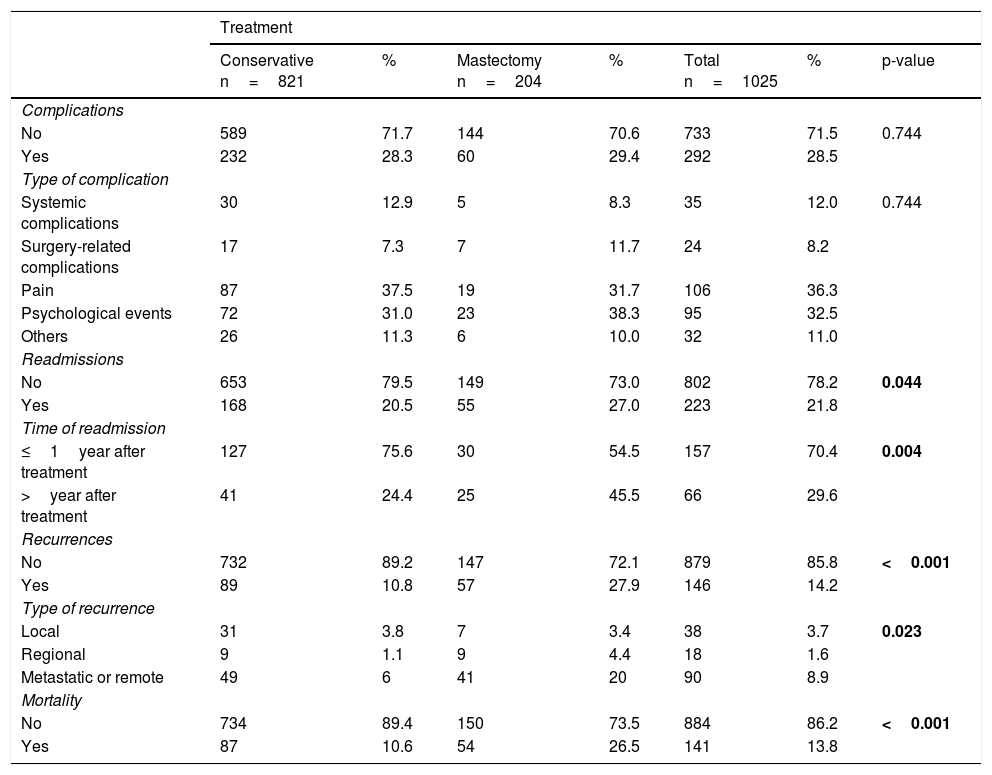
Descriptive analysis of complications, readmissions, recurrence and mortalityComplications, readmissions, recurrences and mortality outcomes by surgical treatment are shown in Table 2.
Descriptive analysis of complications, readmissions, recurrence and mortality.
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Conservative n=821 | % | Mastectomy n=204 | % | Total n=1025 | % | p-value | |
| Complications | |||||||
| No | 589 | 71.7 | 144 | 70.6 | 733 | 71.5 | 0.744 |
| Yes | 232 | 28.3 | 60 | 29.4 | 292 | 28.5 | |
| Type of complication | |||||||
| Systemic complications | 30 | 12.9 | 5 | 8.3 | 35 | 12.0 | 0.744 |
| Surgery-related complications | 17 | 7.3 | 7 | 11.7 | 24 | 8.2 | |
| Pain | 87 | 37.5 | 19 | 31.7 | 106 | 36.3 | |
| Psychological events | 72 | 31.0 | 23 | 38.3 | 95 | 32.5 | |
| Others | 26 | 11.3 | 6 | 10.0 | 32 | 11.0 | |
| Readmissions | |||||||
| No | 653 | 79.5 | 149 | 73.0 | 802 | 78.2 | 0.044 |
| Yes | 168 | 20.5 | 55 | 27.0 | 223 | 21.8 | |
| Time of readmission | |||||||
| ≤1year after treatment | 127 | 75.6 | 30 | 54.5 | 157 | 70.4 | 0.004 |
| >year after treatment | 41 | 24.4 | 25 | 45.5 | 66 | 29.6 | |
| Recurrences | |||||||
| No | 732 | 89.2 | 147 | 72.1 | 879 | 85.8 | <0.001 |
| Yes | 89 | 10.8 | 57 | 27.9 | 146 | 14.2 | |
| Type of recurrence | |||||||
| Local | 31 | 3.8 | 7 | 3.4 | 38 | 3.7 | 0.023 |
| Regional | 9 | 1.1 | 9 | 4.4 | 18 | 1.6 | |
| Metastatic or remote | 49 | 6 | 41 | 20 | 90 | 8.9 | |
| Mortality | |||||||
| No | 734 | 89.4 | 150 | 73.5 | 884 | 86.2 | <0.001 |
| Yes | 87 | 10.6 | 54 | 26.5 | 141 | 13.8 | |
A total of 292 women (28.5%) experienced at least one complication with no differences according to surgical treatment (p-value 0.744).
It has been registered that 223 women (21.8%) were readmitted after surgical treatment until the end of the follow-up. Readmissions were slightly more frequent in the mastectomy group than in the conservative treatment group (27% and 20.5%, respectively, p=0.04). They predominated during the first year after surgery in patients undergoing conservative surgery (75.6%) but were more evenly distributed during follow-up in patients undergoing mastectomy (54.4% during the first year) (p=0.004).
Regarding cancer recurrence, it affected 146 women (14.2%) and was more frequent in the mastectomy group than in the conservative surgery group (27.9% vs. 10.8%, p<0.001). The most frequent type of recurrence was the local one in conservative treatment (3.8%) and the metastatic recurrence in the mastectomy group (20%).
The mortality rate for this cohort with a follow-up period of 13years was 13.8% (n=141), and was higher in the mastectomy group than in the conservative surgery group (26.5% vs. 10.6%, p<0.001).
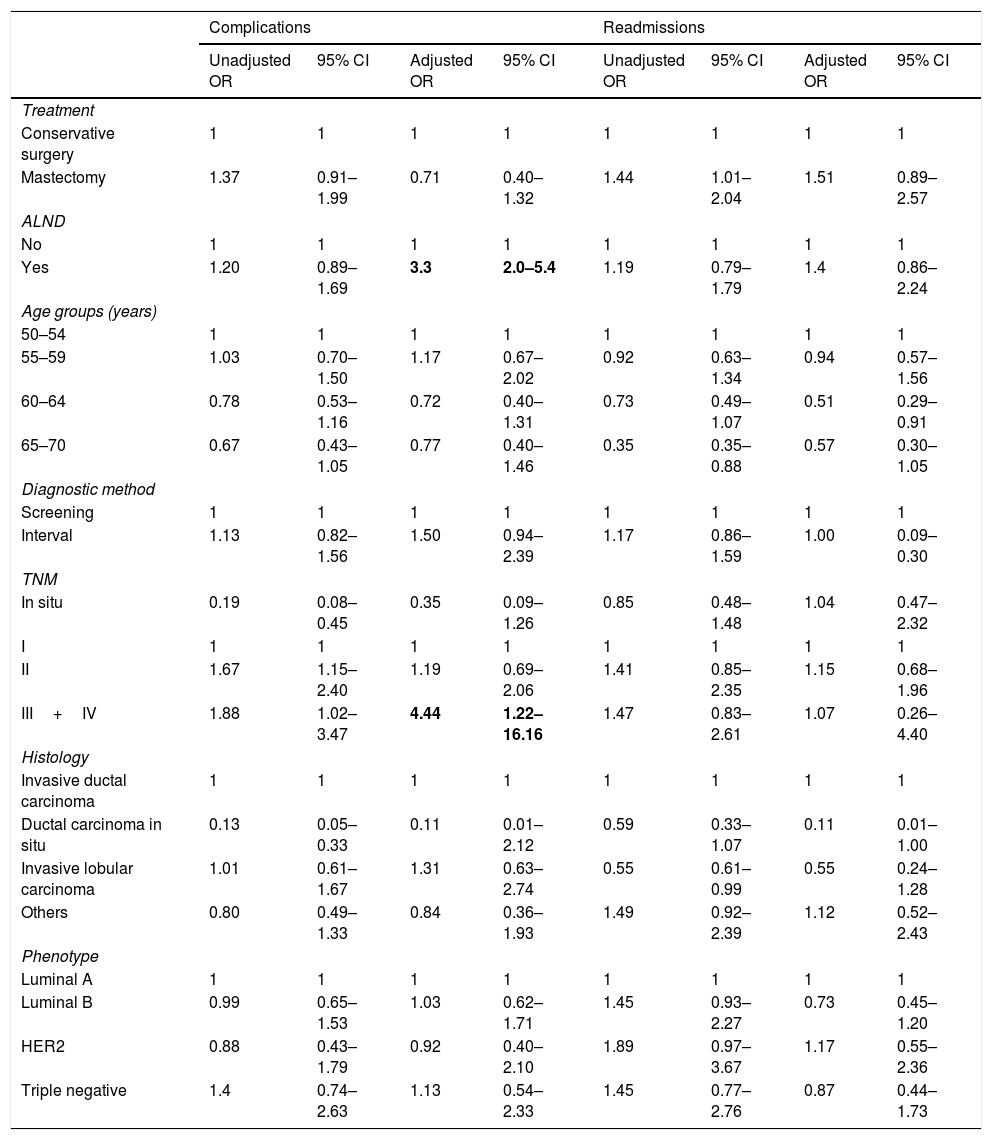
Univariate and multivariate adjusted analysis of complications, readmissions, recurrence and mortalitySurgical treatment was not associated with complications (ORa=0.71 [95%CI 0.40–1.32]) nor with readmissions in the adjusted analysis (ORa=1.51 [95%CI 0.89–2.57]) (Table 3). The presence of ALND and stage III and IV tumors were associated with the presence of complications (OR a (ALND)=3.3 [95%CI 2.0–5.4] and OR a (TNM)=4.4 [95%CI 1.22–16.16]) but not with readmissions.
Unadjusted and adjusted OR of complications and readmissions after surgical treatment (logistic regression).
| Complications | Readmissions | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | Adjusted OR | 95% CI | Unadjusted OR | 95% CI | Adjusted OR | 95% CI | |
| Treatment | ||||||||
| Conservative surgery | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mastectomy | 1.37 | 0.91–1.99 | 0.71 | 0.40–1.32 | 1.44 | 1.01–2.04 | 1.51 | 0.89–2.57 |
| ALND | ||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.20 | 0.89–1.69 | 3.3 | 2.0–5.4 | 1.19 | 0.79–1.79 | 1.4 | 0.86–2.24 |
| Age groups (years) | ||||||||
| 50–54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 55–59 | 1.03 | 0.70–1.50 | 1.17 | 0.67–2.02 | 0.92 | 0.63–1.34 | 0.94 | 0.57–1.56 |
| 60–64 | 0.78 | 0.53–1.16 | 0.72 | 0.40–1.31 | 0.73 | 0.49–1.07 | 0.51 | 0.29–0.91 |
| 65–70 | 0.67 | 0.43–1.05 | 0.77 | 0.40–1.46 | 0.35 | 0.35–0.88 | 0.57 | 0.30–1.05 |
| Diagnostic method | ||||||||
| Screening | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Interval | 1.13 | 0.82–1.56 | 1.50 | 0.94–2.39 | 1.17 | 0.86–1.59 | 1.00 | 0.09–0.30 |
| TNM | ||||||||
| In situ | 0.19 | 0.08–0.45 | 0.35 | 0.09–1.26 | 0.85 | 0.48–1.48 | 1.04 | 0.47–2.32 |
| I | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| II | 1.67 | 1.15–2.40 | 1.19 | 0.69–2.06 | 1.41 | 0.85–2.35 | 1.15 | 0.68–1.96 |
| III+IV | 1.88 | 1.02–3.47 | 4.44 | 1.22–16.16 | 1.47 | 0.83–2.61 | 1.07 | 0.26–4.40 |
| Histology | ||||||||
| Invasive ductal carcinoma | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ductal carcinoma in situ | 0.13 | 0.05–0.33 | 0.11 | 0.01–2.12 | 0.59 | 0.33–1.07 | 0.11 | 0.01–1.00 |
| Invasive lobular carcinoma | 1.01 | 0.61–1.67 | 1.31 | 0.63–2.74 | 0.55 | 0.61–0.99 | 0.55 | 0.24–1.28 |
| Others | 0.80 | 0.49–1.33 | 0.84 | 0.36–1.93 | 1.49 | 0.92–2.39 | 1.12 | 0.52–2.43 |
| Phenotype | ||||||||
| Luminal A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Luminal B | 0.99 | 0.65–1.53 | 1.03 | 0.62–1.71 | 1.45 | 0.93–2.27 | 0.73 | 0.45–1.20 |
| HER2 | 0.88 | 0.43–1.79 | 0.92 | 0.40–2.10 | 1.89 | 0.97–3.67 | 1.17 | 0.55–2.36 |
| Triple negative | 1.4 | 0.74–2.63 | 1.13 | 0.54–2.33 | 1.45 | 0.77–2.76 | 0.87 | 0.44–1.73 |
Adjusted by: treatment, ALND, age, TNM, phenotype, histology, diagnostic method and program. ALND: Axillary lymph node dissection.
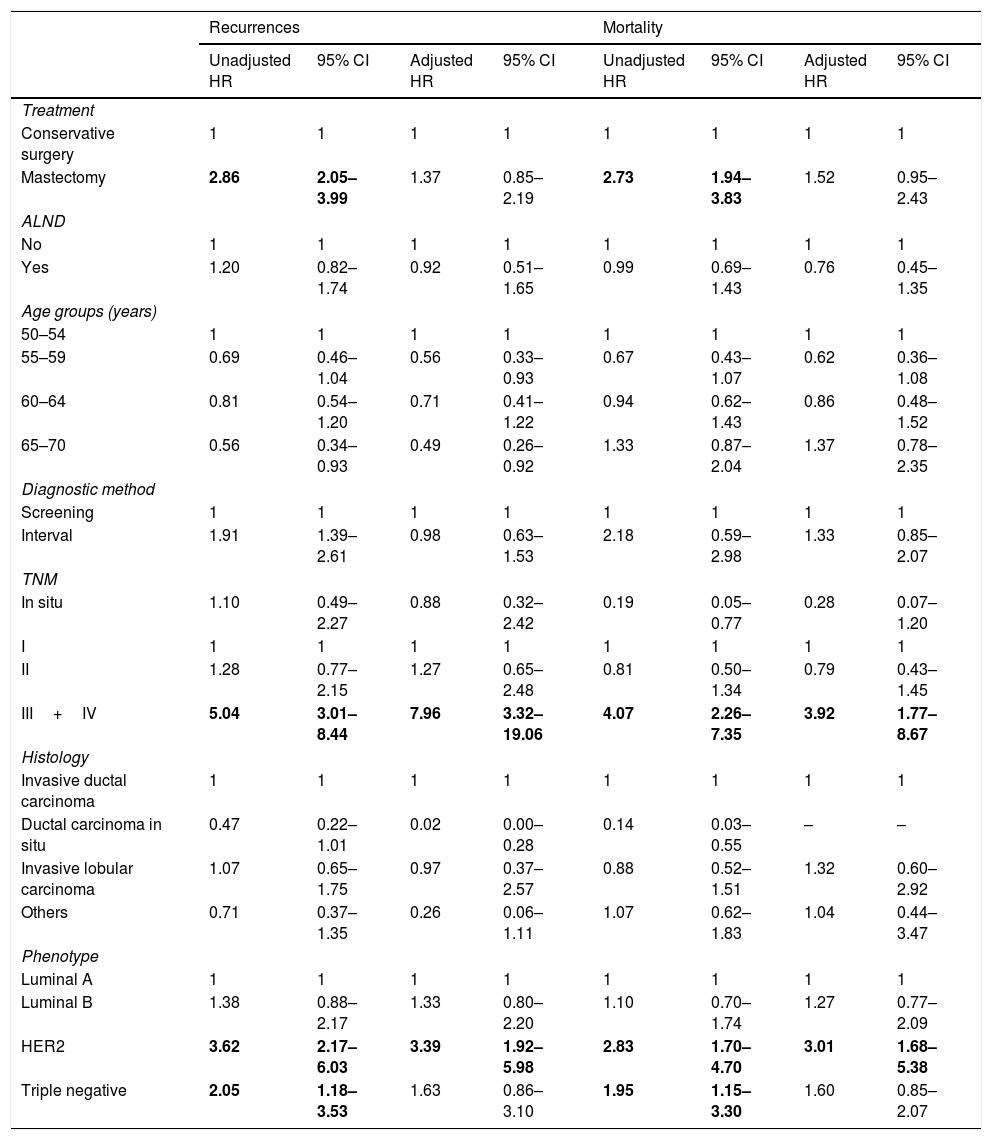
The HR analysis (Table 4) showed that mastectomy was associated with recurrences and mortality in the unadjusted analysis but the association did not remain significant after adjustment (HRa=1.37 [95%CI 0.85–2.19] and HRa=1.52 [95%CI 0.95–2.43]) respectively). Among the other variables, only stage III/IV and HER2 phenotype had a statistically significant association with recurrences and mortality in the adjusted analysis, with the highest HRa found for stages III and IV and the risk of recurrences (HRa=7.96 [95%CI 3.32–19.06]).
Unadjusted and adjusted HR of recurrences and mortality (COX).
| Recurrences | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | Adjusted HR | 95% CI | Unadjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Treatment | ||||||||
| Conservative surgery | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mastectomy | 2.86 | 2.05–3.99 | 1.37 | 0.85–2.19 | 2.73 | 1.94–3.83 | 1.52 | 0.95–2.43 |
| ALND | ||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.20 | 0.82–1.74 | 0.92 | 0.51–1.65 | 0.99 | 0.69–1.43 | 0.76 | 0.45–1.35 |
| Age groups (years) | ||||||||
| 50–54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 55–59 | 0.69 | 0.46–1.04 | 0.56 | 0.33–0.93 | 0.67 | 0.43–1.07 | 0.62 | 0.36–1.08 |
| 60–64 | 0.81 | 0.54–1.20 | 0.71 | 0.41–1.22 | 0.94 | 0.62–1.43 | 0.86 | 0.48–1.52 |
| 65–70 | 0.56 | 0.34–0.93 | 0.49 | 0.26–0.92 | 1.33 | 0.87–2.04 | 1.37 | 0.78–2.35 |
| Diagnostic method | ||||||||
| Screening | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Interval | 1.91 | 1.39–2.61 | 0.98 | 0.63–1.53 | 2.18 | 0.59–2.98 | 1.33 | 0.85–2.07 |
| TNM | ||||||||
| In situ | 1.10 | 0.49–2.27 | 0.88 | 0.32–2.42 | 0.19 | 0.05–0.77 | 0.28 | 0.07–1.20 |
| I | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| II | 1.28 | 0.77–2.15 | 1.27 | 0.65–2.48 | 0.81 | 0.50–1.34 | 0.79 | 0.43–1.45 |
| III+IV | 5.04 | 3.01–8.44 | 7.96 | 3.32–19.06 | 4.07 | 2.26–7.35 | 3.92 | 1.77–8.67 |
| Histology | ||||||||
| Invasive ductal carcinoma | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ductal carcinoma in situ | 0.47 | 0.22–1.01 | 0.02 | 0.00–0.28 | 0.14 | 0.03–0.55 | – | – |
| Invasive lobular carcinoma | 1.07 | 0.65–1.75 | 0.97 | 0.37–2.57 | 0.88 | 0.52–1.51 | 1.32 | 0.60–2.92 |
| Others | 0.71 | 0.37–1.35 | 0.26 | 0.06–1.11 | 1.07 | 0.62–1.83 | 1.04 | 0.44–3.47 |
| Phenotype | ||||||||
| Luminal A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Luminal B | 1.38 | 0.88–2.17 | 1.33 | 0.80–2.20 | 1.10 | 0.70–1.74 | 1.27 | 0.77–2.09 |
| HER2 | 3.62 | 2.17–6.03 | 3.39 | 1.92–5.98 | 2.83 | 1.70–4.70 | 3.01 | 1.68–5.38 |
| Triple negative | 2.05 | 1.18–3.53 | 1.63 | 0.86–3.10 | 1.95 | 1.15–3.30 | 1.60 | 0.85–2.07 |
Adjusted by: treatment, ANLD, age, TNM, histology, phenotype, diagnostic method, program and diagnóstico date. ALND: Axillary lymph node dissection.
In this study of women participating in breast cancer screening programs diagnosed with breast cancer no statistically significant difference were observed on the overall risk of complications, readmissions, recurrence and mortality between performing a conservative surgery or a mastectomy. TNM (stages III–IV) and phenotype were the factors that had a greater impact on recurrence and mortality, while TNM (stage III–IV) and ALND had the greater impact on complications.
ComplicationsComplications after surgical treatment were present in 28.5% of women, the most frequent being pain and psychological events. As previously described in a study in this population,9 the prevalence of pain is consistent with the results of other studies with values ranging from 10% to 50% or more.10
The results of our analysis showed a prevalence of surgery-related complications (seroma or/and wound infection) of 8.2%. Seroma is a common complication after breast cancer surgery, occurring at rates ranging from 3% to 85%.11 Breast surgery is categorized as a low-morbidity procedure and has been described as a clean operation, but is associated with a highly variable incidence rate of wound infection (1.5%–25%).12 In our study, no differences were found in the frequency of complications depending on the type of surgical treatment.
As expected, statistically significant differences were found in the stage-adjusted analysis: stages III and IV had a strong relationship with the presence of complications in adjusted analysis and the performance of an ALND involved a higher risk of complications, probably because seroma and wound infection are increased when the surgery involved lymph node dissection. This finding is particularly of interest in an era where the tendency is to avoid unnecessary ALND, with new protocols following the publications of Dr. Guliano et al.13 However mortality and recurrences were equivalent between N0 patients who received SNB versus N1/N2/N3 patients who underwent ANLD, in our cohort including all stages and all histologic/phenotypic kind of tumors.
ReadmisionsThe percentage of readmissions is used as a quality and hospital safety indicator.14 In addition, admission to hospital is undoubtedly one of the most disabling situations a patient can face, both in the physical and psychological spheres.15
Readmissions after surgical treatment occurred in 21.8% of women, being slightly higher in the mastectomy group (27%) than in the conservative treatment group (20.5%) but without finding statistically significant differences. Similar percentages were reported by a study performed in the United States where breast-conservative surgery had a readmission rate due to reoperation of 21.6%.16 In previous study about readmissions from the same population,17 readmissions risk was not increased by surgical approach but rather by the complications themselves.
RecurrencesPrevious reports indicate that about 10% of women have a recurrence in the first 5years after surgical treatment.18 In our study, with a longer follow-up until 13years (mean of 8.5years), breast cancer recurrence affected 14.2% of women. We included women with all stages of the disease and women in stages III and IV had the highest risk of recurrences, as expected and consistent with the evidence,19 along with patients with HER2-positive tumors.20 Women included in our study were diagnosed between 2000 and 2009 and treatment for HER2 was introduced in 2004–2005, which could explain this result, as observed in other studies where women not receiving chemotherapy or trastuzumab for HER2-postitive tumors had a significantly higher risk of recurrence.20 As it was observed in another study,19 recurrence was more frequent in patients who underwent a mastectomy, but the association disappeared after adjustment by tumor characteristics.
MortalityThe mortality rate in this cohort with a follow-up of up to 13years was 13.8%, which is within the range described in other studies.21 Mortality was higher in the group of women receiving mastectomy, which are the group with a less favorable prognosis. But no difference in the adjusted analysis for risk of mortality was observed according to surgical procedure. Evidence in the literature is inconclusive for mortality according to the surgical technique: most studies comparing mastectomy and conservative surgery plus radiotherapy have reported similar results in terms of survival22,23 even in patients with tumors >5cm.24 However, other studies suggested better survival among women treated with conservative surgery compared with mastectomy.25,26 In contrast, there are an old metanalysis that indicate that mastectomy might provide a slightly overall survival benefit compared with breast-conservative.27
As with the most recurrent phenotypes, mortality is higher in HER2-positive and triple negative tumors, but only the statistical significance for HER2-positive tumors was maintained in the adjusted model. This study was conducted at a time when the use of monoclonal antibodies was not standardized. A recent study28 has shown that early-stage tumors (T1N0) have the same risk of mortality regardless of phenotypic subtype, including HER2-positivity.
Limitations and strengthThis study has some limitations. It is based in a cohort of women participating in a population breast cancer screening program, and consequently all participants were aged between 50 and 70years at diagnosis. This hampers comparisons with studies including women of all ages, but also lends homogeneity to the sample. The women were diagnosed between 2000 and 2009 and since then, treatment improvements have been introduced, the most important being the introduction of sentinel node biopsy and treatment for HER2-positive tumors. Neoadjuvant and adjuvant treatment have a key role in improving breast cancer survival, not taking into account the possible variability in the application of systemic treatment may introduce bias in the analysis, although this study was performed at a time when chemotherapy schemes were limited and all centers followed the same indication protocols. Moreover, information on complications and readmissions were obtained from the medical history, which might have introduced information bias. However, the clinical records review was done by trained professionals, following a common protocol, and the final models were adjusted by different screening programs. This study did not evaluate either monetary costs or patients' quality of life after surgical treatment. However, few studies have analyzed complications, readmissions, recurrence and mortality in the same multicenter work, with a fairly long follow-up period, taking into account tumor characteristics (including all TNM stages), diagnostic method and type of treatment in more than 1000 patients.
ConclusionSurgeons are concerned about surgical variability in breast cancer and its related long-term outcomes. To contribute decision-taking about surgical treatment it is important to have complete information about all possible outcomes, taking into account long-term effects.
This study supports current evidence that the results of different surgical treatment are similar: as long as safe oncological surgery is performed, breast-conserving surgery and mastectomy are equally effective in terms of complications, readmissions, recurrence and mortality adjusted by individual tumor and patients age. This finding allows freer adaptation to professional and health system circumstances, and the needs and desires of each patient with the certainty that personalized surgery will not influence the prognosis of the disease, allowing us to focus on patient's life quality.
The stage of breast cancer is the variable with the greatest weight related with presence of complications, readmissions, recurrence and mortality. Population screening programs are the only way to diagnose breast cancer at early stages, so this article supports their continuity and implementation.
FundingThis work was supported by the following Grants from Instituto de Salud Carlos III, FEDER (PI16/00244) and REDISSEC, FEDER (PI16/0001/0013) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics approval and consent to participateStudy data were collected using a protocol approved by the ethics committee of Parc de Salut Mar (CEIC-Parc de Salut MAR), Barcelona. Specific patient consent was not required because we used retrospective data from screening participants who had previously signed information release documents. All methods were performed in accordance with the relevant guidelines and regulations.
We thank Joana Ferrer from Santa Caterina Hospital, Marisa Baré from Corporació Sanitària Parc Taulí and Teresa Barata from Canary Islands Health Service for their participation in the CAMISS cohort data collection. Also we would like to thank Dr. Pau Moreno and Dr. David Parés from Germans Trias' Hospital for their suggestions and contributions to this article, as well as Mr. Arnau Sans for his support.