In the present study, the degradation of Reactive Yellow 17 (RY17) azo dye in aqueous solution was performed using UV light and iron ions as activators of peroxydisulfate (S2O82− or PDS). The effect of several parameters affecting the degradation process was studied and the optimum conditions are found to be: [RY17] = 10 mg/L, [Fe2+] = 0.05 mM, [S2O82−] = 1 mM and the pH = 3. The obtained results showed that the removal degrees of RY17 using different processes such as S2O82−/Fe2+, S2O82−/UV and S2O82−/Fe2+/UV was 63.3%, 81.0% and 95.4% respectively within 20 min of the degradation. Based on theses removal degrees, the degradation efficiency of the RY17 increases in the order: S2O82−/Fe2+/UV > S2O82−/UV > S2O82−/Fe2+ > UV > S2O82−. The experimental data were analyzed using the first-order, second-order and Behnajady-Modirshahla-Ghanbery (BMG) kinetic models, and the kinetic data were in good agreement with the BMG model. Under the optimal conditions, the comparative study of the RY17 degradation using two salts Na2S2O8 and K2S2O8 showed a strong similarity. The total mineralization was monitored using COD and TOC techniques. The results showed that the peroxydisulfate activated by UV and Fe2+/UV system is efficient for the degradation and mineralization of RY17; it could also be an alternative for the treatment of the real wastewater contaminated by azo dyes.
Wastewaters generated from various industries, and containing dyes represents a threat to aquatic life and environment. Dye effluents may present an ecotoxic risk and have potential dangers of bioaccumulation for humans and other living organisms [1,2]. Synthetic dyes are extensively used in different processing industries such as textile leather, printing, cosmetic, drug and food. Among the synthetic dyes released in effluents from textile industries, azo dyes, which represent almost 50% of about ~700 000 tones of dyes produced in the world. Azo dyes are one of the more detrimental classes because it is highly persistent in the aquatic environment, due to its chemical composition, involving aromatic rings, azoic linkages and amino groups [3–5]. Various technologies such as adsorption, ion exchange, reverse osmosis, coagulation flocculation and advanced oxidation processes (AOPs) are used for removing the dyes from wastewater; theses later have received great attention for the oxidative destruction of refractory compounds. In addition, they have the potential to completely oxidize organic contaminants to CO2, H2O and mineral salts. Their advantages for the treatment of dye wastewater include fast reaction, complete treatment and being pollution-free [6–8].
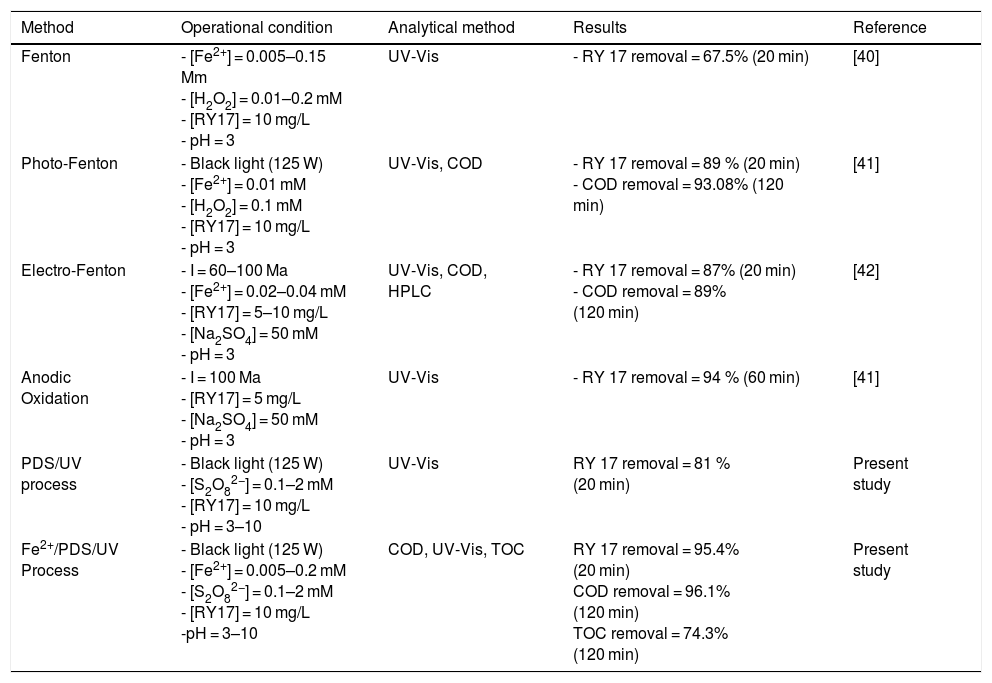
Recently, Peroxydisulfate Oxidation (PDSO) was proved to be a novel and promising technique for the treatment of wastewaters, especially for the removal of organic pollutants. The peroxydisulfate is a strong oxidant (redox potential equal to 2.01 V) and kinetically slows when reacting with a large numbers of organic compounds. However, it was found that the peroxydisulfate anion can be thermo-chemically activated by transition metal ions, such as Fe2+,which leads to the generation of sulfate radicals (SO4•−) oxidant (redox potential is Eo ≈ 2.6 V) [9,10]. The reduction of peroxydisulfate anion leads to the production of sulfate anions [11].
The generated radical (SO4•−) is a stronger oxidant with a kinetically fast reacting tendency. The peroxydisulfate chemistry can be summarized in the following equations [12,13]:
This oxidant has been used for the treatment of organic contaminants such as Orange G [14], Coprofloxacin, Sulfamethoxazole [15], Dithyl Phthalate [16] and Catechol [17]. Compared to the other oxidants, Peroxydisulfate is a solid chemical sufficiently stable below ambient temperature (25 °C), very soluble in water, and it's easy for transport and storage [18]. However, at ambient temperature, peroxydisulfate is slowly reacting with contaminants in water, its activation is therefore necessary for accelerating the degradation process. Peroxydisulfate can be activated using Fe2+ ions because they are nontoxic, cheap and effective.
The activation of peroxydisulfate by using Fe2+ ions has been previously introduced into wastewater treatment; this process has many similarities to the Fenton systems which can help us to better understand the degradation mechanism [19]. Once the sulfate radicals SO4•− is generated, a series of reactions are propagated involving the formation of other active species, in particular the hydroxyl radical (HO•). The mechanism describing the Fe2+ activated peroxydisulfate process includes primarily the following reactions [15]:
The aim of the present work is to remove RY17 dyes from aqueous solution using the process of peroxydisulfate activated with ferrous ion. During this study, several parameters affecting the degradation process were studied, such as: The evaluation of the pH effect on the S2O82−/Fe2+and S2O82−/UV processes, the determination of Na2S2O8 concentration effect and Fe2+ dosage on the degradation efficiency. The investigation of the discoloration kinetics by Fe2+/S2O82− at optimal conditions, and the comparative study of RY17 oxidation using two salts Na2S2O8 and K2S2O8 were also studied. The TOC and COD analysis were done in order to prove the complete RY17 mineralization. To the best of our knowledge, no studies have been carried out on the application of the peroxydisulfate process activated with ferrous ion for the degradation of the selected dye RY17.
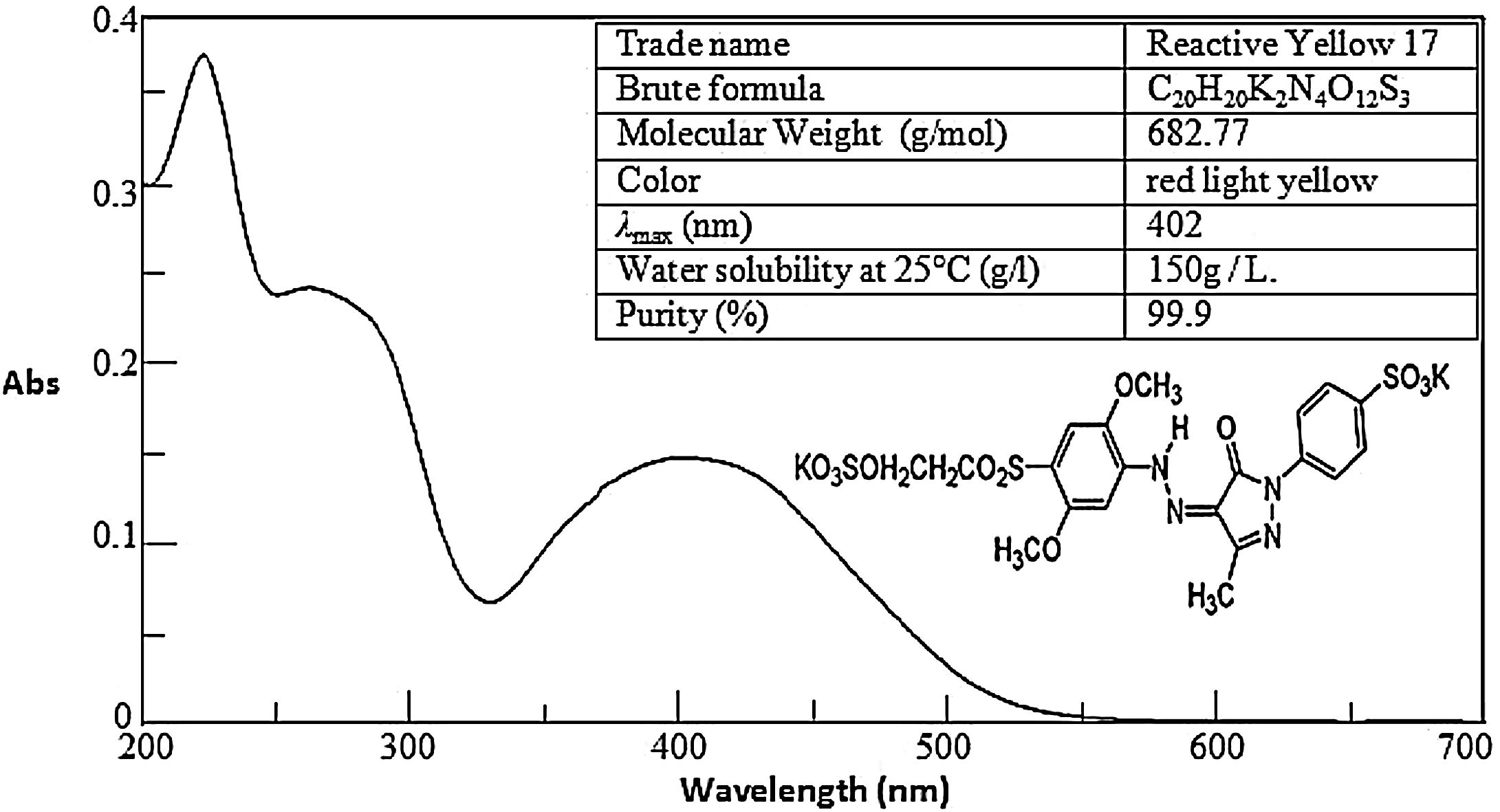
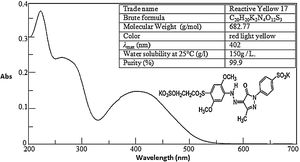
2Materials and methods2.1MaterialsAll chemicals used are reagent grade: RY17 of 99.9% purity. They are kindly supplied by the National Research Center in Egypt. Sodium peroxydisulfate (Na2S2O8, ≥99%) is reagent grade and Sulfuric acid (H2SO4, 98%), Sodium hydroxide, Iron (II) sulfate heptahydrate (FeSO4.7H2O, ≥98%), ethanol (EtOH, 99.8%) and potassium Sodium peroxydisulfate (K2S2O8, ≥99%) are of analytical grade bought from Sigma–Aldrich and Merck products. Aqueous solutions are prepared by using distilled water. The UV–vis spectra with main characteristics are provided in Fig. 1.
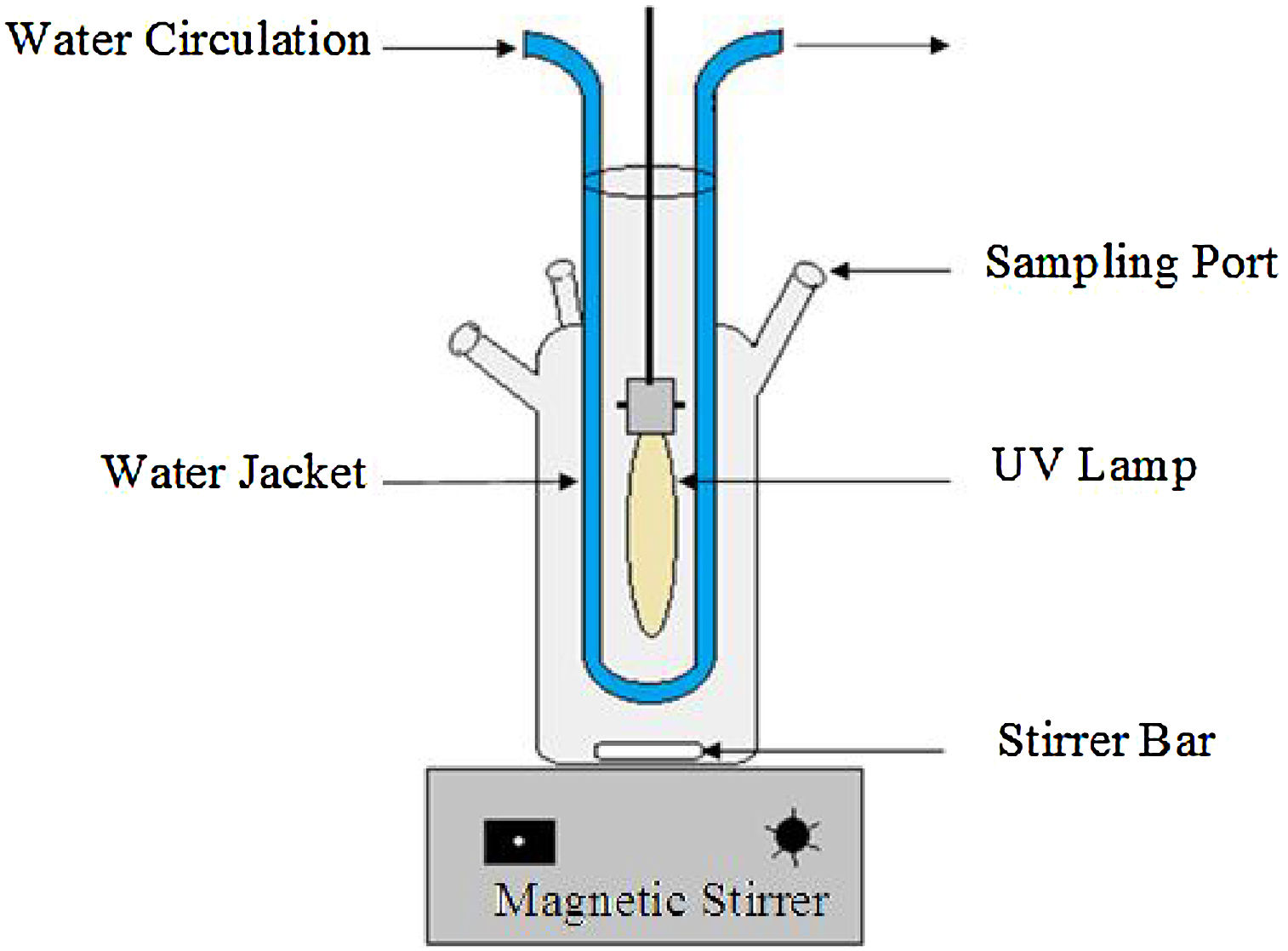
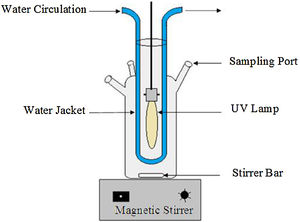
2.2Experimental procedureAs shown in Fig. 2, all experiments were carried out in a cylindrical Pyrex reactor with an internal diameter of 80 mm and a height of 120 mm. A high-pressure mercury lamp (125 W, Philips HPK) emitting 365.5 nm UV light with protection against quartz tubes was placed in the center of the closed reactor. In a typical reaction experiment, a 500 mL of the RY17 solution (10 mg/L) was added to the reactor. The reaction temperature was maintained at 23 °C using a cooling water jacket around the reactor. Desirable solution pH was adjusted by sulfuric acid H2SO4 or sodium hydroxide NaOH using a pH meter (HANNA Instrument Ph209, HI 1332). The appropriate amounts ferric salt (catalyst) and Sodium Peroxydisulfate are introduced entirely into the photoreactor just before starting the UV lamp. At each designated sampling time, 3 mL of the sample was collected from the reactor periodically using a syringe and the reaction was stopped by adding a drop of ethanol, a well-known radical quenching agent, subsequently filtered immediately through 0.45 mm nylon filter (Millipore) membrane before the analysis.
2.3Analytical methodsThe UV–VIS spectra of the dye were recorded from 200 to 800 nm using a UV–VIS spectroscopy (JASCO V-630 Spectrophotometer). The maximum absorbance wavelength (λmax) of RY17 is 402 nm. Therefore, the concentration of azo dyes in water was determined by the absorption intensity at λmax. Chemical oxidation demand (COD) was tested by using colorimetric method, as described in the Standard Methods for the Examination of Water and Wastewater (5220-D; colorimetric) [20]. The total organic carbon (TOC) was also measured using a TOC analyzer (Shimadzu, model TOC-L CPH).The dye decolorization (degradation efficiency DE) used is calculated as follows:
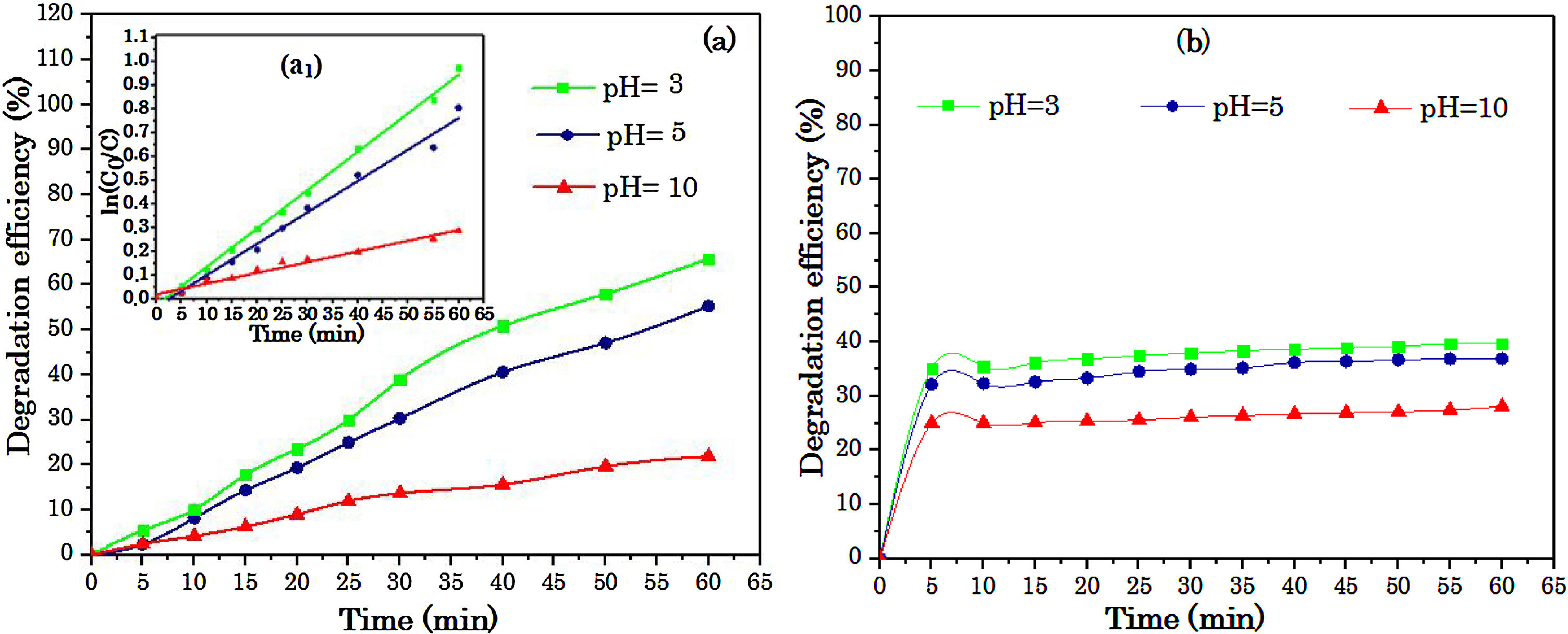
where the C0 and Ct were the concentrations of dye solution at time 0 and t.3Results and discussion3.1Effect of operational parameters3.1.1Effect of pHThe pH of aqueous solution has a significant influence on the S2O82−/Fe2+ and S2O82−/UV processes via changing the ionization degree of the organic pollutants, the surface characteristics, as well as the degree of activity and solubility reaction agents [21]. Fig. 3(a) shows the influence of initial pH on the RY17 degradation using S2O82−/UV process. As can be seen in the figure, the acid pH favored the withdrawal of dye. For the value of pH = 3, the degradation efficiency of RY17 reaches to more than 60% after 60 min of the degradation, for pH = 5 and pH = 10 the degradation efficiency decreases and we note a low efficiency for pH = 10. The degradation efficiency of RY17 after 60 min increases in the following order : pH 3 > pH 5 > pH 10. It is also interesting to note that RY17 is most rapidly degraded at pH = 3. In the S2O82−/UV process, the total radical’s concentration can depend heavily on pH. On the other side, in addition to sulfate radicals, high pH may produce hydroxyl radicals through sulfate radical transformation by Eq. (4). According to Fig. 3(a1), the degradation rate coefficient (k) with Na2S2O8 dose of 0.1 mM for the pH values of 3, 5, and 10 were 0.0162, 0.0134, and 0.0042 min−1, respectively. These results show that the k value at pH 3 was 4 times higher than at pH 10. Under acidic conditions, and according to Eqs. (13) and (14), SO4•− can be generated from acid-catalyzation [22], and react with RY17 to enhance the degradation efficiency of RY17.
Liang and Su [23] studied the inter-conversion from sulfate radical to hydroxyl radical by the reaction Eq. (4), and found that SO4−• gradually prevails at pH < 7. Both of SO4−• and OH are present at pH = 9 with a dominance of •OH at pH = 12. Accordingly, the concentration of SO4−• reacting with RY17 may be lower at pH more than 5, which could be explain the slower degradation efficiency of RY17 at pH = 10. At pH = 5, the three reactions compete with each other: Eqs. (10), (15) and (16) and their simultaneous occurrence may decrease the degradation of RY 17, that is why at pH = 3 achieved a higher degradation than at pH = 5.
Degradation experiments were also studied at pH ranged from 3 to 10 to assessing the degradation efficiency of RY 17 with the S2O82−/Fe2+process. The results are given in Fig. 3(b). The degradation of RY17 decreases for the pH value more than 3, because of different reasons : iron precipitation (Eq. (17)) and decrease in the production of free radicals derived from the electrostatic repulsion forces between oxidant and catalyst with negative surface charges and peroxydisulfate molecules of SO52− and HSO5−[24,25]. Furthermore, the previous studies reported on peroxydisulfate process showed that the used catalyst was FeSO4[26,27]. In this sense, the best results were obtained when the initial solution pH = 3.
3.1.2Influence of catalyst concentrationThe rate of disappearance of RY17 is given by Eq. (18). The integration of this differential equation gives Eq. (19) considering a quasi-stationary state for the concentration of radicals SO4−•, that is, their non-accumulation in the solution. The kinetic law can be written:
The integration of Eq. (18) gives:
The determination of apparent constant of the RY17 degradation is calculated from the slope of the curve ln[RY17]0/[RY17] = f(t) (Fig. 4(a)).
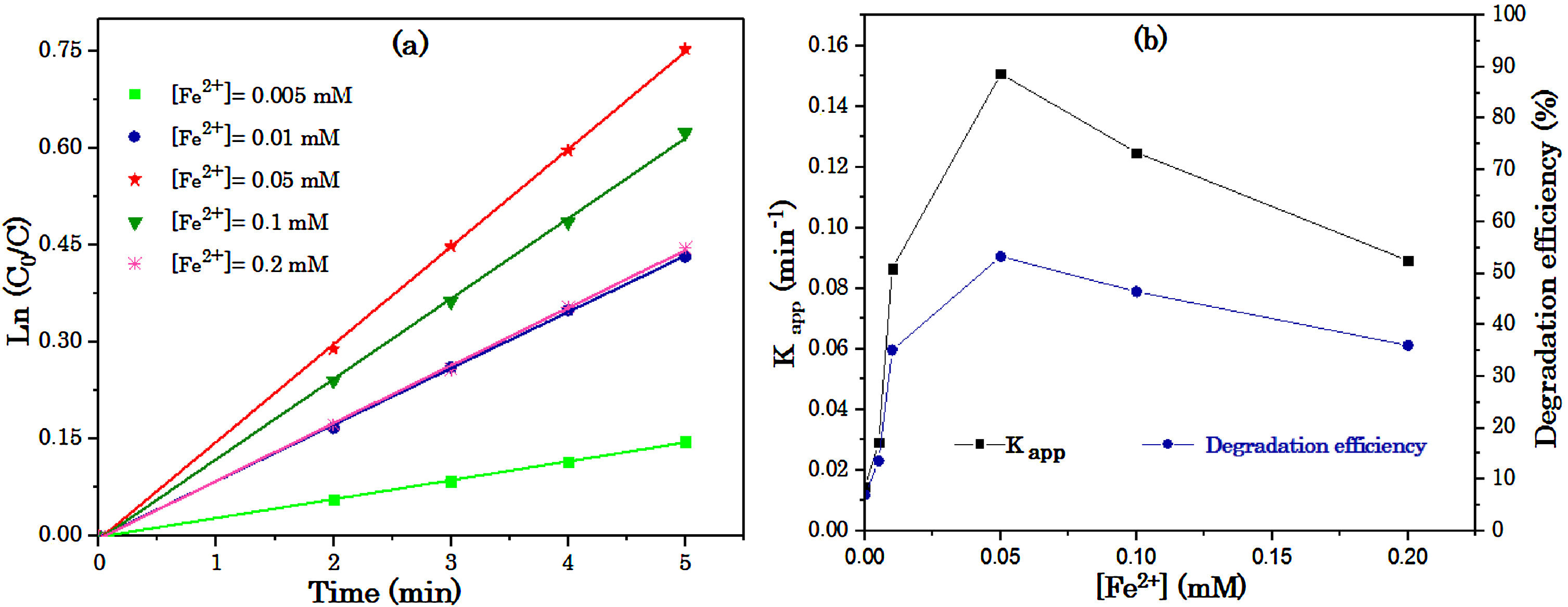
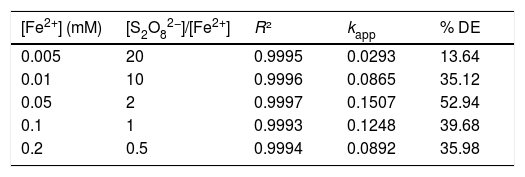
Fig. 4 (a) shows that the degradation kinetics of RY17 follows a pseudo-first order. This is in agreement with the values of the obtained correlation coefficients. It can be also seen that the values of the apparent constants (kapp) increase significantly when Fe2+ concentration increases, until the concentration of 0,05 mM, then it decreases until the concentration of 0.2 mM as shown in Table 1.
Table 1 summarizes for different concentrations of Fe2+, apparent kinetic constants and degradation efficiencies during 5 min of treatment. The best obtained result is found for [Fe2+] = 0.05 mM corresponding to the ratio 2. This ratio gives a better kinetic constant (0.15 min−1) and a better degradation performance (52.9%).
Iron concentration is also an important parameter in the S2O82−/Fe2+ process because it directly affects the performance of sulfate radical SO4•-, by catalytically decomposing S2O82− as shown in Eq. (5), but it can also act as a scavenger of sulfate radicals [28,29], while also acting as scavengers of radicals SO4−• if it is in excess.
Fig. 4 (b) shows the degradation efficiency and the evolution of the apparent kinetic constant of the degradation of RY17 by the radicals SO4−• depending on the initial iron concentration. The effect of different iron concentrations ranging from 0.005 to 0.2 mM on the elimination efficiency of RY17 using the S2O82−/Fe2+ process was studied at an optimal pH of 3 and 0.1 mM of S2O82−. Indeed, increasing the concentration of Fe2+ ions between 0.005 and 0.05 mM leads to a significant increase in the discolouration from 13.6 to 52.9% compared to 7% without addition of catalyst. Thus, the value of the apparent kinetic constant is in the range of 0.15 min−1. Therefore, the optimal catalyst concentration (Fe2+) is 0.05 mM. This value is the most appropriate for subsequent experiments, since the significant change was not observed by a further increase in the quantity of catalyst. This phenomenon could possibly be explained by the fact that the decomposition rate of S2O82− increases substantially due to the presence of additional redox-active centers (ions Fe2+) [30]. However, the use of an additional concentration of 0.05 to 0.2 of Fe2+ ions reduced the degradation efficiency of RY17 from 53 to 39% after 5 min of treatment, which may be due to the trapping effect of SO4•− in the iron species (Eq. (7)).
The high concentration of catalyst Fe2+ in the solution is not in favor of a better degradation rate, as activators of persulfate, the ultimate oxidizing power is limited by: (a) an excessive amount of Fe2+ can act as a scavenger of SO4•− (Eq. (7)), which explains the decrease in the degradation rate of RY17 in the S2O82−/Fe2+ process. (b) A high concentration of Fe2+ also promotes the parasitic reaction between ferric ions and sulfate radicals formed to the detriment of the reaction S2O82−/Fe2+ in the medium. (c) The rapid conversion of Fe2+ to Fe3+ limits the ultimate capacity for oxidation of ferrous-peroxydisulfate process [31]. (d) The generation of excess radicals SO4•− followed by the disappearance of the SO4•− species without decomposition of the pollutant due to the combination of the species of SO4•− (Eq. (6)).
The results indicate that the catalytic activity of the ferrous ion allows it to be an effective agent in the degradation of RY17 using sodium peroxydisulfate.
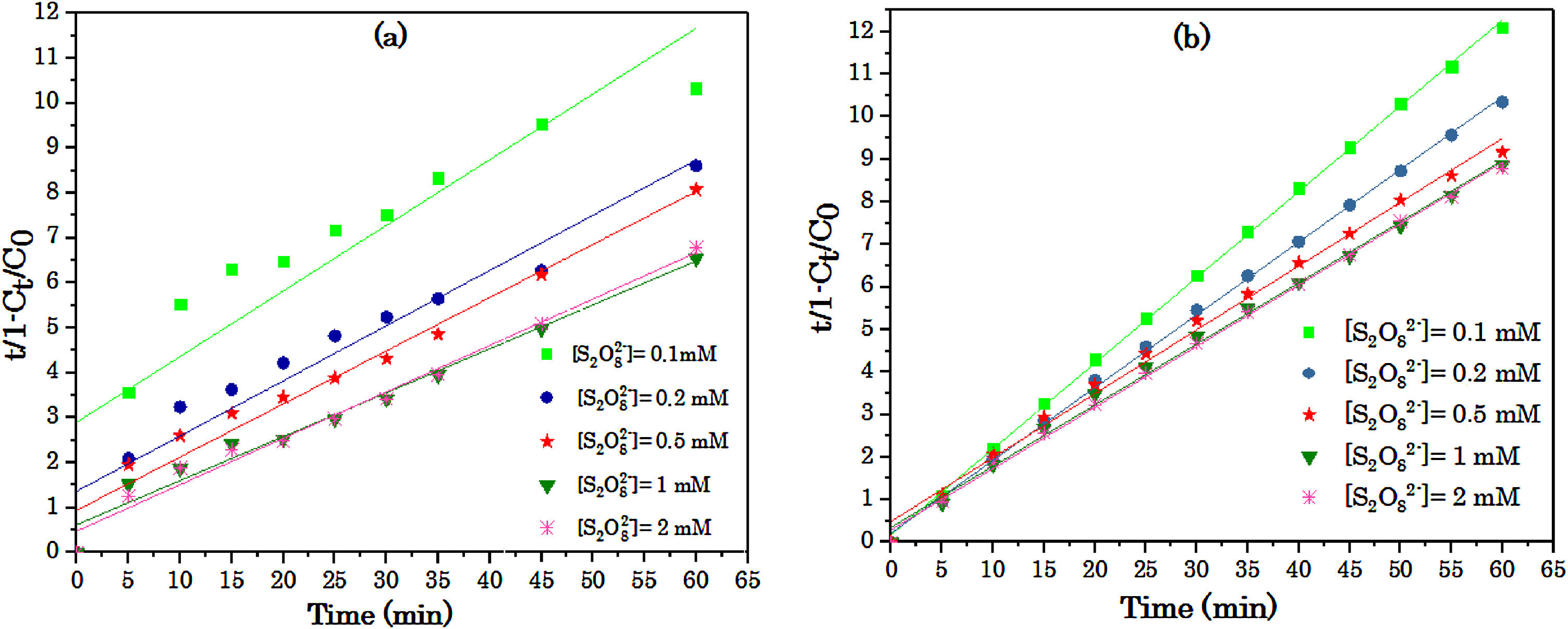
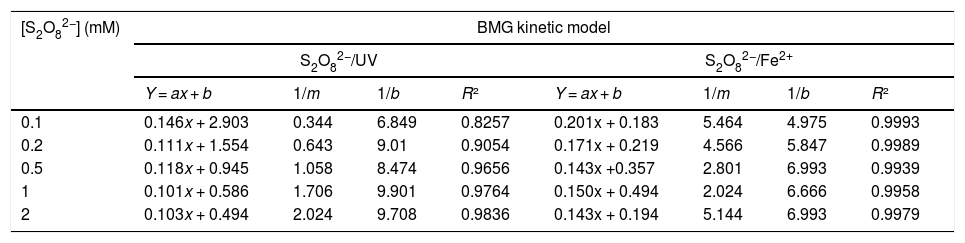
3.1.3Effect of S2O82− concentrationInitial S2O82− concentration can play an important role as an oxidizing agent in the S2O82−/UV and S2O82−/Fe2+ process due to the production of a broad number of SO4−•. Fig. 5 shows the effect of S2O82− concentration on the RY17 removal. At any particular peroxydisulfate concentration, the degradation of the RY17 is presented in the form: BMG kinetics by S2O82−/Fe2+ and S2O82−/UV process. The parameters of kinetic model at different peroxydisulfate concentrations are summarized in Table 2. The effect of different concentrations of S2O82- on the RY17 degradation efficiency is investigated in the range of 0.1–2 mM peroxydisulfate using S2O82−/UV and S2O82−/Fe2+processes for 10 mg/L of RY17 solution with the optimum value of pH fixed at 3.0, and the iron concentration of 0.05 mM. According to the Fig. 5 and Table 2, the degradation of RY17 increases significantly with increasing the peroxydisulfate concentration from 0.1 to 2 mM. This phenomenon can be explained by the fact that more reactive radicals are generated for the degradation of RY 17 at higher S2O82− concentrations. Herein, by increasing the concentration of S2O82−, more S2O82− molecules could reach the surface of dye and subsequently react with Fe2+, which improves the degradation of RY17. But, further increase in S2O82− concentration to 1.0 mM decreased slightly the elimination of RY17. The higher peroxydisulfate concentration provides more sulfate radicals which act in favor of target contaminants degradation [32,33]. Therefore, 1.0 mM is considered as an optimum dose of S2O82− and applied for next experiments. The experiments with the catalyst were done for 3 times and the reproducibility was about 2.8%.
The parameter of BMG kinetic model of the degradation of RY 17at different S2O82−concentrations by: (a) S2O82−/UV and (b) S2O82−/Fe2+.
| [S2O82−] (mM) | BMG kinetic model | |||||||
|---|---|---|---|---|---|---|---|---|
| S2O82−/UV | S2O82−/Fe2+ | |||||||
| Y = ax + b | 1/m | 1/b | R² | Y = ax + b | 1/m | 1/b | R² | |
| 0.1 | 0.146x + 2.903 | 0.344 | 6.849 | 0.8257 | 0.201x + 0.183 | 5.464 | 4.975 | 0.9993 |
| 0.2 | 0.111x + 1.554 | 0.643 | 9.01 | 0.9054 | 0.171x + 0.219 | 4.566 | 5.847 | 0.9989 |
| 0.5 | 0.118x + 0.945 | 1.058 | 8.474 | 0.9656 | 0.143x +0.357 | 2.801 | 6.993 | 0.9939 |
| 1 | 0.101x + 0.586 | 1.706 | 9.901 | 0.9764 | 0.150x + 0.494 | 2.024 | 6.666 | 0.9958 |
| 2 | 0.103x + 0.494 | 2.024 | 9.708 | 0.9836 | 0.143x + 0.194 | 5.144 | 6.993 | 0.9979 |
So, it was noticed that any enhancement in the concentrations of applied S2O82− did not increase continuously the elimination of the contaminant due to the possibility of reaction of sulfate and hydroxyl radicals (Eq. (10)), the recombination of both sulfate and hydroxyl radicals (Eqs. (6) and (9)) and peroxydisulfate scavenging reactions (Eqs. (8) and (11)) [34]. However, at low concentration of S2O82−, an adequate number of SO4−• radicals cannot be produced, which contributes to low oxidation rate, and reduction of removal efficiency.
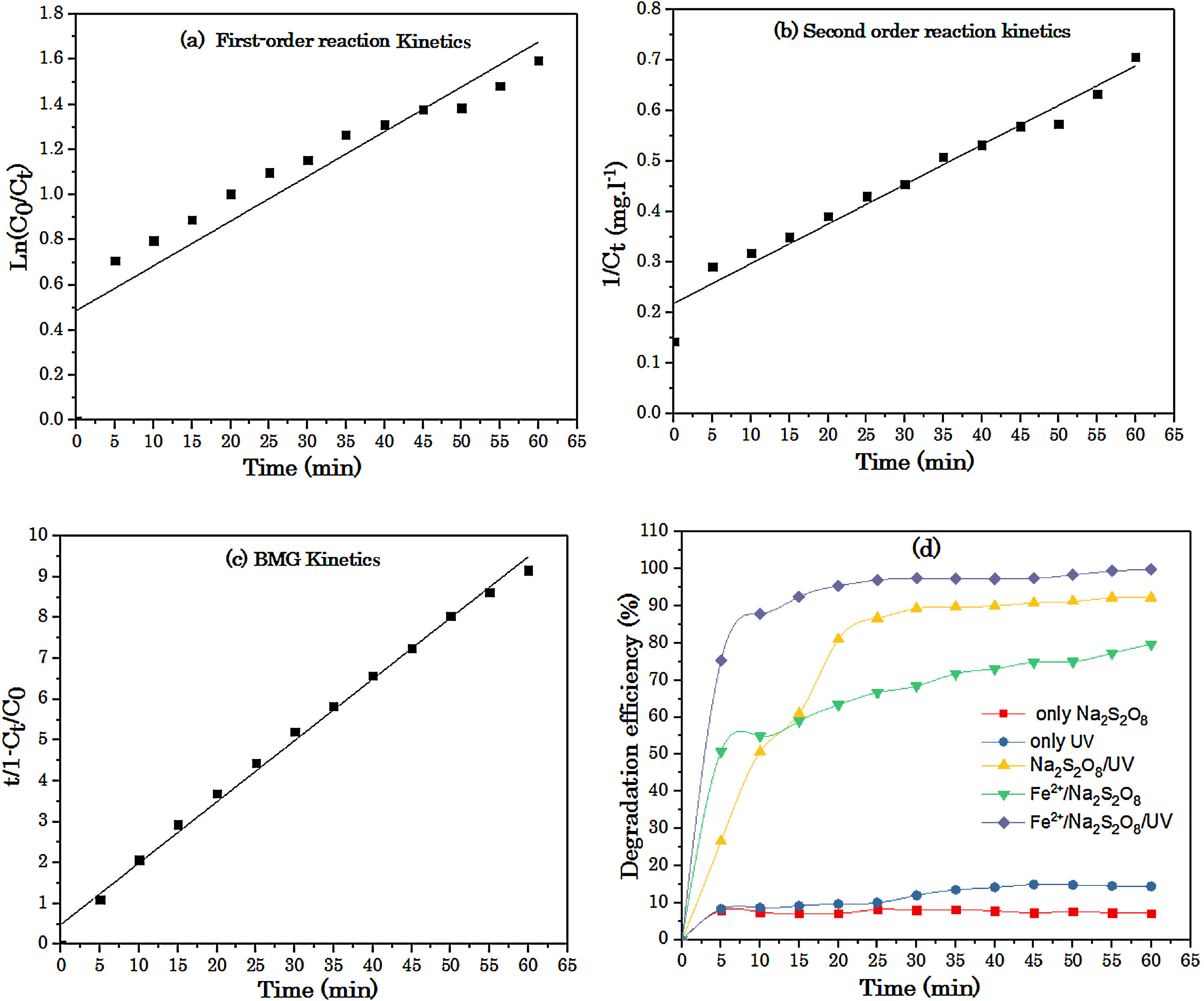
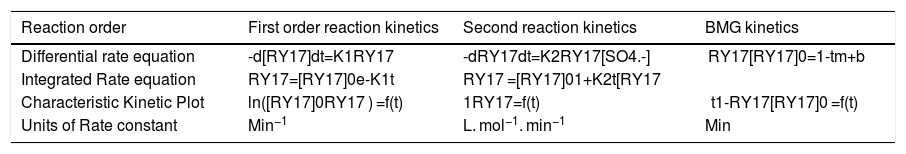
3.2Kinetic study at optimum conditionIn the kinetic study, first and second order reaction kinetics are used to study the degradation kinetic of the RY 17 by the S2O82−/Fe2+process. The obtained kinetic parameters are illustrated in Fig. 6(a)–(c). As can be seen in the figure, the BMG kinetics model shows a higher correlation coefficient (~0.99) compared to that based on the first-order (~0.83) and the second-order (~0.96). The results indicate that the BMG kinetics is the best model to describe the degradation of RY17 by the S2O82−/Fe2+process. The linear forms equations of first-order, second-order and BMG models are given in Table 3.The BMG mathematical model to simulate the reaction kinetics has proposed by Behnajady, Modirshahla and Ghanbery [35]. Previous studies reported that these results are consistent with the results of the decolonization of other contaminants in aqueous solution by Fenton process [36,37].
(a) First-order reaction kinetics, (b) second order reaction kinetics, (c) BMG kinetics, for degradation of RY17 by S2O82−/Fe2+process, and (d) Kinetics comparison between applied processes for RY17 removals from aqueous solution. [S2O82−] = 1 mM, [Fe2+] = 0.05 mM, [RY17] = 10 mg/L, pH = 3.
Linear forms equations of the first-order, second-order and BMG reaction kinetics.
| Reaction order | First order reaction kinetics | Second reaction kinetics | BMG kinetics |
|---|---|---|---|
| Differential rate equation | -d[RY17]dt=K1RY17 | -dRY17dt=K2RY17[SO4.-] | RY17[RY17]0=1-tm+b |
| Integrated Rate equation | RY17=[RY17]0e-K1t | RY17 =[RY17]01+K2t[RY17 | |
| Characteristic Kinetic Plot | ln([RY17]0RY17 ) =f(t) | 1RY17=f(t) | t1-RY17[RY17]0 =f(t) |
| Units of Rate constant | Min−1 | L. mol−1. min−1 | Min |
Where k1 and k2 represent the apparent kinetic rate constant of pseudo first and second-order kinetic model, respectively; (t) is the reaction time, [RY17] is the concentration of RY17 at time t, [RY17]0 is the initial concentration of RY17 at time t = 0, (m) and (b) are two constants characteristic relating to the reaction kinetics and oxidation capacity, and (k) is the observed degradation rate coefficient (Table 4).
Fig. 6(d) presents the operational experiments realized for RY 17 degradation using different processes: S2O82−, UV, S2O82−/UV, S2O82−/Fe2+ and S2O82−/Fe2+/UV. The optimum conditions for the experimental results are : pH = 3, the iron concentration is 0.05 mM, the S2O82− concentration is 1 mM and the RY17 concentration is 10 mg/L (see Fig. 6). Note that the degradation efficiencies of the individual processes (UV, S2O82−) are lower than those of the simultaneous processes, that can be associated with the lower oxidation potential of UV and peroxydisulfate and the lack of effectiveness of these to remove contaminants [25].
The obtained degradation efficiencies of RY17 by S2O82−, UV, Fe2+/S2O82−, S2O82− /UV and Fe2+/S2O82−/UV processes are, respectively, 6.2%, 9.6%, 63.3%, 81.0% and 95.4%, during 20 min of reaction. On the other hand, the better performance of the simultaneous processes can be attributed to the formation of reactive oxidizing specie radical SO4●− playing an important role in the oxidative catalysis of RY17. These results indicate that the peroxydisulfate molecules decomposed effectively in the presence of UV light rather than Fe2+. Furthermore, when iron ions, UV light and peroxydisulfate are utilized together, we can reach the highest degradation efficiency of RY17 (95.4%). This increase might be due to the presence of both activators (Fe2+ and UV) resulting in efficient decomposition of S2O82− and also to the presence of radical SO4●− in the solution [30]. In S2O82−/UV and S2O82−/Fe2+/UV process, both Fe2+ and UV and only UV light plays a significant role in the S2O82− decomposition to produce the free radical SO4●−. The results confirm that UV and Fe2+/UV have high potential in activation of peroxydisulfate molecules and have therefore an effective in the catalytic oxidation of azo dye via generation of reactive species. According to these results, the degradation efficiency of RY17 dye increases in the following order: S2O82−/Fe2+/UV > S2O82−/UV > S2O82−/Fe2+> UV > S2O82−.
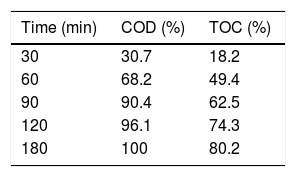
3.3The mineralization studyThe mineralization assessment was studied through COD and TOC analysis during the degradation process of RY17 in aqueous solution. In order to confirm the mineralization of RY17 using S2O82−/Fe2+/UV process, Table 6 present the results of COD and TOC analysis as a function of degradation time in optimum experimental conditions (0.05 mM iron dosage, 1 mM PDS dose, pH 3 and T = 25 °C). As listed in Table 5, around 100% of RY17 was degraded within 60 min. Using S2O82−/Fe2+/UV process, the mineralization percentage was 68.2 and 49.4% respectively for COD and TOC analysis and these percentages increase with degradation time. These results are probably caused by the specific oxidation pathway of the dye. Additionally, ecological toxicity of intermediate products could be avoided when the contact time was enough to mineralize the target pollutants into non-toxic CO2 and H2O by S2O82−/Fe2+/UV process.
3.4Comparative study of the degradation and mineralization of RY17 between two salts Na2S2O8 and K2S2O8The peroxydisulfate is normally available as a salt associated with ammonium, sodium, or potassium. Salari et al. [13] reported that potassium peroxydisulfate (K2S2O8) gives a better result in photo-oxidative removal of some organic materials than (NH4)2S2O8 showing instability due to the oxidation of the ammonium and ammonia by peroxydisulfate [38].
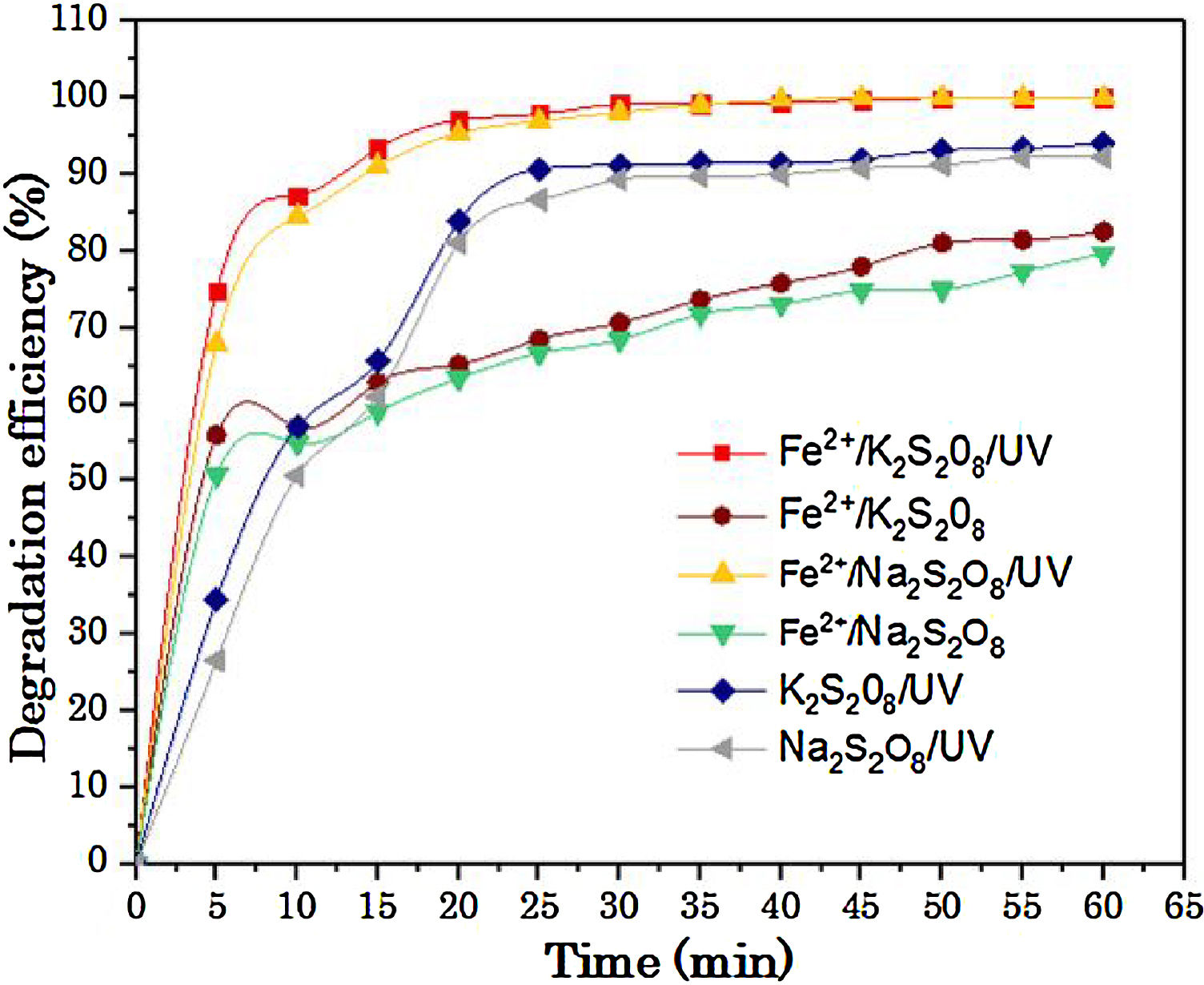
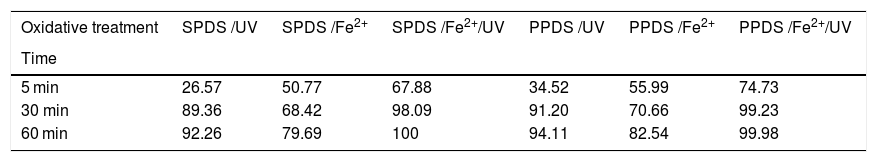
In addition, as reported by other researcher [39], the sodium peroxydisulfate is preferred for use in the field. Because, it is sufficiently stable at or below ambient temperature (25 °C), while it is stored in the solid form. With that, and the inadequacy of adding ammonia to water, K2S2O8 and Na2S2O8 are the best choice for using in the UV/peroxydisulfate oxidation process. Moreover, potassium peroxydisulfate and sodium peroxydisulfate are much less expensive than other oxidants such as hydrogen peroxide. From Fig. 7 and Table 6, the efficiency of K2S2O8/Fe2+and Na2S2O8/Fe2+processes increases rapidly with UV irradiations in the first minutes.
Comparison of the degradation efficiency (%) of RY17 between Na2S2O8 and K2S2O8.
| Oxidative treatment | SPDS /UV | SPDS /Fe2+ | SPDS /Fe2+/UV | PPDS /UV | PPDS /Fe2+ | PPDS /Fe2+/UV |
|---|---|---|---|---|---|---|
| Time | ||||||
| 5 min | 26.57 | 50.77 | 67.88 | 34.52 | 55.99 | 74.73 |
| 30 min | 89.36 | 68.42 | 98.09 | 91.20 | 70.66 | 99.23 |
| 60 min | 92.26 | 79.69 | 100 | 94.11 | 82.54 | 99.98 |
*PPDS: Potassium peroxydisulfate; *SPDS:Sodium peroxydisulfate.
The RY17 discoloration with K2S2O8 salt shows a strong degree of similarity with Na2S2O8 salt. Accordingly, there is no salt effect. RY 17 mineralization degree in S2O82−/Fe2+/UV process was studied using two salts Na2S2O8 and K2S2O8. In this regards, the oxidation experiments were carried out for 10 mg/L RY 17 concentrations in optimum experimental conditions (0.05 mM iron dosage, 1.0 mM PDS dose and pH 3).
3.5Comparison with the other advanced oxidation methodsAmong different methods, the advanced oxidation process is a promising treatment for the removal of different azo dyes, especially RY 17. As shown in Table 7, many researchers have surveyed the RY17removal in aqueous solution through various advance oxidation processes. In comparison to the other studies (Table 7), the UV light with iron ions as activators of peroxydisulfate process is an effective method for the removal of RY 17, and it provide good removal efficiency. Additionally, the advantages of this method such as moderate cost, high stability, and aqueous solubility make it more suitable
Summary of the degradation processes applied in treatment of RY 17.
| Method | Operational condition | Analytical method | Results | Reference |
|---|---|---|---|---|
| Fenton | - [Fe2+] = 0.005–0.15 Mm - [H2O2] = 0.01–0.2 mM - [RY17] = 10 mg/L - pH = 3 | UV-Vis | - RY 17 removal = 67.5% (20 min) | [40] |
| Photo-Fenton | - Black light (125 W) - [Fe2+] = 0.01 mM - [H2O2] = 0.1 mM - [RY17] = 10 mg/L - pH = 3 | UV-Vis, COD | - RY 17 removal = 89 % (20 min) - COD removal = 93.08% (120 min) | [41] |
| Electro-Fenton | - I = 60–100 Ma - [Fe2+] = 0.02–0.04 mM - [RY17] = 5–10 mg/L - [Na2SO4] = 50 mM - pH = 3 | UV-Vis, COD, HPLC | - RY 17 removal = 87% (20 min) - COD removal = 89% (120 min) | [42] |
| Anodic Oxidation | - I = 100 Ma - [RY17] = 5 mg/L - [Na2SO4] = 50 mM - pH = 3 | UV-Vis | - RY 17 removal = 94 % (60 min) | [41] |
| PDS/UV process | - Black light (125 W) - [S2O82−] = 0.1–2 mM - [RY17] = 10 mg/L - pH = 3–10 | UV-Vis | RY 17 removal = 81 % (20 min) | Present study |
| Fe2+/PDS/UV Process | - Black light (125 W) - [Fe2+] = 0.005–0.2 mM - [S2O82−] = 0.1–2 mM - [RY17] = 10 mg/L -pH = 3–10 | COD, UV-Vis, TOC | RY 17 removal = 95.4% (20 min) COD removal = 96.1% (120 min) TOC removal = 74.3% (120 min) | Present study |
In this study, Peroxydisulfate activated using Fe2+ an ion was evaluated as a process for RY17 removal from aqueous solution. The obtained results indicate that S2O82−/Fe2+/UV process provide a good performance compared to S2O82−/UV for RY17 degradation. The degradation efficiency of S2O82−/Fe2+, S2O82−/UV and S2O82−/Fe2+/UV processes was respectively 63.3%, 81.0% and 95.4% within 20 min of reaction. RY17 degradation was found to be affected by changing some parameters such as pH of the solution, initial concentrations of Fe2+ and S2O82− with UV. The optimum conditions for a high RY17 removal were determined at pH = 3, [Fe2+] = 0.05 mM, [S2O82−] = 1 mM, [RY17] = 10 mg/L, and room temperature. Although S2O82− is essential for the generation of the sulfate radicals SO4−•, a very high amount of S2O82− and Fe2+ could reduce degradation efficiency, which may be due to the scavenging effect.
The kinetic studies indicated that the degradation of RY17 was well described by the BMG kinetics models. On other hand, the comparative study of the RY17 degradation efficiency between two salts Na2S2O8 and K2S2O8 shows similar results, and a total mineralization degree was obtained at the optimum conditions. Consequently, S2O82−/Fe2+/UV process can be used for the treatment of water discharge containing azo dyes such as RY17; this process is very effective and less expensive than conventional processes.





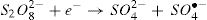
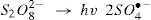
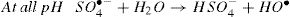
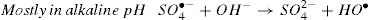
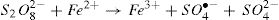
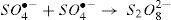
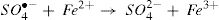
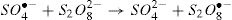
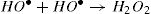
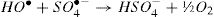
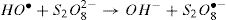
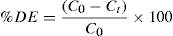
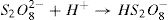
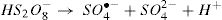
![Effect of initial pH on the degradation of RY17 by: (a) S2O82−/UV, (a1) kinetics of degradation of RY17and (b) S2O82−/Fe2+. [RY17] = 10 mg/L; [S2O82−] = 0.1 mM; [Fe2+] = 0.01 mM. Effect of initial pH on the degradation of RY17 by: (a) S2O82−/UV, (a1) kinetics of degradation of RY17and (b) S2O82−/Fe2+. [RY17] = 10 mg/L; [S2O82−] = 0.1 mM; [Fe2+] = 0.01 mM.](https://static.elsevier.es/multimedia/26036363/0000003000000003/v1_201811230645/S2603636318300356/v1_201811230645/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
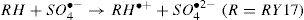
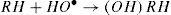
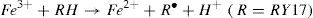
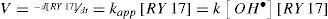
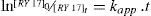
![(a) Determination of order and apparent constant degradation of RY 17 by the S2O82− /Fe2+ process, (b) Evolution of apparent kinetic constant and Efficiency degradation of RY 17 as a function of Fe2+ concentration. [S2O82−] = 0.1 mM, [RY17] = 10 mg/L, pH = 3.0. (a) Determination of order and apparent constant degradation of RY 17 by the S2O82− /Fe2+ process, (b) Evolution of apparent kinetic constant and Efficiency degradation of RY 17 as a function of Fe2+ concentration. [S2O82−] = 0.1 mM, [RY17] = 10 mg/L, pH = 3.0.](https://static.elsevier.es/multimedia/26036363/0000003000000003/v1_201811230645/S2603636318300356/v1_201811230645/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Degradation of RY17 by: (a) S2O82−/UV and (b) S2O82−/Fe2+ at different S2O82− concentrations. [RY17] = 10 mg/L; Fe2+ = 0.05 Mm. Degradation of RY17 by: (a) S2O82−/UV and (b) S2O82−/Fe2+ at different S2O82− concentrations. [RY17] = 10 mg/L; Fe2+ = 0.05 Mm.](https://static.elsevier.es/multimedia/26036363/0000003000000003/v1_201811230645/S2603636318300356/v1_201811230645/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![(a) First-order reaction kinetics, (b) second order reaction kinetics, (c) BMG kinetics, for degradation of RY17 by S2O82−/Fe2+process, and (d) Kinetics comparison between applied processes for RY17 removals from aqueous solution. [S2O82−] = 1 mM, [Fe2+] = 0.05 mM, [RY17] = 10 mg/L, pH = 3. (a) First-order reaction kinetics, (b) second order reaction kinetics, (c) BMG kinetics, for degradation of RY17 by S2O82−/Fe2+process, and (d) Kinetics comparison between applied processes for RY17 removals from aqueous solution. [S2O82−] = 1 mM, [Fe2+] = 0.05 mM, [RY17] = 10 mg/L, pH = 3.](https://static.elsevier.es/multimedia/26036363/0000003000000003/v1_201811230645/S2603636318300356/v1_201811230645/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Comparison of degradation efficiency of RY17 between two salts Na2S2O8 and K2S2O8. [RY17] = 10 mg/L, [S2O82−] = 1 mM, [Fe2+] = 0.05 mM and pH = 3. Comparison of degradation efficiency of RY17 between two salts Na2S2O8 and K2S2O8. [RY17] = 10 mg/L, [S2O82−] = 1 mM, [Fe2+] = 0.05 mM and pH = 3.](https://static.elsevier.es/multimedia/26036363/0000003000000003/v1_201811230645/S2603636318300356/v1_201811230645/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

