This study aims to analyze the benefit of adjuvant radiotherapy (RT) in malignant phyllodes tumor in terms of overall survival and control of local and systemic recurrence. Recently, there is an increasing trend toward the use of adjuvant RT.
Methods and materialFifty-four patients with non-metastatic, malignant phyllodes tumor from January 2001 to December 2015 were included in the study. All patients had undergone margin negative surgical treatment either with a wide local excision [n=6] or simple mastectomy [n=48]. Patients who received adjuvant RT were stratified as Group 1 [n=29] and those who did not receive adjuvant radiation as Group 2 [n=25]. Survival probabilities were estimated by the Kaplan–Meier method.
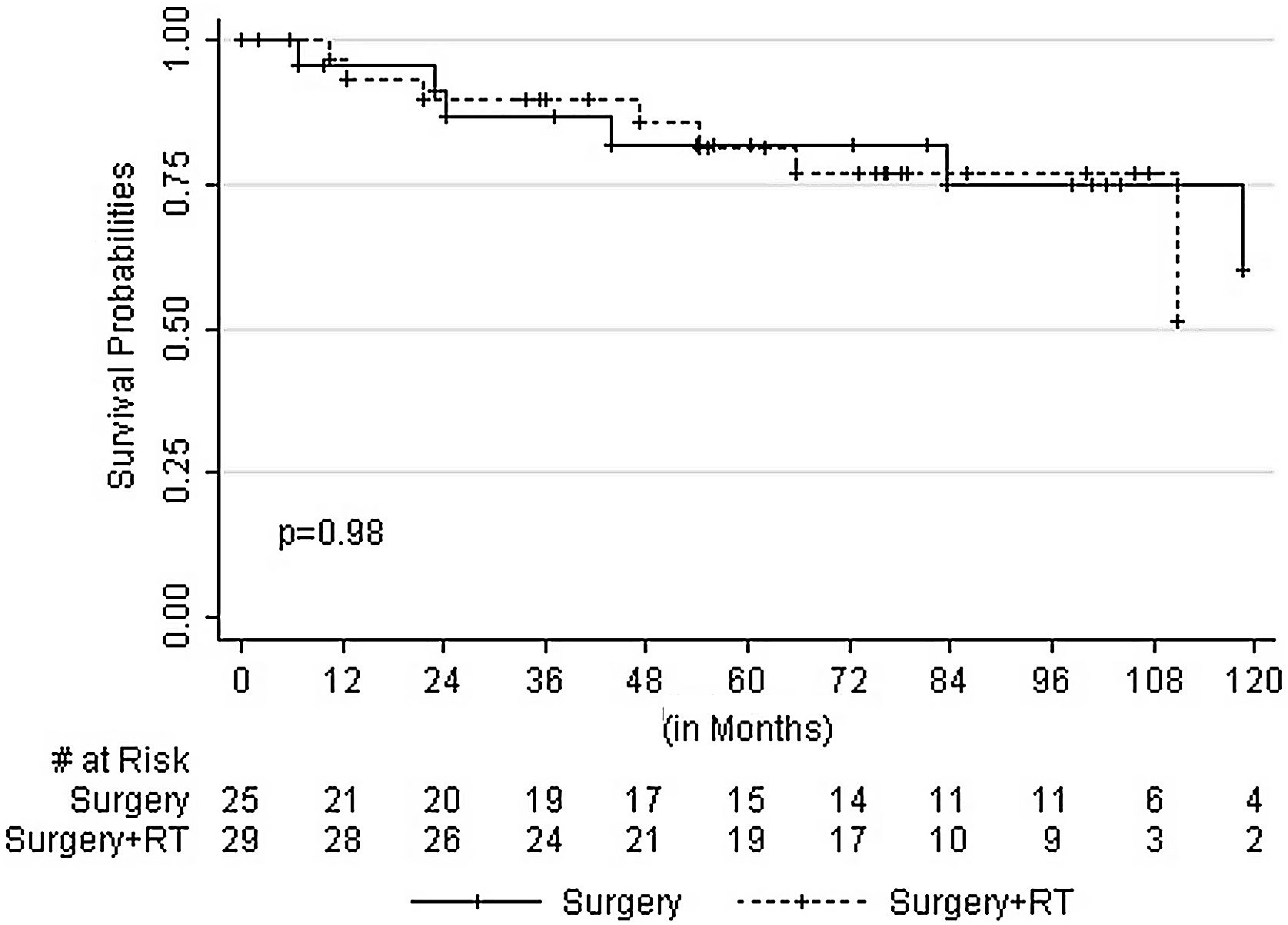
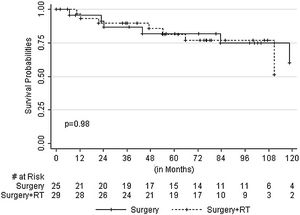
ResultsThe median follow-up was 76 months (2–150 months) with no statistically significant clinicopathologic or treatment factor associated with either local or systemic recurrence. There were seven [local 7.4% and systemic 5.6%] recurrences (3 in Group 1 and 4 in Group 2). The 5 and 10-year overall survival were 82% and 51% in Group 1 and 82% and 62% in Group 2, respectively (p=0.989).
ConclusionThe role of adjuvant radiation in malignant phyllodes tumor in terms of overall survival is doubtful when margins are negative.
El objetivo de este estudio es analizar el beneficio de la radioterapia (RT) adyuvante en el tumor filodes maligno en términos de supervivencia general y control de la recidiva local y sistémica. Existe una tendencia reciente hacia el uso de RT adyuvante.
Métodos y materialSe incluyó en el estudio a 54 pacientes con tumor filodes maligno no metastásico, de enero de 2001 a diciembre de 2015. Todas las pacientes habían sido sometidas a tratamiento quirúrgico de márgenes negativos, bien mediante extirpación local amplia [n=6] o mastectomía simple [n=48]. Las pacientes que recibieron RT adyuvante fueron estratificadas como Grupo 1 [n=29], y aquellas que no recibieron radiación adyuvante como Grupo 2 [n=25]. Las probabilidades de supervivencia fueron calculadas mediante el método de Kaplan-Meier.
ResultadosEl seguimiento medio fue de 76 meses (2-150 meses) sin factor clinicopatológico o terapéutico estadísticamente significativo asociado a la recidiva local o sistémica. Se produjeron siete recidivas [locales 7,4% y sistémicas 5,6%] (tres en el Grupo 1 y cuatro en el Grupo 2). La supervivencia general a cinco y 10 años fue de 82 y 51% en el Grupo 1, y de 82% y 62% en el Grupo 2, respectivamente (p=0,989).
ConclusionesEl rol de la radiación adyuvante en el tumor filodes maligno en términos de supervivencia general es dudoso cuando los márgenes son negativos.
Phyllodes tumor of breast accounts for less than 1% of all the breast neoplasms.1–4 The majority of these tumors occur in women aged between 35 and 55 years.5 Phyllodes tumor is a biphasic neoplasm consisting of both epithelial and stromal components.6 According to the World Health Organization classification of breast tumors,7 they are classified as benign, borderline and malignant phyllodes taking into account stromal cellularity, cellular atypia, stromal overgrowth, mitosis and microscopic tumor borders. There is a need for effective local treatment of malignant phyllodes tumors as these tumors have a relatively low risk of metastasis and death due to disease. The primary treatment for malignant phyllodes tumors is surgical, either a wide local excision or a simple mastectomy. Even though the role of postoperative adjuvant radiation was studied, it was not routinely used except in those undergoing wide local excision or margin positive simple mastectomy. Malignant phyllodes tumor (MPT) usually presents at a young age and with large tumor size. After a wide local excision in malignant phyllodes, adjuvant radiotherapy is used following the principle of adjuvant RT in invasive breast cancer with reduced local and distant recurrences. Recently, a sudden increase in adjuvant radiotherapy has been noticed even after mastectomy as phyllodes arise from stromal elements, and some recommend following treatment guidelines of soft tissue sarcoma.8
AimThis study aims to analyze the benefit of adjuvant radiotherapy in margin negative malignant phyllodes tumor in terms of overall survival and control of local and systemic recurrence.
Subjects and methodsThe medical records of 77 consecutive patients diagnosed with phyllodes tumor at the institute from January 2001 to December 2015 were considered for this retrospective study. Fifty-four non-metastatic, margin negative patients with malignant phyllodes tumor were included for analysis. They were followed up till August 2018. The data captured the age, menopausal status, initial diagnosis (by trucut biopsy or excision biopsy), definitive surgical procedure, final histopathological diagnosis, size of tumor and margin status including recurrence (local/systemic).
All patients underwent surgery, and forty-eight had undergone simple mastectomy (SM), and six had wide local excision (WLE). Patients who received adjuvant radiotherapy (RT) were stratified as Group 1 and those who did not receive adjuvant radiation as Group 2. In our center, the indication for radiotherapy before 2009 was after WLE and in patients who had positive margins after SM. From 2010 onwards, all patients after WLE and following SM with a tumor size more than 5cm or with positive margins received adjuvant radiotherapy. The planning target volume was only the chest wall following SM (total dose of 5000cGy, 200cGy/day×5 fractions per week) and after WLE, to the whole breast with a boost to tumor bed (5000cGy to entire breast followed by a boost of 1000cGy to tumor bed 200cGy/day×5 fractions per week). Patients were treated with 6MV photon beam therapy using a linear accelerator. All patients with positive margins received adjuvant radiation and were excluded from our study.
The patients were followed up once every three months for the first three years and after that every six months for the next two years and once a year after five years of completion of treatments. All the patients were clinically examined during each visit to rule out local recurrence. Also, chest radiograph and mammogram were performed annually. Other additional imaging was done if needed, based on symptoms. At the time of censoring, the last follow-up date is the patient's last visit or death. In patients not reported for scheduled reviews, they were contacted over phone. The patient's status was recorded as alive or dead, including the probable cause of death as informed by the attendant. The time of telephonic interview was recorded as the date of last follow up. The Institutional Ethics Committee approval was obtained for the study.
Statistical analysisStatistical software STATA 11.2 version was used to analyze data. The disease-free survival (DFS) is defined as the time from the first diagnosis to the first recurrence. The time elapsed in months between the initial diagnosis and closing date of the study (August 2018) or death, whichever is the earliest is defined as overall survival (OS). Kaplan–Meier method was used to estimate survival probabilities. The log-rank test was used to compare the survival probabilities according to the prognostic factors. The cox-proportional hazard model was employed to identify prognostic factors. A p-value of <0.05 was considered significant.
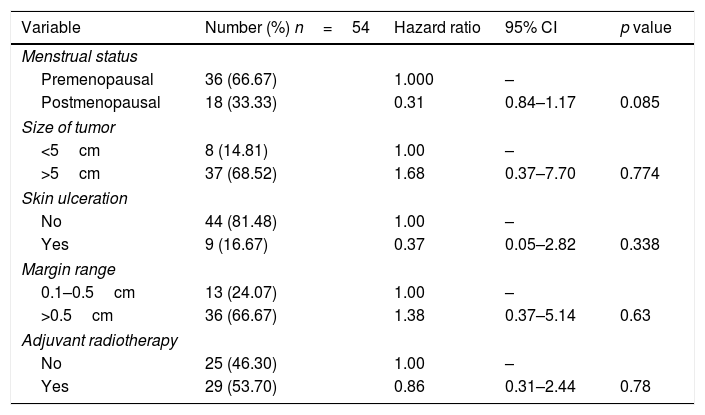
ResultsThe clinicopathological and treatment details of the patients with malignant phyllodes tumor included in our study are shown in Table 1. The median age at diagnosis was 40 years (range 17–67 years). Preoperative histopathological examination was with a trucut biopsy in 45 patients and excision biopsy in 9 patients. The trucut biopsy was confirmative of MPT in 50% of the patients. All the patients underwent surgical treatment either with a simple mastectomy or wide local excision. The median size of the tumor was 10cm (range 2–30cm). The margin status was negative in 49 (90.7%), range of margin was 0.1 to >1cm, and unknown in 5 (9.3%). Skin involvement with ulceration was seen in 9 (16.7%), without ulceration in 44 (81.5%), and unknown skin involvement in one (1.9%). The mean time for recurrence was 10.2 months (range 5.5 months to 69.7 months). There were no statistically significant clinicopathologic or treatment factor associated with recurrence.
Cox proportional hazard survival analysis.
| Variable | Number (%) n=54 | Hazard ratio | 95% CI | p value |
|---|---|---|---|---|
| Menstrual status | ||||
| Premenopausal | 36 (66.67) | 1.000 | – | |
| Postmenopausal | 18 (33.33) | 0.31 | 0.84–1.17 | 0.085 |
| Size of tumor | ||||
| <5cm | 8 (14.81) | 1.00 | – | |
| >5cm | 37 (68.52) | 1.68 | 0.37–7.70 | 0.774 |
| Skin ulceration | ||||
| No | 44 (81.48) | 1.00 | – | |
| Yes | 9 (16.67) | 0.37 | 0.05–2.82 | 0.338 |
| Margin range | ||||
| 0.1–0.5cm | 13 (24.07) | 1.00 | – | |
| >0.5cm | 36 (66.67) | 1.38 | 0.37–5.14 | 0.63 |
| Adjuvant radiotherapy | ||||
| No | 25 (46.30) | 1.00 | – | |
| Yes | 29 (53.70) | 0.86 | 0.31–2.44 | 0.78 |
Twenty-nine patients (54%) were in Group 1, and twenty-five (46%) were in Group 2. Six patients had WLE, and 48 patients had undergone SM. Twelve patients with clinically significant nodes underwent axillary sampling, but all the nodes were pathologically negative. In the WLE group of six patients, the median size of the tumor was 4cm. Out of the six patients who underwent WLE, three patients were in Group 1. In the patients who had undergone WLE, there was no local recurrence, irrespective of whether RT was received or not. The margin range was 0.1–2cm. In the SM group of 48 patients, the median size of the tumor was 10cm. The margin range in SM was 0.1–3cm. Out of the 48 patients who had undergone SM, 26 patients were in Group 1 and 22 patients in Group 2. In both the WLE and SM group of patients, there was no recurrence in twenty-six (89.7%) of the patients in Group 1, and twenty-one (84%) of the patients in Group 2.
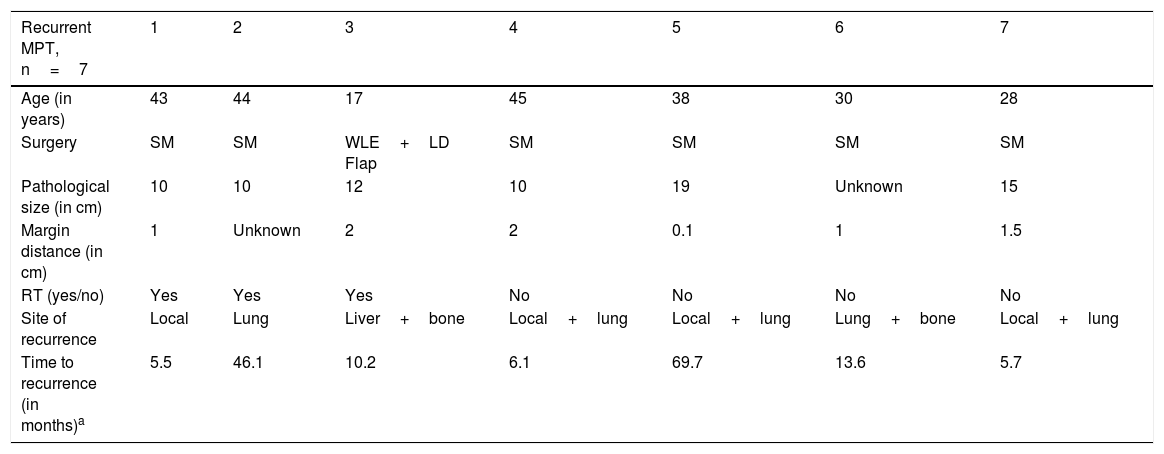
The median follow-up period was 76 months (range 2–150 months). In Group 1 MPT, three recurrences were observed in patients, irrespective of WLE or SM. One had local recurrence at six months (tumor size 10cm, SM done, margins negative). Two had systemic recurrence, one in the lung following SM, and the other in liver and bone after WLE. Four recurrences were observed in Group 2. Out of the four patients with recurrence in Group 2, three patients had presented initially with local recurrence. Also, there was co-existent systemic recurrence (median tumor size 10cm, SM done, the median time for recurrence 10 months) and one had systemic recurrence only at 13 months (size of tumor not known as SM done outside) (Table 2). Age, type of surgery, size, margin distance and adjuvant radiotherapy did not show any clear relationship to survival (Table 1). The 5 and10 year DFS were 78% and 60% in Group 1, 78% and 52% in Group 2 respectively (p=0.707).
Details of recurrent malignant phyllodes.
| Recurrent MPT, n=7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age (in years) | 43 | 44 | 17 | 45 | 38 | 30 | 28 |
| Surgery | SM | SM | WLE+LD Flap | SM | SM | SM | SM |
| Pathological size (in cm) | 10 | 10 | 12 | 10 | 19 | Unknown | 15 |
| Margin distance (in cm) | 1 | Unknown | 2 | 2 | 0.1 | 1 | 1.5 |
| RT (yes/no) | Yes | Yes | Yes | No | No | No | No |
| Site of recurrence | Local | Lung | Liver+bone | Local+lung | Local+lung | Lung+bone | Local+lung |
| Time to recurrence (in months)a | 5.5 | 46.1 | 10.2 | 6.1 | 69.7 | 13.6 | 5.7 |
RT – radiotherapy, MPT – malignant phyllodes tumor, SM – simple mastectomy, WLE+LD flap – wide local excision+latissimus dorsi flap.
Age, size of the tumor, type of surgery, margin distance and adjuvant radiotherapy were not predictive for local recurrence. However, local recurrence observed in tumor size of more than 10cm was not statistically significant with a p-value of 0.774. Patients were censored at the last follow-up date as alive or dead. Three patients were lost to follow up, and 14 patients were dead. The cause of death in seven patients was due to recurrent disease with progression. The cause of death was due to elderly age in two patients, and the cause of death was unknown in five patients. The 5 and 10-year overall survival were 82% and 51% in Group 1 and 81.9% and 61.9% in Group 2 respectively (p=0.989) (Fig. 1).
DiscussionMalignant phyllodes tumor of the breast is a rare neoplasm. The usual presenting symptom is a palpable lump with a history of a sudden increase in the tumor's size. It commonly presents as a large tumor with skin ulceration due to pressure necrosis. Core needle biopsy is preferable for preoperative diagnosis of phyllodes tumor as it is minimally invasive and has replaced excision biopsy for initial diagnosis.9–14 In those who undergo wide local excision the reported mean age at diagnosis was 45 years and mean size of the tumor was 4cm.15–18 However despite most of the tumors presenting as large tumors, there is a low incidence of local recurrence and distant metastasis. In literature, the reported incidence of local recurrence is 10–65% (in our study 7.4%) and distant recurrence is 5-40% (in our study 5.6%).15–25 In large-sized tumors wide local excision or simple mastectomy was done. There is no role of axillary dissection,20 and it is not routinely recommended.
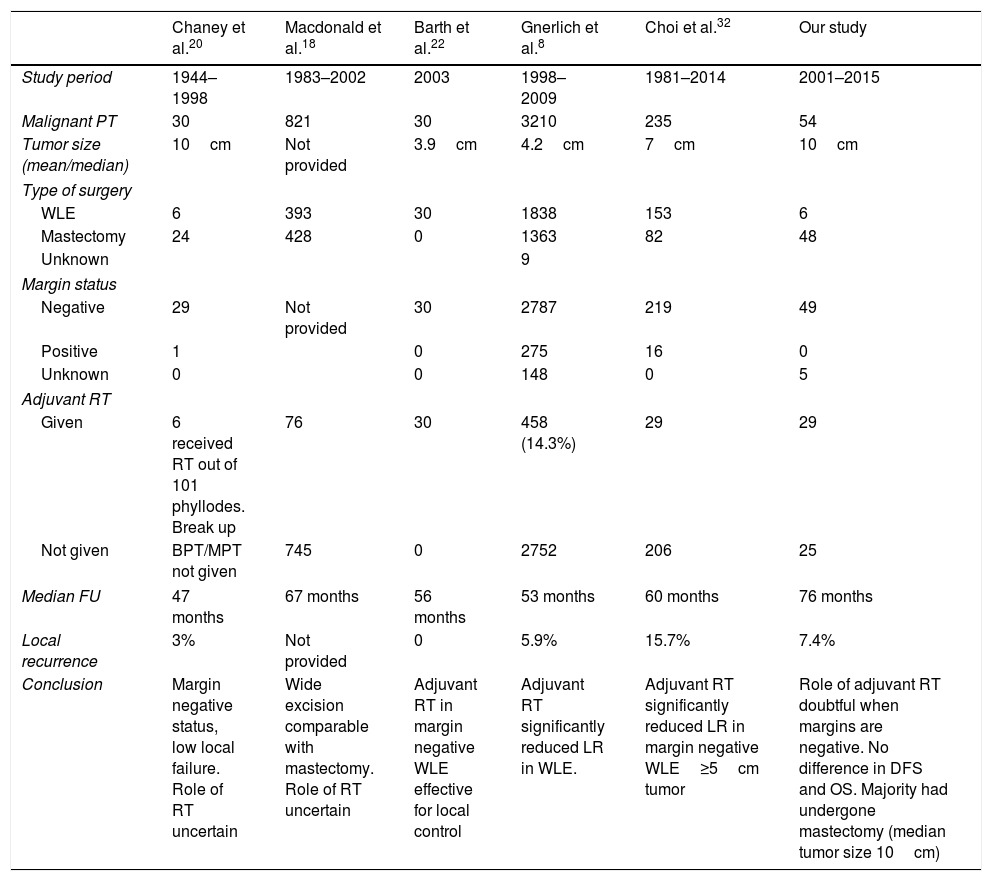
Studies have shown lower relative disease-free local relapse with the addition of radiation (80% with RT Vs 59% without RT), after a margin negative wide local excision. In mastectomy patients, the relative disease-free local relapse with and without radiation was 92% Vs 78%. It was the first prospective study reported which showed the benefit of radiation following wide local excision with negative margin.22 Another study has shown there has been over utilization of radiotherapy after mastectomy with lower relative disease-free local relapse and no increase in OS or DFS.8 Pandey et al. have shown that adjuvant RT decreased the risk of local recurrence (LR) and improved survival. The margin of excision is an essential factor for recurrence.26 Chaney et al. have shown that the addition of radiation in borderline or malignant phyllodes minimized local recurrence.20 Pezner et al. retrospectively analyzed the effect of radiation in malignant phyllodes of the breast and recommended adjuvant RT for tumor sizes of >2cm.24 Zeng et al., in a systematic review and meta-analysis of effects of adjuvant radiotherapy in borderline and malignant phyllodes tumor, suggested that the addition of radiation after breast-conserving surgery decreased local recurrence rates. They also observed that adjuvant RT after mastectomy showed no survival benefit and that RT was beneficial in margin positive patients.27 Mallick et al., in a study of multimodality approach for malignant and borderline phyllodes tumor from a tertiary care center, concluded that RT was beneficial in tumors more than 10cm or positive margins.28 It has been reported that a margin of less than 1cm has an increased risk of local recurrence.16 However, another study has stated that the evidence supporting larger margins is limited, and margin length did not influence local recurrence risk.29 An analysis of 35 patients from a single institution suggested that surgery is the mainstay treatment and postoperative radiotherapy decreased local recurrence in specific presentations.30 Reports have shown a lack of association with the size of the tumor and local recurrence.5,29 Recurrent malignant phyllodes tumor can be of a more malignant phenotype than the initial tumor.12 In a recent systematic review and meta-analysis published with 54 studies and 9234 cases, the reported pooled LR rate was 18% for MPT. The risk factors identified for local recurrence were tumor-related characteristics like mitosis, stromal cellularity, atypia etc., in addition to the surgical margin. This review also mentions the highly debated role of RT in phyllodes tumor; however, due to limited data, this study did not assess RT as a risk factor.31 A positive margin and WLE both significantly correlated with a higher LR risk.31,32 A comparative table of a few studies with MPT treated with radiotherapy is shown in Table 3.
Comparison of studies in MPT treated with radiotherapy.
| Chaney et al.20 | Macdonald et al.18 | Barth et al.22 | Gnerlich et al.8 | Choi et al.32 | Our study | |
|---|---|---|---|---|---|---|
| Study period | 1944–1998 | 1983–2002 | 2003 | 1998–2009 | 1981–2014 | 2001–2015 |
| Malignant PT | 30 | 821 | 30 | 3210 | 235 | 54 |
| Tumor size (mean/median) | 10cm | Not provided | 3.9cm | 4.2cm | 7cm | 10cm |
| Type of surgery | ||||||
| WLE | 6 | 393 | 30 | 1838 | 153 | 6 |
| Mastectomy | 24 | 428 | 0 | 1363 | 82 | 48 |
| Unknown | 9 | |||||
| Margin status | ||||||
| Negative | 29 | Not provided | 30 | 2787 | 219 | 49 |
| Positive | 1 | 0 | 275 | 16 | 0 | |
| Unknown | 0 | 0 | 148 | 0 | 5 | |
| Adjuvant RT | ||||||
| Given | 6 received RT out of 101 phyllodes. Break up | 76 | 30 | 458 (14.3%) | 29 | 29 |
| Not given | BPT/MPT not given | 745 | 0 | 2752 | 206 | 25 |
| Median FU | 47 months | 67 months | 56 months | 53 months | 60 months | 76 months |
| Local recurrence | 3% | Not provided | 0 | 5.9% | 15.7% | 7.4% |
| Conclusion | Margin negative status, low local failure. Role of RT uncertain | Wide excision comparable with mastectomy. Role of RT uncertain | Adjuvant RT in margin negative WLE effective for local control | Adjuvant RT significantly reduced LR in WLE. | Adjuvant RT significantly reduced LR in margin negative WLE≥5cm tumor | Role of adjuvant RT doubtful when margins are negative. No difference in DFS and OS. Majority had undergone mastectomy (median tumor size 10cm) |
PT, phyllodes tumor; WLE, wide local excision; RT, radiotherapy; BPT, benign/borderline phyllodes tumor; MPT, malignant phyllodes tumor; FU, follow up; OS, overall survival; DFS, disease free survival.
The debate, to consider adjuvant radiotherapy for MPT is yet to be defined due to the rarity of the tumor, coupled with no prospective studies. Also, as it arises from stromal elements with increased concern for local recurrence, radiotherapy is now widely used because of fear of recurrences. Majority of radiation oncologists receive such references from the surgeon for an opinion on radiation. It is a fact that recurrences are aggressive with fewer treatment options. Except for breast-conserving surgery and positive margins as an indication for radiotherapy, the benefit of adjuvant radiotherapy after a margin negative mastectomy in MPT is not clear. The limitation of our study is that it is retrospective and less number of subjects analyzed. The reported median tumor size in our subset was 10cm, and out of the fifty-four patients, forty-eight had undergone a simple mastectomy. Our study's advantage is that it is from a single institution comparing the disease-free survival and overall survival with and without adjuvant radiotherapy in MPT following surgery with negative margins.
ConclusionThe role of adjuvant radiation in malignant phyllodes tumor is doubtful when margins are negative. The 5 and 10-year overall survival in both the RT versus no RT groups were not statistically significant. There is a need to identify high-risk factors as predictors for local and systemic recurrence, including survival benefit with adjuvant radiotherapy in future studies.
Ethical approvalThis article does not contain any interventional studies on human participants performed by any authors. Institutional Ethics Committee approval was obtained.
FundingThis work has not received any funding.
Conflict of interestThe authors declare that they have no conflict of interest.