We are currently contending with a significant consequence of the SARS-CoV-2 virus pandemic, termed post-COVID syndrome, which has escalated into another global crisis. Conservative estimates suggest that at least 76 million individuals worldwide are grappling with this condition, based on a projected 10% incidence rate among those previously infected. Daily, the tally of cases associated with this syndrome continues to mount. The emergence of long COVID has spurred intensified research endeavors, yet the current volume of ongoing studies falls short, and patient prognosis remains largely unchanged. Consequently, there is an urgent need for enhanced research methodologies to tackle this emerging condition effectively and to mount a robust response to the crisis. Management of post-COVID syndrome primarily revolves around symptomatic relief and rehabilitation strategies aimed at ameliorating the most prevalent symptoms that profoundly impact patients’ quality of life, such as fatigue, dyspnea, and loss of taste and smell. Despite sustained investigative efforts, specific treatments, including steroid therapies, have thus far failed to yield clinically significant outcomes. In this review, we delve into the multifaceted nature of post-COVID syndrome, exploring its impact, current management strategies, and the imperative for advancing research to better understand and address this pressing global health challenge.
Actualmente estamos enfrentando una importante secuela de la pandemia del virus SARS-CoV-2, el síndrome post-COVID, y esto representa otra crisis global, se estima que actualmente al menos 76 millones de personas en todo el mundo tienen esta condición, esto basado en una incidencia conservadora del 10% de los casos de personas infectadas en el mundo con el nuevo coronavirus, pero los casos aumentan diariamente. Con el impacto que está causado el COVID prolongado, las investigaciones se han acelerado, pero aun los estudios son insuficientes, y no se ha logrado mejorar el pronóstico de estos pacientes. Por lo que, para tratar de asegurar una adecuada respuesta a esta crisis, se requieren investigaciones con diseños adecuados que permitan enfocar mejor esta nueva condición. El tratamiento de esta afección está basado en el manejo sintomático y en la rehabilitación de los síntomas más frecuentes y que han afectado la percepción de una buena calidad de vida de los pacientes como la fatiga, la disnea y la pérdida de olfato y gusto, ya que tratamientos específicos como esteroides no han demostrado resultados clínicamente significativos.
On May 5th, 2023, the Emergency Committee of the World Health Organization (WHO) declared the end of the SARS-CoV-2 pandemic, which began on January 30th, 2020. It is estimated that this international health emergency claimed the lives of almost seven million people over the span of 3 years, but the statistic could be much higher. Despite the pandemic being over, COVID-19 continues to be a threat to public health, as cases of virus infection continue to be reported in the world every day. The emergence of new variants increases the likelihood of new outbreaks, which in turn increase in the number of cases and the number of deaths.1
The pandemic prompted many changes in our daily lives.2 There is a high number of individuals that continue to face psychological, emotional, and economic distress following the establishment of control measures such as quarantines, border closures, school closures, among others.3 Studies report a high prevalence of symptoms of depression, anxiety, and stress associated with the COVID-19 pandemic.4
Following recovery from acute COVID-19, a proportion of patients experience long-term symptoms, which may be different from the symptoms of the initial episode of COVID-19.5 Throughout the pandemic, the sequelae of SARS-CoV-2 infection have received different terms, including long COVID, post-COVID-19 condition, post-COVID syndrome, post-acute sequelae of SARS infection, or post-acute COVID-19 syndrome (PACS).6
Patients with more severe manifestations of the SARS-CoV-2 disease spectrum are those who subsequently develop post-COVID syndrome. The incidence of this condition varies in studies, but it is estimated that post-COVID syndrome is present in 10–30% of the patients who were not hospitalized for the initial infection, in 50–70% of hospitalized patients,7,8 and in 10–12% of those vaccinated.9,10
Patients with PACS suffer long-term effects on various body systems, such as the pulmonary, cardiovascular, and nervous systems. Additionally, many patients exhibit different psychological effects that affect their quality of life.11 The syndrome encompasses multiple adverse outcomes such as the onset of new conditions, including cardiovascular, thrombotic, and cerebrovascular diseases,12 type 2 diabetes,13 and dysautonomia.14 A significant proportion of patients are not able to return to work15 and require an increase in medical care. The medical cost of treating these sequelae of SARS-CoV-2 is still incalculable.16
The etiology of this syndrome remains undetermined. Nonetheless, researchers have proposed various hypotheses, including persistent immune dysregulation, continued presence of SARS-CoV-2 within tissues, reactivation of latent viruses such as herpesvirus, microbiota dysregulation, autoimmunity, endothelial dysfunction, and neurological effects of the virus.17–20 Considering the diverse clinical presentations of post-COVID syndrome, these differing pathophysiological mechanisms likely correspond to each affected system.
DefinitionThe persistence of symptoms following the acute phase of SARS-CoV-2 infection has been defined according to different terms, including long COVID, post-COVID-19 condition, post-COVID syndrome, post-acute sequelae of SARS infection, or post-acute COVID-19 syndrome (PACS).6
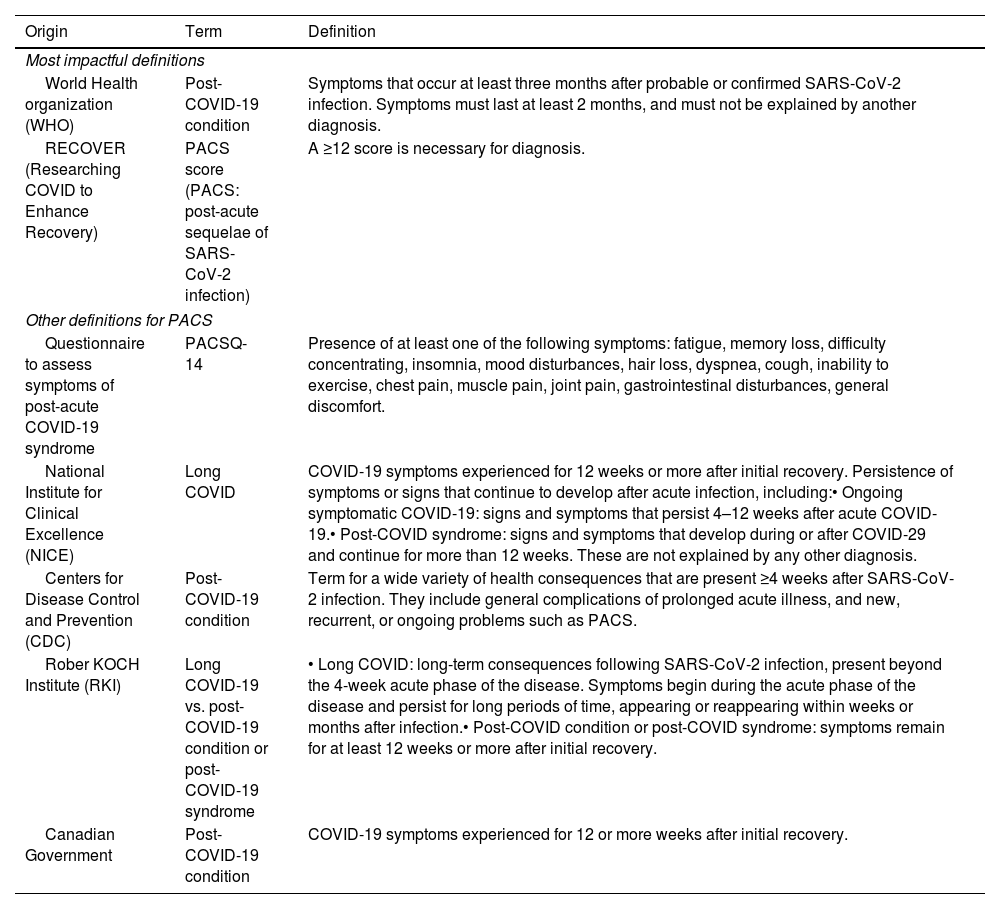
The wide variety of symptoms has resulted in different definitions for this condition (Table 1).21–23
Long COVID definitions.
| Origin | Term | Definition |
|---|---|---|
| Most impactful definitions | ||
| World Health organization (WHO) | Post-COVID-19 condition | Symptoms that occur at least three months after probable or confirmed SARS-CoV-2 infection. Symptoms must last at least 2 months, and must not be explained by another diagnosis. |
| RECOVER (Researching COVID to Enhance Recovery) | PACS score (PACS: post-acute sequelae of SARS-CoV-2 infection) | A ≥12 score is necessary for diagnosis. |
| Other definitions for PACS | ||
| Questionnaire to assess symptoms of post-acute COVID-19 syndrome | PACSQ-14 | Presence of at least one of the following symptoms: fatigue, memory loss, difficulty concentrating, insomnia, mood disturbances, hair loss, dyspnea, cough, inability to exercise, chest pain, muscle pain, joint pain, gastrointestinal disturbances, general discomfort. |
| National Institute for Clinical Excellence (NICE) | Long COVID | COVID-19 symptoms experienced for 12 weeks or more after initial recovery. Persistence of symptoms or signs that continue to develop after acute infection, including:• Ongoing symptomatic COVID-19: signs and symptoms that persist 4–12 weeks after acute COVID-19.• Post-COVID syndrome: signs and symptoms that develop during or after COVID-29 and continue for more than 12 weeks. These are not explained by any other diagnosis. |
| Centers for Disease Control and Prevention (CDC) | Post-COVID-19 condition | Term for a wide variety of health consequences that are present ≥4 weeks after SARS-CoV-2 infection. They include general complications of prolonged acute illness, and new, recurrent, or ongoing problems such as PACS. |
| Rober KOCH Institute (RKI) | Long COVID-19 vs. post-COVID-19 condition or post-COVID-19 syndrome | • Long COVID: long-term consequences following SARS-CoV-2 infection, present beyond the 4-week acute phase of the disease. Symptoms begin during the acute phase of the disease and persist for long periods of time, appearing or reappearing within weeks or months after infection.• Post-COVID condition or post-COVID syndrome: symptoms remain for at least 12 weeks or more after initial recovery. |
| Canadian Government | Post-COVID-19 condition | COVID-19 symptoms experienced for 12 or more weeks after initial recovery. |
The WHO definition was formulated through a Delphi methodology involving an international panel comprising 265 patients, researchers, and WHO officials. This condition is frequently characterized by symptoms such as fatigue, dyspnea, cognitive impairment, and other manifestations that impede the patient's ability to perform daily activities. These symptoms may manifest following the initial recovery from an acute episode of COVID-19 or persist beyond the acute phase. Additionally, they may exhibit fluctuations or relapses over time.22 The publication of this definition acknowledged the potential for modification in light of new research findings.
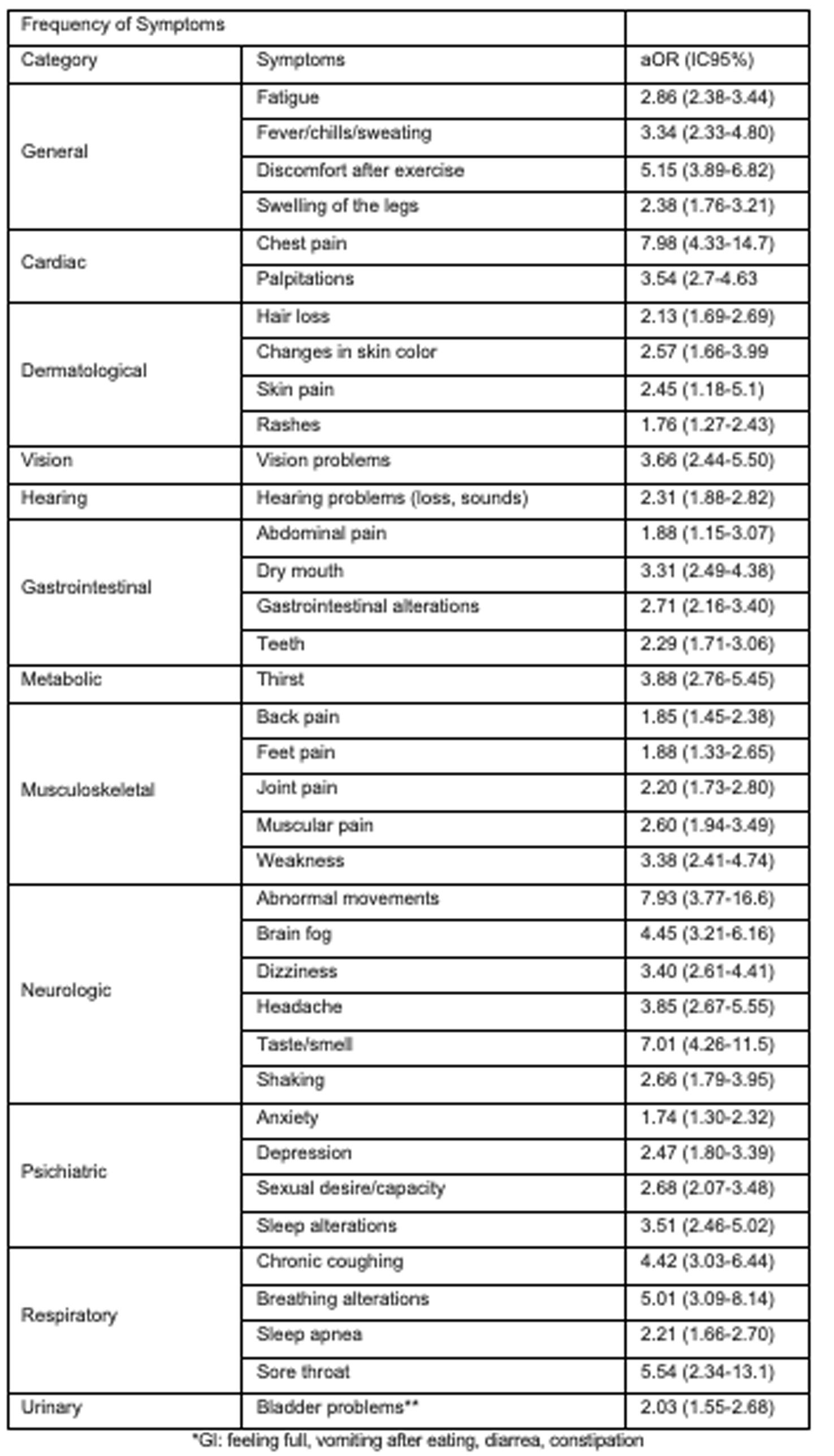
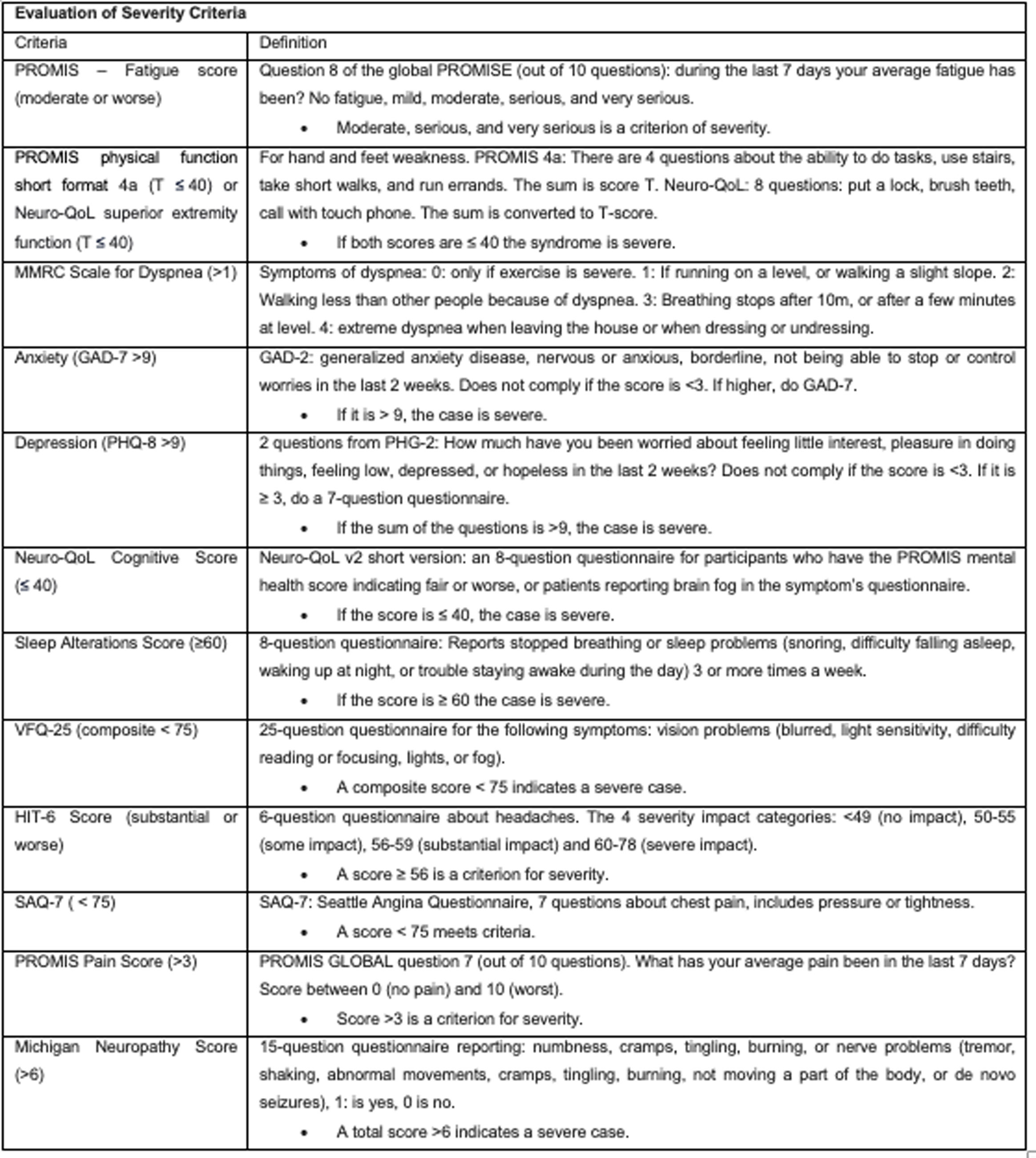
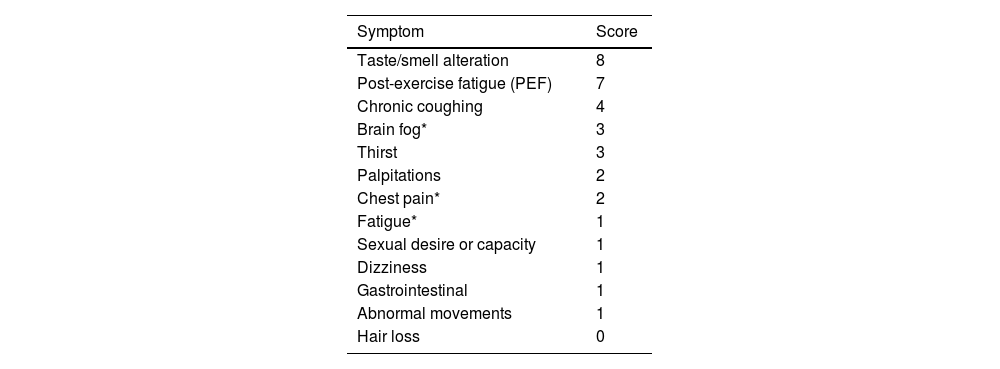
Recently, a new definition of post-acute sequelae of SARS-CoV-2 infection (PACS) was introduced, based on symptom descriptions within a prospective cohort study.23 Out of the 44 symptoms assessed, those with a prevalence of 2.5% or higher were considered. Adjusted odds ratios (aOR) were calculated using weighted logistic regression based on infection status (see Appendix 1). PACS identification was performed using LASSO (least absolute shrinkage and selection operator) with balanced weights. Each symptom was assigned a score derived from estimated coefficients (dividing the log OR by 0.10 and rounding to the nearest integer), and participants received a cumulative score based on the sum of symptoms. Those surpassing the threshold were classified as PACS-positive, while those below were considered nonspecific for PACS. The LASSO method identified 12 symptoms, each assigned a score ranging from 1 to 8. The optimal threshold for the PACS score was determined as ≥12. Although this threshold and methodology represent a significant advancement in PACS detection, ongoing updates and adjustments may still be necessary (see Table 2 and Appendix 2).
Establishing a universal definition for this syndrome is imperative for accurate diagnosis, effective research, and the development of targeted treatments.
PrevalenceThe epidemiology of this condition varies between studies, given differences in region, population type, follow-up time, identification, and reporting of cases. Systematic reviews and meta-analyses have determined a global prevalence of 43%. This prevalence differs between hospitalized and non-hospitalized patients (54% and 34%, respectively). The prevalence is generally higher in women (49%) than in men (37%). The Asian continent has the highest prevalence for this syndrome (51%).24 Other studies report that 80% of people with a confirmed diagnosis of COVID-19 persist with at least one alteration in imaging, laboratory samples, and the presence of signs and/or symptoms.25
There have been many risk factors associated with the development of this condition. Tsampasian et al. published a systematic literature review analyzing 41 studies with a total of 860,783 patients to determine risk factors associated with PACS. Female sex, adulthood, high body mass index (BMI), and exposure to tobacco smoke were the main factors associated with persistent symptoms 3 months or more after acute infection.26 In addition to identifying similar risk factors in their meta-analysis, Maglietta et al. found that the degree of severity of acute infection is factor directly related to the onset of PACS. In the same review, Tsampasian et al. identify that two doses of vaccination against SARS-CoV-2 has a lower risk of developing PACS compared to patients without a vaccination schedule (OR 0.57; 95% CI 0.43–0.76).27
Aside from the factors directly associated with patients, viral conditions also seem to influence the onset of post-COVID syndrome. In their observational study, Thaweethai et al. identified that the persistent symptoms were significantly more frequent in patients with pre-Omicron infections. This observation gave rise to the hypothesis that different viral variants have a relationship with the development of this sequel.23
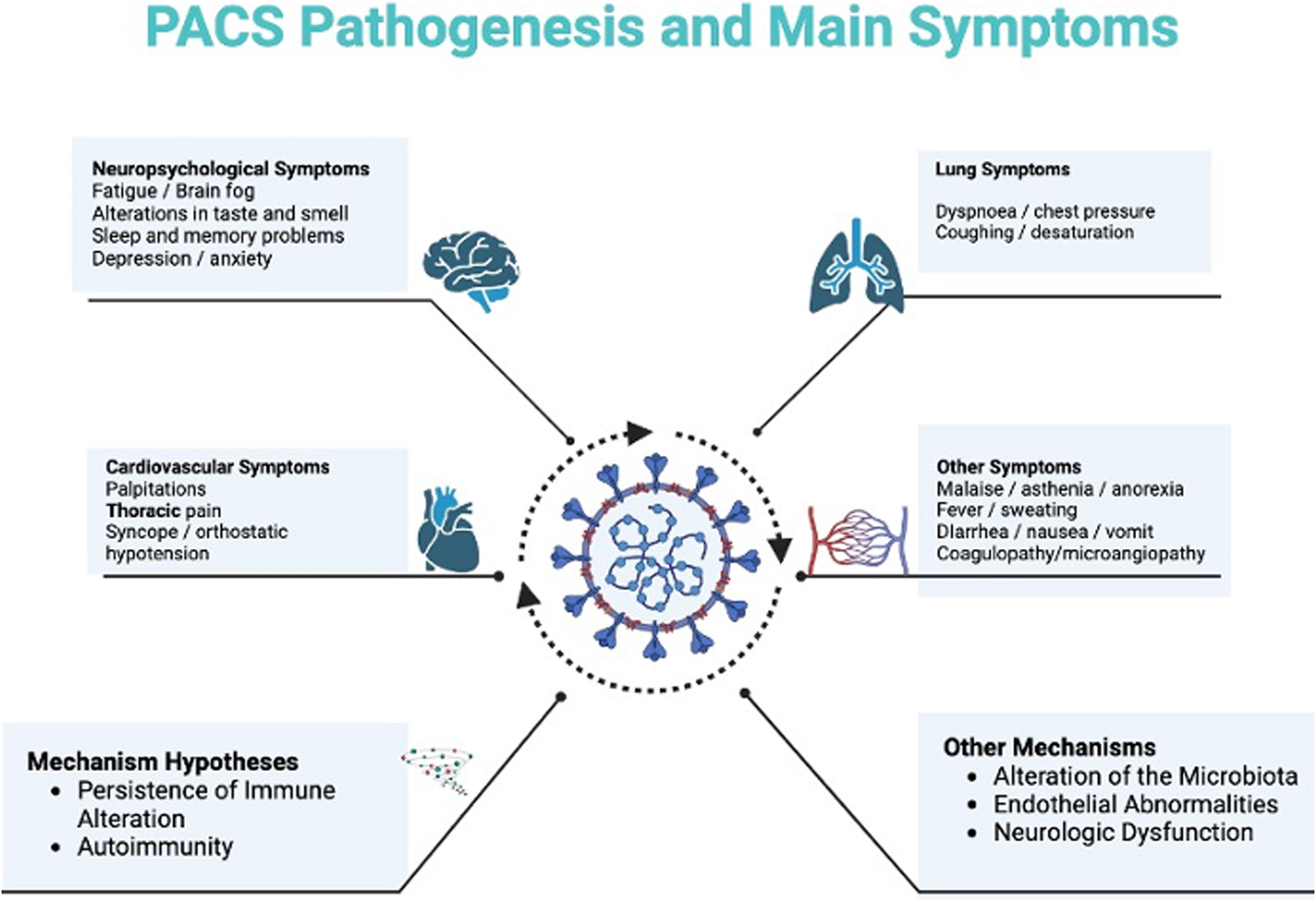
PathophysiologyThere are several theories aiming to offer a plausible explanation for the emergence of this complex condition. Among them, the theory concerning the alteration of the immune response seems to be pivotal. Other theories, such as autoimmunity and the role of microbiota, are also noteworthy (see Fig. 1). This review will delve into several of these theories.
Alteration of the immune responseDuring COVID-19, the inadequate innate immune response (cytokine storms) and the impairment of the adaptive immune response (alteration of T and B cells) play a very important role in the systemic manifestations and variable spectrum of disease, explaining the high mortality and organ dysfunction witnessed in severe cases.21 One of the strongest hypotheses for the cause of PACS is the persistence of this altered immune response,28 which works as a continuum, leading to a sustained alteration of the system and organ damage.
To the date, few studies have evaluated the persistence of elevated cytokines and the alteration of T and B cells in the etiology of PACS. Persistence of the altered immune response has been found in some studies,20,28–30 but not in others.31
Elevated levels of cytokines IL-1B, IL-6, and TNF-α have been found to persist in patients for long periods of time.32 IL-6 and TNF-α are proinflammatory cytokines that contribute to the recruitment, activation, and differentiation of leukocytes, as well as the maturation of B cells and the expansion of T helper cells. Peluso et al. evaluated how this immune response could be related to the sequelae in patients with SARS-CoV-2 infection. His team evaluated 121 volunteer patients confirmed by positive PCR tests. Out of the 121, 73 patients reported persistent symptoms. Researchers found that those 73 patients presented significantly higher levels of TNF-α and IP-10. Similarly, IL-6 levels were 29% higher during early recovery and 44% higher in late recovery, compared to patients without subsequent symptoms. The study also found that patients with severe post-COVID syndrome (exhibiting 25% more persistent symptoms) presented higher levels of IL-6 than those with fewer symptoms.30
Other studies have shown that an insufficient immune response with inadequate or no antibody production in the acute phase of COVID-19 can be a predictor of the development of PACS. This was described by García-Abellán et al., in a prospective longitudinal study of 146 COVID-19 patients. According to the study's COVID-19 Clinical Symptom Questionnaire (CSQ), patients exhibiting low peak IgG levels at 2- and 6-months post-infection had worse clinical outcomes than those with normal IgG levels.33
However, the humoral immune response is only part of the hypotheses behind these inflammatory mechanisms. In a study published by Patterson et al., it was demonstrated that patients with PACS symptoms had a 15-month-post-infection elevation of non-classical monocytes (CD14 dim CD16+) and intermediate monocytes (CD14+ and CD16+) compared to healthy controls. These monocytes have been implicated in maintaining the integrity of the blood–brain barrier as well as in phagocytosis against viral insults.34
T lymphocytes can assume a different cytokine profile, leading to the appearance of phenotypic and functional defects that can limit the response of CD8+ lymphocytes and the viral clearance to chronic infection.35 Autopsy studies suggest that the perivascular invasion of CD68+ macrophages and CD8+ lymphocytes, alongside the marked activation of astroglia, could perpetuate the disruption of the blood–brain barrier.36
Afrin et al. propose that mast cell activation plays a fundamental role in this inflammatory cascade. According to their studies, mast cell activation syndrome could be the cause of the hyperproduction of cytokines and the recruitment of other inflammatory cells.37
AutoimmunityAutoimmunity plays an increasingly important role in the pathophysiology of this condition. Since 2020, Zhang et al. described the presence of anticardiolipin antibodies as well as antibodies against B2-glycoprotein in patients with COVID-19 developing coagulopathy. These findings indicated the participation of autoimmunity in the infection.38 Likewise, Sacchi et al. demonstrated the development of autoimmunity in a cohort of 40 patients with the presence of ANA, ASCA, ANCA and anticardiolipins.39
The presence of autoantibodies has been shown to be higher in patients with PACS than in the general population, as demonstrated by Dr. Rojas’ clinical and serological study, taking place in Bogotá (Colombia) from March to May 2021. The study included 100 non-vaccinated patients exhibiting persistent symptoms four weeks post-SARS-CoV-2 infection. The study found that the presence of autoantibodies correlated with anti-SARS-CoV-2 antibodies, suggesting that this activation contributes to the appearance of autoimmunity.40
Anaya et al. demonstrated that latent autoimmunity (presence of autoantibodies without clinical symptoms or without meeting classification criteria for autoimmune disease) was present in a group of COVID-19 hospitalized patients with high disease severity.41 This polyautoimmunity was proven to persists during PACS (it was not transient).19
MicrobiotaAnother theory that supports PACS is related to the intestinal microbiota. In the last decade, multiple studies have mentioned the importance of the intestinal microbiota in health and disease.42
The role of the intestinal microbiota in the initiation, adaptation, and regulation of the immune response has been well established.43 However, recent studies have focused on determining the role of the lung microbiota (an organ that was previously considered sterile and aseptic) in diseases such as COPD, cystic fibrosis, asthma, among others.44–46 The relationship between the G.I. tract and the lungs could be another determining factor for the onset of disease, since it has been found that changes in the composition of the intestinal microbiota are associated with greater susceptibility to respiratory diseases and changes in the lung tissue.47,48
Although acute COVID-19 mainly affects the respiratory tract, some patients suffer from gastrointestinal manifestations. Viral particles have even been found in patients’ gastrointestinal tract.49,50 Moreover, patients with SARS-CoV-2 infection have also shown signs of altered fecal microbiota, with a decrease in Bifidobacterium, Lactobacillus, and Eubacterium, and an increase in pathogenic bacteria such as Corynebacterium and Ruthenibacterium.51
In their study, Su et al. found an incomplete recovery of the intestinal microbiota in a cohort of SARS-CoV-2 infected patients in the 6–14 months following the manifestation of PACS symptoms.52 Bacteria of the genus Prevotella and Veillonella predominated in the microbiota of these patients a year after infection. These genera have previously been shown to induce an inflammatory response,53,54 as well as decrease beneficial bacteria such as Bifidobacterium adolescentis and B. pseudocatenulatum that reduce inflammation and neurological symptoms.55,56
The role of the microbiome in PACS is yet to be deciphered. More studies are necessary to determine whether interventions to recover homeostasis of the bacterial flora will have any impact on the improvement of symptoms in patients with PACS.
ManifestationsThis syndrome can involve different organ systems such as the cardiovascular, gastrointestinal, respiratory, neurocognitive, and musculoskeletal system. It can similarly lead to alterations in smell and taste. Its symptoms have a wide spectrum ranging from general feelings of fatigue, fever, dizziness, dyspnea, cough, pulmonary embolism, hair loss, gastrointestinal alterations, chest tightness, palpitations, tachycardia, difficulty falling asleep, anxiety, depression, among others.57
There are five types of manifestations that are related to the patient's condition, the state prior to severity, and the characteristics of the presentation and evolution of the symptoms.58
- •
Type I: patients with symptoms related to infection, organ damage, and comorbidities.
- •
Type II: patients with symptoms that persist more than six weeks after the onset of the disease.
- •
Type III: patients with initial recovery who develop subsequent symptoms lasting less than three months or more than six months.
- •
Type IV: asymptomatic carriers who present symptoms for less than three months or more than six months.
- •
Type V: patients with mild symptoms or asymptomatic carriers who suddenly die within 12 months.
Different studies have identified that fatigue is the most reported symptom for PACS (with 30–72% of patients reporting this symptom). However, a relationship between its development and the severity of the infection has not yet been established. This symptom is explained by different alterations such as systemic inflammation and immune mechanisms (release of cytokines and reactive oxygen species), as well as direct infection of the virus in the muscle (inflammation and damage of muscle fibers and neuromuscular junction).59
Patients with PACS may present persistent radiological alterations such as ground-glass, dyspnea, bronchiectasis, and pulmonary fibrosis, being this last one the most serious complication. Factors such as advanced age, severe infection, elevated D-dimer levels, acute respiratory distress syndrome, smoking, prolonged mechanical ventilation, history of cardiovascular or pulmonary disease, and alcoholism can serve as predictors for the onset of these manifestations.60
Cardiovascular alterations are explained largely by the presence of angiotensin-converting enzyme 2 (ACE2) receptors. Studies suggest that the damage after acute infection is due to the persistence of the virus in the heart, which explains the chronic inflammation, myocardial injury, myocarditis, cardiomyopathy, and cardiac arrhythmias.60,61
Neurological manifestations of PACS are perhaps the most common.62 In general, alteration of the blood–brain barrier, activation of glial cells, infiltration of the leukocyte parenchyma, and direct neural invasion by the virus play an important role. These contribute to the precipitation or exacerbation of neurodegenerative and neuroinflammatory disorders, alterations in memory, taste and smell, headache, among others. The psychiatric symptoms of PACS are explained by the pathophysiological mechanisms described, but the isolation established as disease management is also highlighted as an important factor for the development of diseases such as depression, anxiety, and insomnia. Critical illness, long-term ventilatory support, and acute respiratory distress syndrome have negative cognitive effects.59 The activation of neuroinflammatory mechanisms, including the activation of microglia, lead to persistent neuroinflammation and subsequent neuronal damage represented by neurocognitive and neuropsychiatric disorders.63 Some reports show evidence of not only microscopic changes, but of decreases in the volume of gray matter and decreases in cerebral flow in frontal areas and the limbic system.64 Anosmia and ageusia, symptoms of relevance in the diagnosis of SARS-CoV-2 infection, seem to have a direct relationship with viral invasion at the neuroepithelial level. However, the persistence of these symptoms seems to be related to chronic neuroepithelial inflammation and the presence of viral particles, as demonstrated by Melo et al. in animal studies.65
Other conditions to consider – differential diagnosticsMyalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)A multisystem neuroimmune disease that often begins during or after an infection.17 This condition has been difficult to address and there are different approaches for diagnosis in adults. Most follow the Institute of Medicine's definition (now called the National Academy of Medicine)66:
The core symptoms are as follows: (i) A notable decline or limitation in the ability to engage in activities at pre-illness levels (occupational, educational, social, or personal), accompanied by profound fatigue lasting at least 6 months, which is not attributable to excessive exertion and remains unalleviated by rest; (ii) post-exertional fatigue (PEF); and (iii) non-restorative sleep. Furthermore, either (i) cognitive impairments or (ii) orthostatic intolerance must also be present.
The onset of ME/CFS has been associated with infections. In their systematic review and meta-analysis, Hwang et al. report the following as associated viruses: human herpesvirus 7 (HHV-7), parvovirus B19, borna virus disease (BDV), enterovirus, and Coxsackie virus B.67 Following the 2003 severe acute respiratory syndrome (SARS) pandemic, chronic symptoms such as fatigue and psychiatric disorders were reported.68
The sequelae following SARS-CoV-2 infection are defined, and researchers have also found similarity and overlap of symptoms between PACS and ME/CFS.69,70 A study conducted by Wong et al. found that out of 29 ME/CFS symptoms, 25 (86%) were reported in at least one long COVID study.71
An international study spanning 56 countries and involving 3762 patients discovered that symptoms like fatigue, post-exertional malaise (PEM), and cognitive impairments persist seven months post-initial diagnosis of SARS-CoV-2 infection. Notably, all of these symptoms are significant in the context of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).15 Similarly, a study led by Bonilla et al. found that 43% of COVID-19 patients met symptomatic criteria for ME/CFS after six months of infection.72
Both ME/CFS and long COVID exhibit shared pathophysiological features, including altered immune responses, heightened autoimmunity, metabolic changes, and neurological and autonomic dysfunctions. Hence, it is crucial to consider this condition as a potential diagnosis associated with the sequelae of COVID-19 infection.69
Post-intensive care syndromeAt the beginning of the SARS-CoV-2 pandemic, between 32% and 42% of hospitalized patients required transfer to the ICU. These patients presented multiple secondary complications leading to organ dysfunction and prolonged ICU stay. The fatality reports are around 50%, with a range between 40.8% (NIMV only) and 71.6% (support with IMV, vasoactive agents and new renal replacement).73,74
Patients that manage to overcome the critical illness often report symptom sequelae following the ICU stay. This condition has been denominated post-intensive care syndrome (PICS).75
There is no consensus on the definition of PICS. Nevertheless, this term is used to describe the physical, cognitive, and mental health impairments that follow critical illness and persist beyond the acute phase of hospitalization. PICS is often applied to disease survivors. This condition can lead to physical deterioration, weakness, and alterations in lung and/or physical function. It can similarly lead to chronic alterations in memory, attention, mental processing speed, and mental status, while prompting the onset of psychiatric symptoms such as anxiety, post-traumatic stress, depression, among others.76
PICS is a condition that should be considered when evaluating patients with sequelae of critical SARS-CoV-2 infection. Establishing which complications are caused directly by the ICU visits, and which are caused by long COVID or EN/CFS is necessary for adequate diagnosis.77–79
TreatmentCurrently, studies have focused on the symptomatic management and rehabilitation. These practices generally target symptoms such as fatigue, dyspnea, anosmia, ageusia, among others. Specific pharmacological therapies have not shown generate significant improvement in patients, so their use is not necessarily recommended.80
During the pandemic, treatment with steroids (specifically dexamethasone) reduced mortality in patients with acute manifestations of the disease, especially those requiring oxygen. Milne and colleagues found in a case–control study that patients receiving dexamethasone during the acute phase of the disease were less likely to experience PACS symptoms at 8-month follow-ups than patients who did not receive the treatment.81
Several treatments have been tested for the management of pulmonary fibrosis. Kerget et al. carried out a prospective, randomized study comparing two antifibrinolytics (nintedanib and pirfenidone) in patients with pulmonary fibrosis secondary to post-COVID-19 syndrome. The study found that both treatment groups presented a significant increase in parameters like lung function tests, distance traveled in walking test, and oxygen saturation. The treatment groups similarly exhibited a decrease in heart rate and a decrease in the radiological changes score (at the lung level). However, the antifibrinolytics led to a significant increase in gastrointestinal adverse effects, with the most noticeable changes in the nintedanib group.82
Given their role in modulating systemic inflammation and immunity, multiple multivitamins and nutritional supplements have been tested for treatment in these patients.83 Vitamin D has been described as an important factor for an adequate immune response and musculoskeletal recovery. Its low levels have been associated as a risk factor for the development of PACS.84 Studies are underway with supplementation of Coenzyme Q10 (NCT04960215), Vitamin B3 (NCT04809974), and essential fatty acids such as Omega-3 (NCT05121766), among others.
Rehabilitation with aerobic exercises, resistance exercises, respiratory physiotherapy, and relaxation techniques can be effective in improving muscle strength, improving walking ability, and improving quality of life. A randomized study including 72 elderly patients who suffered from SARS-CoV-2 infection demonstrated that 6-week rehabilitation program improved lung function parameters, quality of life, and anxiety in this population.85
VaccinationVaccination has been effective at reducing the severity of acute COVID-19 disease and preventing the onset of the post-acute COVID syndrome. The World Health Organization reported that by the end of November, 2023, 13,595,721,080 COVID-19 vaccines were administered worldwide.2,86 To the date, vaccination is the only measure with solid foundations that has been shown to prevent unfavorable outcomes related to infection with the SARS-CoV-2 virus. Vaccines have had a positive impact on the natural course of the disease, reducing rates of hospitalization (in general wards and intensive care unit) and reducing the number of deaths arising from complications.87
A systematic review and meta-analysis carried out in 2022 shows that vaccinated patients have a lower risk of developing PACS (RR=0.71 (95% CI 0.58–0.87, p<0.01).88 The study also found that only patients who received two doses of the vaccine achieved a protective effect. There was no difference in outcome when comparing the time when the vaccination was performed (before vs. after the infection/disease).
Variables influencing vaccine response must be considered when analyzing the persistence of symptoms after the initial infection. One of such variables is the number of doses. Between March of 2020 and April of 2022, an observational study was carried out in nine hospitals in Italy. A total of 2560 healthcare workers participated, 29% with a PCR diagnosis of COVID-19 (asymptomatic patients included) and 31% (95% CI 27.7–34.5%) presenting PACS symptoms. The number of vaccine doses was correlated to a lower prevalence of post-COVID-19 syndrome (41% in unvaccinated, 30% in patients with one dose, 17.4% in patients with two doses, and 16% in patients with three doses).89 A similar study in Israel compared PACS symptoms in 951 patients with infection and 2437 patients without previous infection. The study found that patients with at least two doses of the vaccine had a substantial decrease in PACS symptoms compared to patients without the complete vaccine scheme.90
The time of the application of the vaccine seems to have no impact on the onset of PACS, given the lack of differences between patients getting their vaccination before and after the infection and/or disease. However, a cohort study in the United Kingdom, carried out between February and September of 2021, demonstrated that patients vaccinated after COVID-19 infection have a decrease in the probability exhibiting symptoms such as loss of smell, loss of taste, and problems sleeping.91
ConclusionsSignificant knowledge gaps persist regarding post-COVID syndrome, underscoring the necessity for continued research efforts to understand its behavioral patterns and elucidate the underlying pathogenic processes. Further exploration of the relationship between this syndrome and the presence of autoantibodies is crucial for gauging disease severity and anticipating subsequent sequelae in affected patients. Establishing a comprehensive monitoring and surveillance plan is imperative to swiftly detect the emergence of this syndrome among patients requiring intensive care unit (ICU) attention. Early diagnosis and intervention can effectively mitigate complications and prevent the deterioration of patients’ quality of life. Moreover, there must be a heightened emphasis on addressing the mental health ramifications of this disease and future pandemics. Implementing preventive measures can significantly alleviate post-traumatic stress, anxiety, and depression among affected individuals. A novel methodological approach is warranted to adequately analyze the factors associated with long COVID, its differential diagnoses, and the development of effective treatment strategies.
Conflict of interestsThe authors do not declare any conflict of interest for this article.
The authors express their gratitude for the financial support provided by Grupo Sura, Grupo ISA, Minera de Cobre Quebradona – Anglogold, Mineros Aluvial S.A., Corbeta, Sumicol Corona, Fundación Fraternidad Medellín, Nutresa, Fundación Bancolombia, Zijin (Continental Gold), Fundación Sofia Pérez, Gran Colombia Gold, and the Pintuco Foundation.
They also appreciate the logistical support and resources provided by the Government of Antioquia, Grupo Sura, the National University of Colombia-Medellín, the St. Vincent Foundation Hospital, and the University of Wisconsin-Madison, which included transportation, facilities, equipment, installed capacity, and additional support for human resources and laboratory services.