COVID 19 & vaccines: Development and practice
More infoThe new coronavirus is still a life-threatening menace, because of its changing nature and capacity to produce many mutations to bypass the immune system. The vaccination is the first effective weapon against COVID-19.
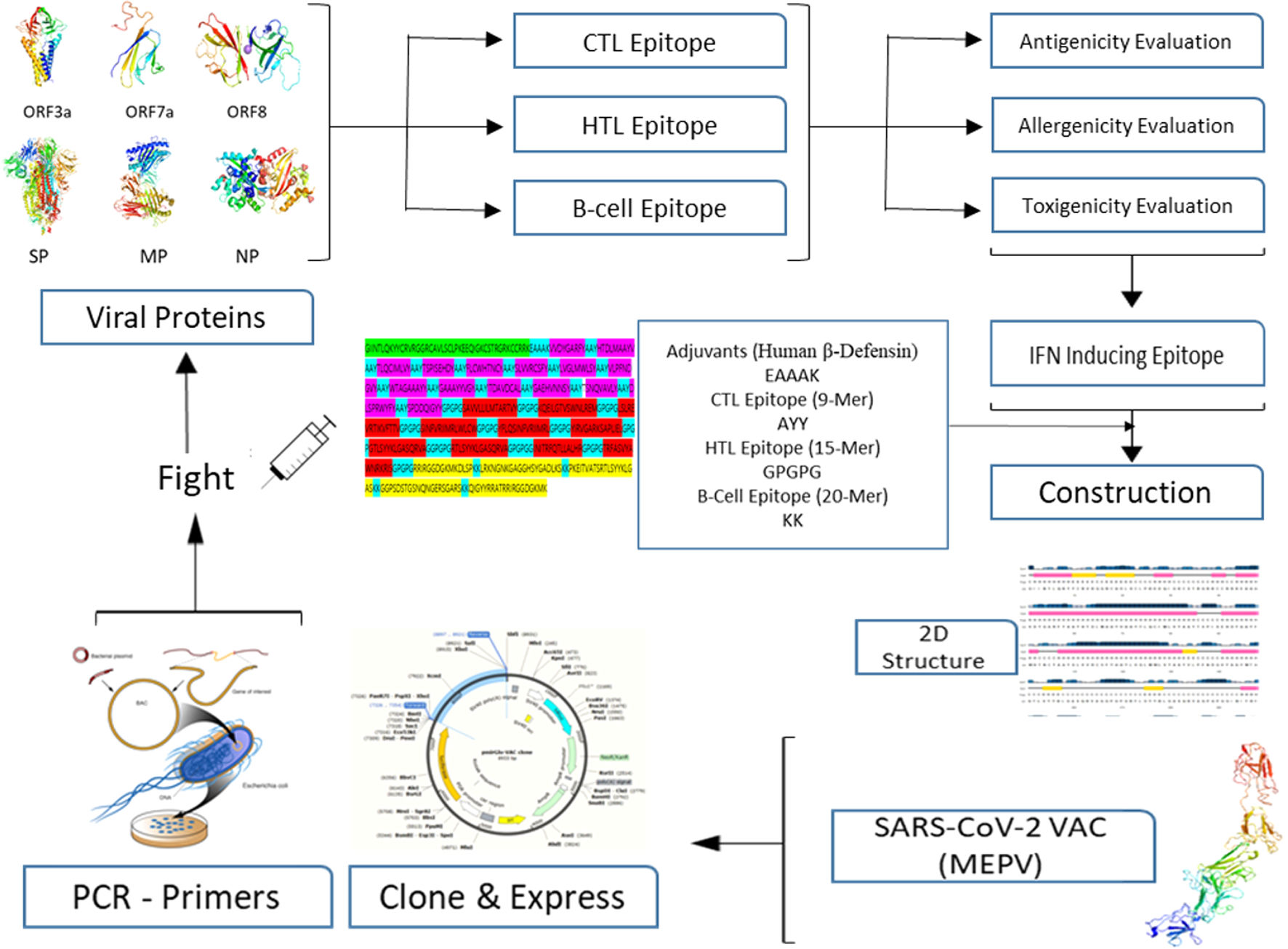
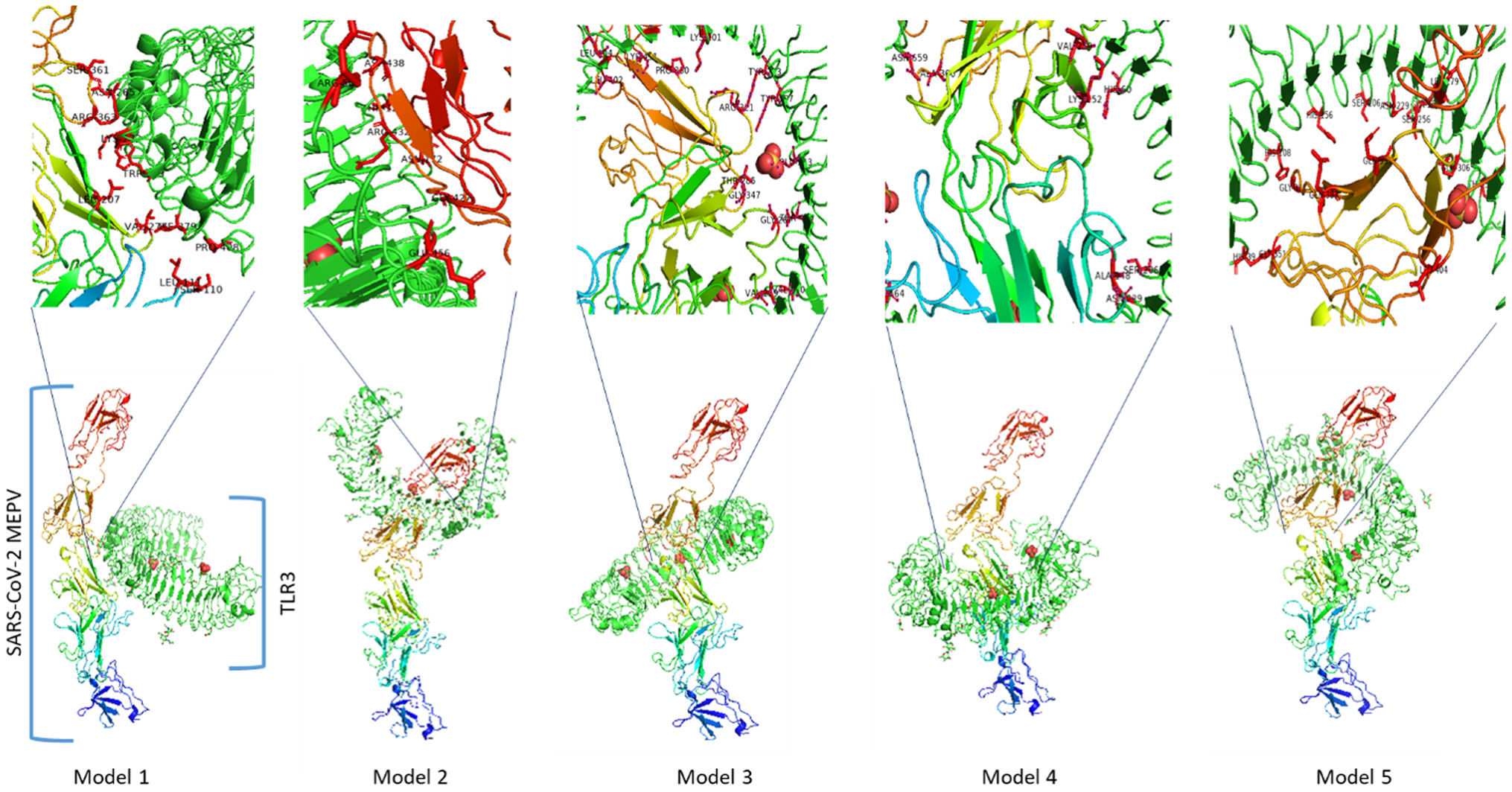
AimThe study's goal was to design a multi-epitope peptide vaccine (MEPV) for a mix of Omicron and Delta Coronavirus strains using immuno-chemoinformatics tools.
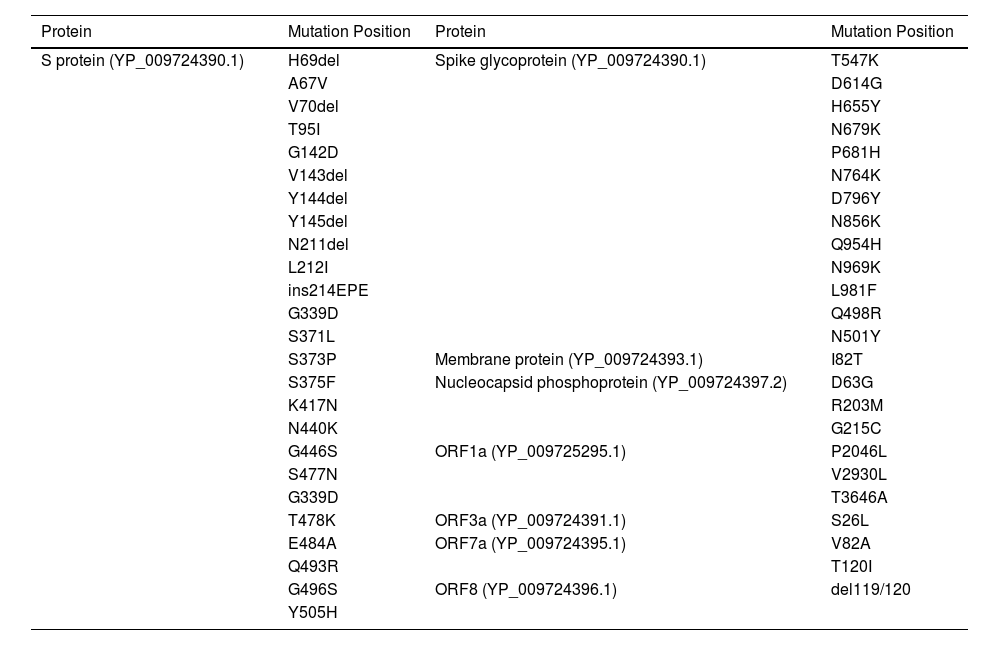
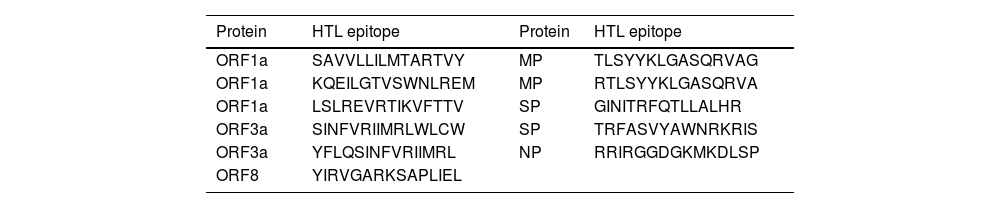
MethodsTo create the vaccine epitopes, seven proteins from the Omicron and Delta coronavirus strains were selected (ORF1a, ORF3a, surface protein, membrane protein, ORF7a, ORF8, and nucleocapsid protein). Antigenicity, toxicity, and allergenicity of the epitopes were evaluated.
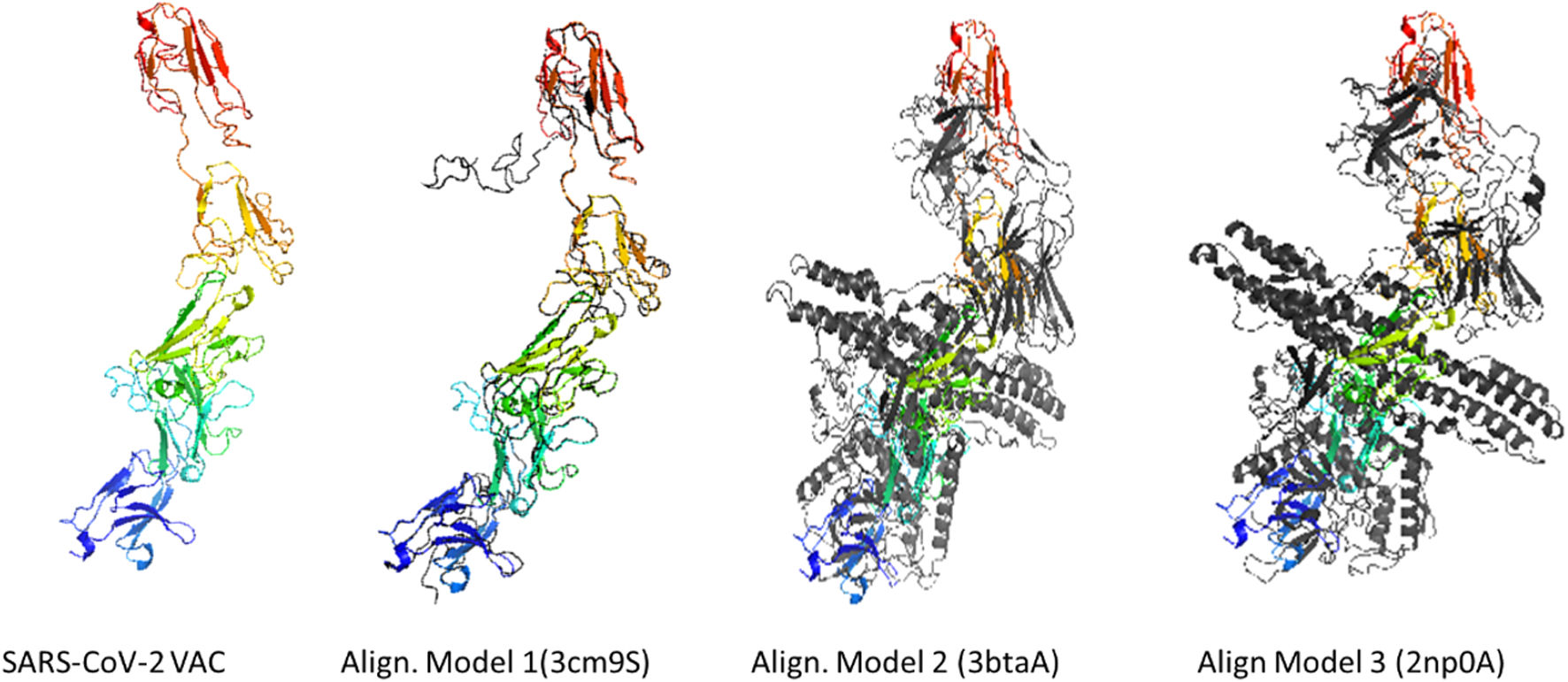
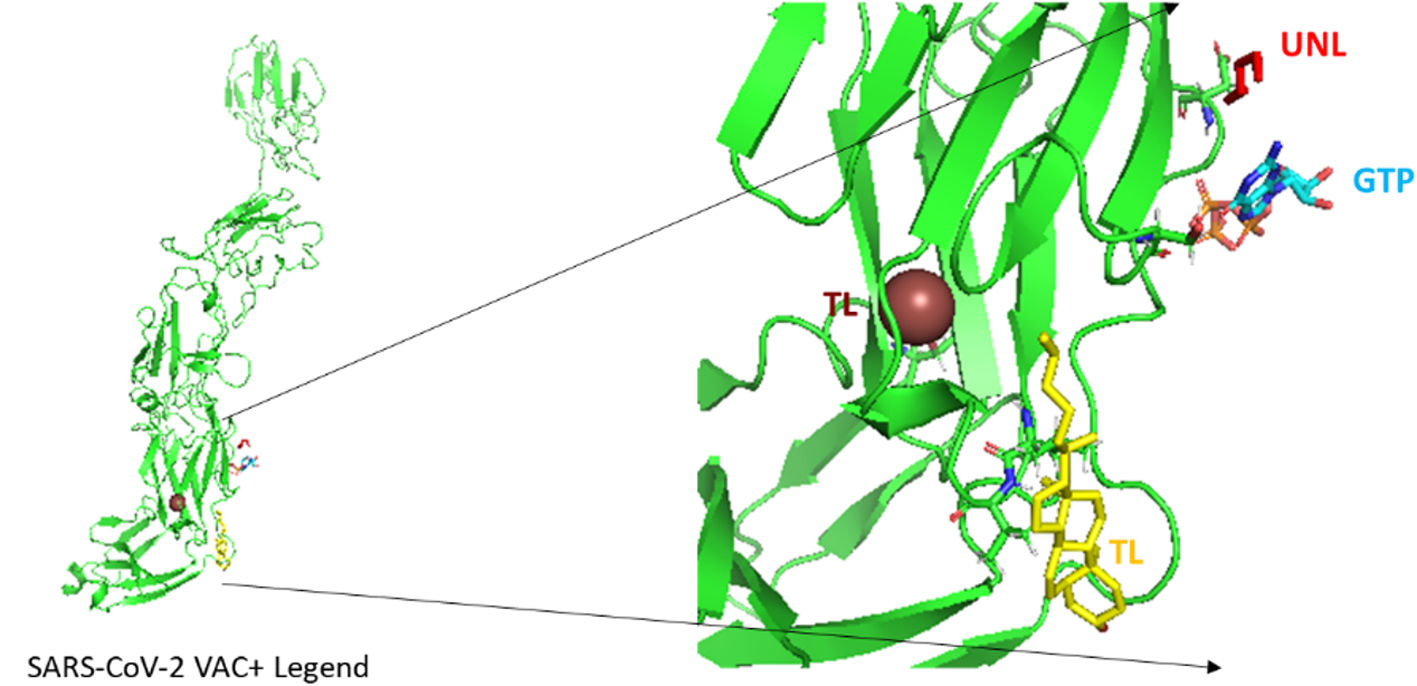
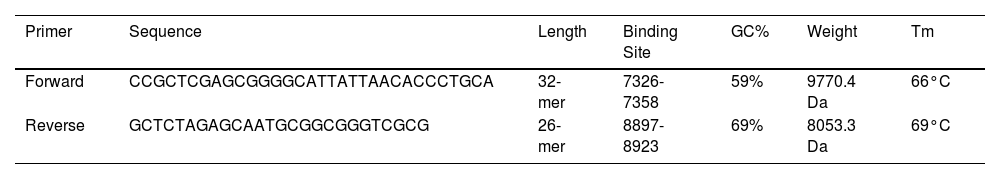
ResultsThe designed vaccine is made up of 534 amino acids that are homogeneous, antigenic, and non-toxic. Sticky restriction enzymes (XhoI and XbaI) were used to incorporate the MEPV into the pmirGLO luciferase vector. SnapGene server was used to create primers for PCR testing. Developing the MEPV is a terrific cost-effective strategy. The created MEPV's physiochemical properties have been determined to be basic, hydrophobic, and stableImmunogenicity and immune response profiles of the developed vaccine candidate were better assessed using in silico immunological simulations.
ConclusionsWe advocate moving the built vaccine to the biological validation step, where it may test our findings using appropriate model organisms.
el nuevo coronavirus sigue siendo una amenaza mortal debido a su naturaleza cambiante y su capacidad de producir muchas mutaciones para eludir el sistema inmunitario. La vacunación es la primera arma eficaz contra el COVID-19.
Objetivoel objetivo del estudio era diseñar una vacuna peptídica multiepítopo (MEPV) para una mezcla de cepas de Omicron y Delta Coronavirus utilizando herramientas inmunoquimioinformáticas. Métodos: Para crear los epítopos de la vacuna, se seleccionaron siete proteínas de las cepas de coronavirus Omicron y Delta (ORF1a, ORF3a, proteína de superficie, proteína de membrana, ORF7a, ORF8 y proteína de nucleocápside). Se evaluaron la antigenicidad, toxicidad y alergenicidad de los epítopos.
ResultadosLa vacuna diseñada está compuesta por 534 aminoácidos que son homogéneos, antigénicos y no tóxicos. Se usaron enzimas de restricción pegajosas (XhoI y XbaI) para incorporar el MEPV en el vector de luciferasa pmirGLO. El servidor SnapGene se utilizó para crear cebadores para las pruebas de PCR. Desarrollar el MEPV es una excelente estrategia rentable. Se ha determinado que las propiedades fisicoquímicas del MEPV creado son básicas, hidrofóbicas y estables. Se utilizaron simulaciones inmunológicas in silico para evaluar mejor la inmunogenicidad y el perfil de respuesta inmunitaria de la vacuna candidata generada.
ConclusionesAbogamos por pasar la vacuna construida al paso de validación biológica, donde puede probar nuestros hallazgos utilizando organismos modelo apropiados.