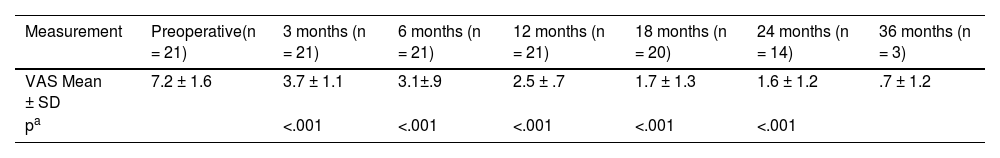to assess clinical safety and postoperative audiological outcomes in postlingual deafness Spanish speaking patients, who underwent surgery with Nurotron™ cochlear implant.
Material and methodsRetrospective descriptive case series study. We performed follow-up of complications and audiological measurements before and after cochlear implantation. Patients with bilateral severe to profound sensorineural hearing loss or patients with unilateral deafness with/without tinnitus were included. Repeated-measures within-subjects for assess pure tone thresholds and speech performance (bilingual test) with a detailed monitoring to establish security or adverse effects were performed. Analysis of variance tests, repetitive measures, were used for statistical analysis.
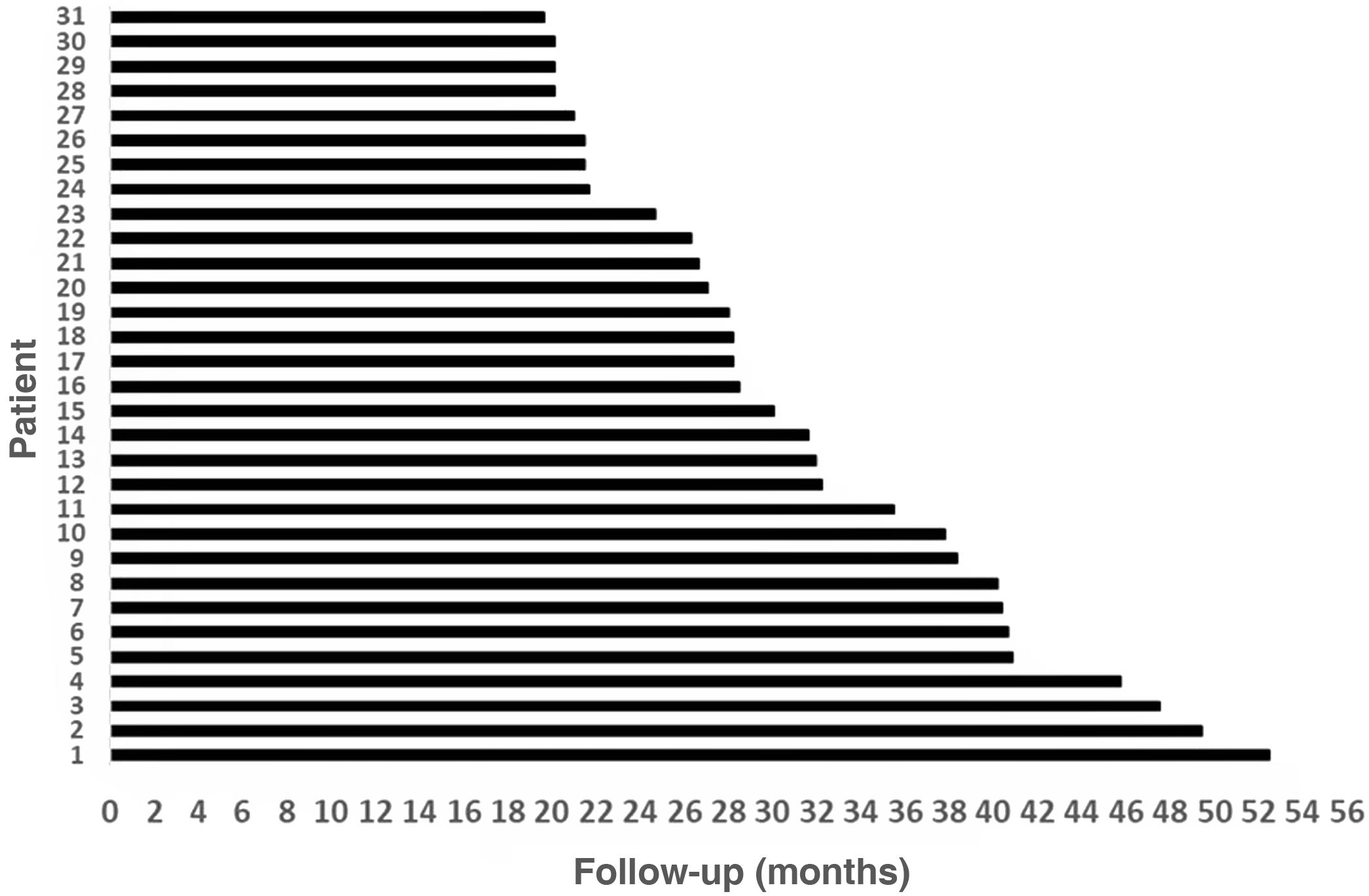
Results31 patients were included, 17 (54.8%) men and 14 (45.2%) women. Mean age at the time of surgery was 49.82 ± 18.8 years. The mean follow-up of the group was 31.56 ± 9.57 months (minimum = 19.6 months and maximum = 52.50 months). As major complication one patient (3.23%) had a hard failure that required removal and re-implantation. 25.8% of the patients presented minor complications, the most frequent being vertigo/unsteadiness in 22.6%.
The mean of language discrimination (free field at 65 dB SPL) was 62.19% ± 16.66; being 69.82% ± 7.35 in the group of severe to profound bilateral sensorineural hearing loss. A statistically significant reduction was observed in patients with tinnitus, assessed using the visual analogue scale, preoperative = 7.2 ± 1,6 vs postoperative (18months postoperative) = 1.7 ± 1.3 (p < .001).
ConclusionsThe Nurotron™ cochlear implant shows satisfactory audiological results, in accordance with what has been reported in the literature. Minor complications were similar to previous studies, but the percentage of hard failure should continue to be observed, which was higher than other reports with comparable follow-up.
evaluar la seguridad clínica y resultados audiológicos postoperatorios en pacientes de habla hispana con sordera postlingual, que recibieron implante coclear Nurotron™.
Materiales y métodosEstudio descriptivo retrospectivo tipo serie de casos. Se hizo seguimiento de complicaciones y mediciones audiológicas antes y después del implante coclear. Se incluyeron pacientes con pérdida auditiva neurosensorial bilateral severa a profunda o pacientes con sordera unilateral con/sin tinnitus. Se realizaron medidas repetidas dentro de los sujetos para evaluar los umbrales de tonos puros y el rendimiento del habla (listados de bisílabas), así como seguimiento clínico para establecer la seguridad del dispositivo. En el análisis estadístico se utilizaron análisis de la varianza y pruebas de medidas repetitivas.
Resultadosse incluyeron 31 pacientes, 17(54,8%) hombres y 14(45,2%) mujeres. La edad media en el momento de la cirugía fue de 49,82 ± 18,8años. El seguimiento medio fue de 31,56 ± 9,57meses (mínimo = 19,6meses y máximo = 52,50meses). Como complicación mayor un paciente (3,23%) tuvo un fallo técnico que requirió remoción y reimplantación. El 25,8% de los pacientes presentaron complicaciones menores, siendo la más frecuente el vértigo/inestabilidad en el 22,6%.
La media de discriminación del habla (campo libre a 65 dB SPL) fue de 62,19% ± 16,66; siendo del 69,82% ± 7,35 en el grupo de hipoacusia neurosensorial bilateral severa/profunda. Se observó una reducción estadísticamente significativa en los pacientes con tinnitus, evaluados mediante la escala analógica visual, preoperatorio = 7,2 ± 1,6 vs postoperatorio (18 meses postoperatorio) = 1,7 ± 1,3 (p < 0,001).
ConclusionesEl implante coclear Nurotron™ muestra resultados audiológicos satisfactorios, acordes con lo reportado en la literatura. Las complicaciones menores fueron similares a estudios previos, pero se debe continuar observando el porcentaje de fallo técnico que fue superior a otros reportes con seguimiento equiparable.
In adults, hearing loss is associated with social isolation, depression, loss of autonomy, decreased productive capacity and neurocognitive alterations.1,2 Some patients can be rehabilitated with hearing aids,1,3 but, for those with severe/profound sensorineural hearing loss (SNHL), the gain with a hearing aid is insufficient and the best treatment option is the cochlear implant (CI).3 In these patients, the main objective is to maintain spoken language as the main form of communication.3
Bilateral severe/profound SNHL was the first indication for cochlear implantation approved by the Food and Drug Administration in adults.1 Subsequently, the indications were extended to patients with severe/profound unilateral SNHL with the other healthy ear (SSD: “Single Sided Deafness”) or with less than severe SNHL in the other ear (asymmetric sensorineural hearing loss).1,3 In cases of SSD or asymmetric SNHL, the additional objectives are tinnitus control, restoration of binaural hearing (localisation of the sound source, improving discrimination of speech in a noisy background) and improvement in quality of life.1,3–5
Despite the recognized benefits of auditory rehabilitation, its use remains low.1,6 In a systematic review on the subject, it was reported that in adults the factors involved with low use of hearing aids could be grouped into three categories: motivational factors, access barriers and factors related to the performance of the hearing device.6 The following are reported to be among the most relevant access barriers: limitations due to the cost of the device and coverage limitations by insurers/health systems.6 In the specific case of CI, one study estimated that less than 10% of adults who are candidates for CI actually receive it.7 Other factors that explain the low use of CI are lack of knowledge of its benefits and selection criteria.1,6–8
As the CI is a high-tech device with complex manufacturing, there are few companies that produce it, maintaining a high cost that has not decreased in the last three decades.8 Nurotron™ is a brand of CI, which obtained approval for use in China in 2011, in the European community in 2012, in Colombia in 2013 and may represent an access alternative for some patients.8 In the initial clinical trial, they reported ∼80% language discrimination at 6 months (sentences in Mandarin) and from then on a plateau phenomenon.8 The authors also mention that the performance of the Nurotron™ CI is comparable to other brands available on the market.8
We conducted the present study in order to describe the clinical safety and audiological outcomes in postlingual Spanish-speaking adult patients who received a Nurotron™ brand CI.
Materials and methodsStudy designThis is a descriptive study of case series in which the results before and after cochlear implantation are compared. Patients operated on from March 2014 to January 2020 were included. Patients received the CI in a tertiary referral centre in Bogotá, Colombia.
SubjectsPatients who received cochlear implantation with the Nurotron™ brand were included. Additional criteria were: age >15 years, acquisition of spoken language before cochlear implantation, native Spanish language, and having outcome measurements for at least 12 months postoperatively. Patients with contralateral CI or who had neurological deficits were excluded. Eligible patients were identified from the study centre’s CI patient files. Demographic, surgery, and clinical follow-up information was obtained retrospectively. The audiological and CI performance results were obtained in the audiology service in charge of activating and programming the devices. Preoperative information was taken from the records of the 6 months (±1 month) prior to surgery and postoperative information included up to the last complete follow-up recorded.
A convenience sample size was used, including all patients who met the inclusion criteria and did not have any exclusion criteria; therefore, no sample size calculation was made.
Since the research was carried out by recording information from medical records, it was a non-risk research study for the patient and complied with the recommendations of the Declaration of Helsinki.9 The patients included in the study received cochlear implantation as part of the treatment indicated by their treating physician and no data that could identify a patient individually was recorded, ensuring the confidentiality and privacy of the study subjects.
Cochlear implantation protocol and follow-upPatients were implanted according to the cochlear implantation protocol of the participating institution. For the language evaluations, disyllable lists were used, in accordance with what was described for the Spanish language.3 In the preoperative evaluation, the percentage of language discrimination (PLD) was obtained in the best amplification conditions when the patient used a hearing aid, otherwise the PLD was obtained at the maximum output of the audiometer (90 dB HL). Adults are considered candidates for CI if on the side to be implanted they present severe/profound SNHL defined as: pure tone average (PTA) of 4 frequencies (.5−3 Khz by air ≥70 dB and PLD < 50%) and were classified into one of the following three indications: 1. Patients with SSD (Group 1); 2. Patients with bilateral severe/profound SNHL (Group 2); 3. Patients with asymmetric SNHL (Group 3). SSD is defined as: diseased ear with deep SNHL (PTA ≥ 90 dB and PLD ≤ 20%) and healthy contralateral ear (PTA ≤ 20dB). Bilateral severe/profound SNHL is defined as: PTA≥70dB and PLD < 50% in both ears. Asymmetric SNHL is defined as: ear to be implanted with PTA ≥ 70dB and PLD < 50% and contralateral ear with mild to severe SNHL (PTA = 21 dB–69 dB) with PLD ≥ 60%. The CI was switched on between 4–6 weeks postoperatively and the follow-ups were: 3 months, 6 months, 12 months and then every 6 months. At each follow-up point, a language evaluation, clinical evaluation, audiological evaluation, and CI reprogramming are performed. At each visit, systematic questioning was made regarding device use, device functioning and also any complications with its use.
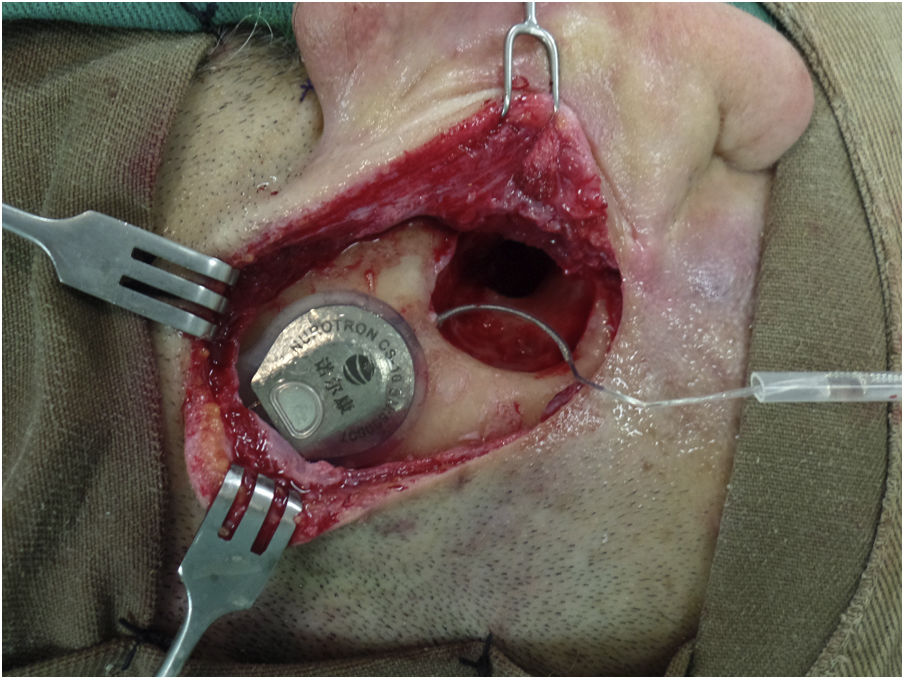
Surgical technique and device implantedThe surgical technique is standard, seeking, whenever possible, to insert the series of electrodes through a round window (RW). In addition, the aim is to minimize tissue trauma, as described by Almario et al.10 In all cases, a bed is made to accommodate the antenna-receiver and the series of electrodes is passed towards the mastoid through a tunnel/channel in order to increase the stability of the internal component (Fig. 1). Perioperative antibiotics are used and asepsis/antisepsis is exhaustive during surgery to reduce the risk of late infections such as biofilms.11 To reduce cochlear trauma, perioperative and topical corticosteroids are used before opening the RW, the milling of the RW niche is done at low speed (2000 rpm), the insertion of the electrode series is done slowly (>30 s) and this is to avoid reinsertion of the electrode. In cases of cochlear obliteration/ossification, a stepwise approach is used until the cochlear lumen is found or a radical cochleostomy is performed, as described by Balkany et al.12
Patient with Nurotron™ cochlear implant.
In a right ear, the antenna-receiver can be seen housed in the bed that is drilled for this purpose in order to increase its stability. The array of electrodes passes from the bed to the mastoid through a tunnel. These measures seek to reduce the risk of displacement/extrusion of the internal component of the cochlear implant. The patient had a canal wall down mastoidectomy cavity with a (due to sequelae of chronic cholesteatomatous otitis media) so for cochlear implantation it was decided to perform a subtotal petrosectomy with closure of the external auditory canal in its lateral third, resection of all epithelial remnants, obliteration of the Eustachian tube with muscle and bone grafts and obliteration of the cavity with muscle and temporal fascia grafts.
The Nurotron™ brand CI used was model CS-10A (Nurotron Biotechnology Inc.; Hangzhou, Zhejiang, China), which has a receiver/stimulator with an array of 24 intracochlear active electrodes and two reference electrodes. A straight electrode was used in all cases (standard electrode, which was the one available at the time of the surgeries), which is 22 mm long, with a distance between contacts of .8 mm and an area of .2 mm2 in each contact. At the tip level, the electrode series measures .70 x .56 mm and at the base level it measures .93 x .68 mm.8 The patients received the Venus® sound processor, which has a behind-the-ear design and establishes communication with the internal component through the radiofrequency antenna.
Audiological measurementsThe preoperative evaluation included tone, air, and bone audiometry, and four-frequency PTA was calculated to define the severity of hearing loss in each ear. These results are confirmed with auditory brainstem potentials or steady state potentials. Speech audiometry is done by air with supra-aural headphones, seeking the maximum PLD in each ear. Subsequently, performance with a hearing aid is evaluated in a free field, with two speakers located 1 m in front of the patient (azimuth 0° and elevation 0°), looking for hearing thresholds and PLD at 65 dB SPL. For Group 1, measurements were made with a hearing aid in the diseased ear and for patients in Groups 2 and 3, each ear was evaluated separately with its respective hearing aid. For patients in Groups 1 and 3, the untested ear was occluded with an insert hearing protector plus masking with white noise at 45 dB through a supra-aural earphone.
Postoperative evaluations were performed in a similar way to that described for the preoperative evaluations, with the CI turned on and performing occlusion plus masking of the better ear for patients in Groups 1 and 3 and without the use of hearing aids in the non-implanted ear for those in Groups 1 and 3 and without the use of hearing aids in the non-implanted ear for patients in Group 2 patients.
Tinnitus evaluationIn patients with tinnitus, symptom severity was assessed using the visual analogue scale (VAS). It was explained to the patients that tinnitus corresponded to “internal noise(s) that they perceived and that there was no external source that generated it” and they were asked to rate its severity from 0 to 10, with 0 being = absence of tinnitus and 10 = “the worst tinnitus you can imagine.” In the preoperative period this was done without the use of a hearing aid and in the follow-up visits it was done with the CI on.
Evaluation and follow-up of complicationsThe cochlear implantation protocol has a pre-designed medical history format that must be completed at each visit. This format has a section in which the presence or absence of complications with the use of the device is specifically recorded. Complications are classified as major and minor. A major complication is one that requires intensive medical treatment, requires surgical revision, and/or causes permanent morbidity in the patient.
Statistical analysisThe quantitative variables (interval/proportional and ordinal) are described by measures of central tendency and dispersion and the nominal variables by frequencies. Comparisons were made with parametric or non-parametric tests, depending on the nature of the variables.
For the main outcomes, complications are presented by frequencies. Audiometric and tinnitus assessment measurements were compared before and after cochlear implantation, using a repeated measures analysis of variance (ANOVA), with statistical significance at p < .05. The analyses were performed with SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA).
The results of the study are presented following the recommendations for reporting observational studies: STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology).13
This research did not receive specific support from public sector agencies, the commercial sector or non-profit entities. The study was approved by the ethics and research committee of the participating institution (code: CEIFUS 3300-22; minutes No 045-22).
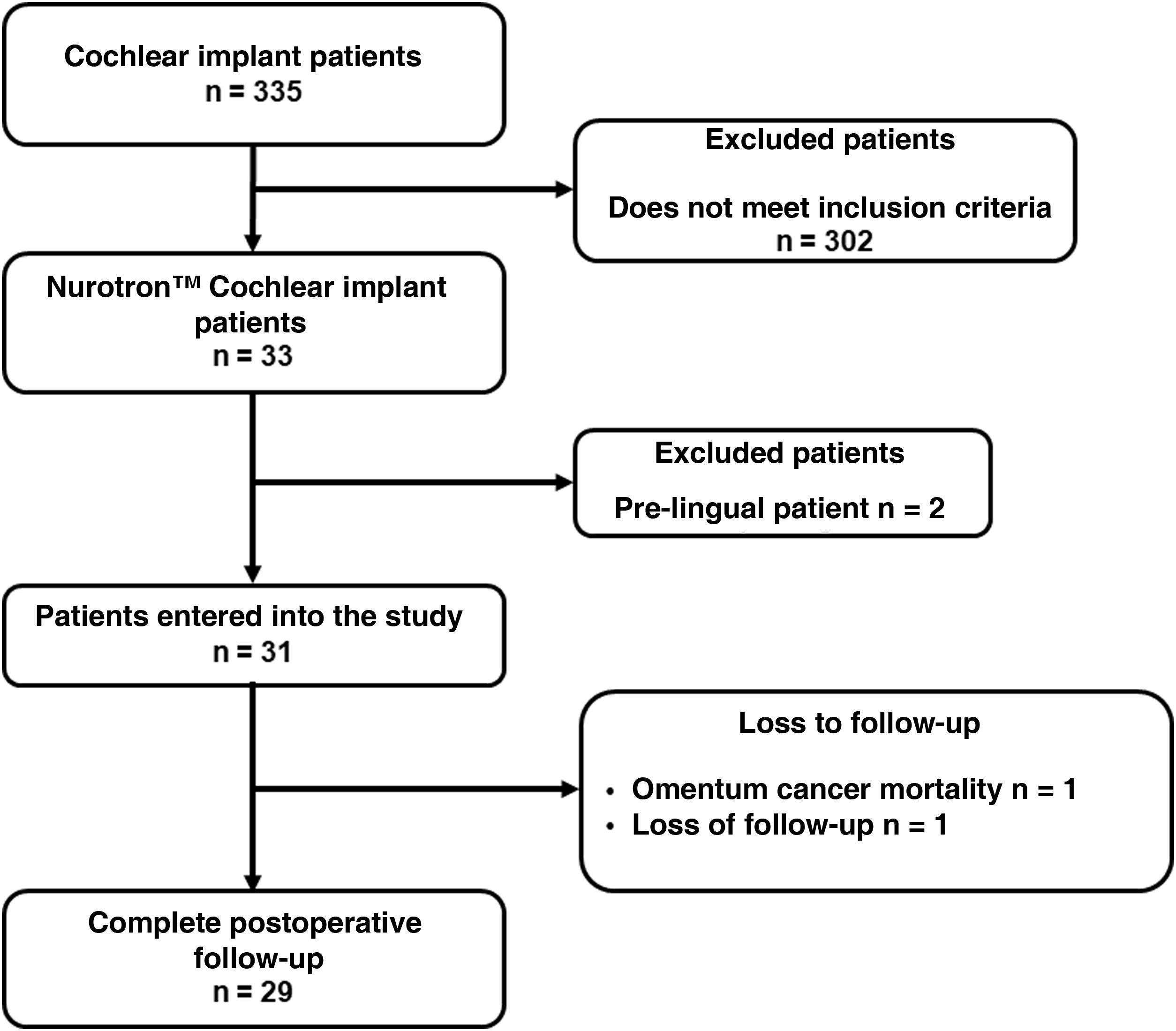
ResultsThirty-three patients who received Nurotrón™ CI were identified, two patients were prelingual and their results were excluded from the analysis. Two patients were lost to follow-up (6.5%), one due to death from a pathology not related to CI and another due to loss of insurance to the health system (Fig. 2). The follow-up of the group was 31.56 ± 9.57months (minimum = 19.6 months, maximum = 52.50 months) (Fig. 3). For the two cases of loss to follow-up, their results are reported up to the time of the last available follow-up. The death of the patient who died was due to omental cancer. This patient had his implant working properly (24 active electrodes and PLD = 60%) and using it routinely.
Table 1 shows the characteristics of the study group and Table 2 presents the clinical findings of the study population. Surgeries were primary and were performed unilaterally in all patients. The duration of severe/profound hearing loss before CI was 49.52 ± 46.64 months. 14 patients (45.2%) used hearing aids before receiving the CI and used them for 122.14 ± 144.83 months. SSD was the most common indication (38.7%, n = 12), which is related to sudden deafness as the most common cause in our series. In general, the ear that received the CI had profound SNHL (PTA = 92.7 dB ± 11.3; PLD = 7.87%±11.4). Tinnitus was a frequent symptom, occurring in 21 patients (67.7%), with moderate to severe intensity (VAS = 4–9). In the preoperative imaging study, 15 patients (48.4%) underwent MRI + CT, 9 (29%) only CT and 7 (22.6%) only MRI. In these studies, one patient (3.2%) with dilated vestibular aqueduct and two with complete cochlear ossification (6.5%) were identified.
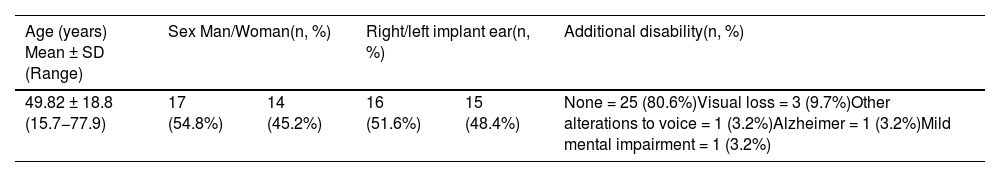
Characteristics of the study population (n = 31).
| Age (years) Mean ± SD (Range) | Sex Man/Woman(n, %) | Right/left implant ear(n, %) | Additional disability(n, %) | ||
|---|---|---|---|---|---|
| 49.82 ± 18.8 (15.7−77.9) | 17 (54.8%) | 14 (45.2%) | 16 (51.6%) | 15 (48.4%) | None = 25 (80.6%)Visual loss = 3 (9.7%)Other alterations to voice = 1 (3.2%)Alzheimer = 1 (3.2%)Mild mental impairment = 1 (3.2%) |
SD = Standard deviation.
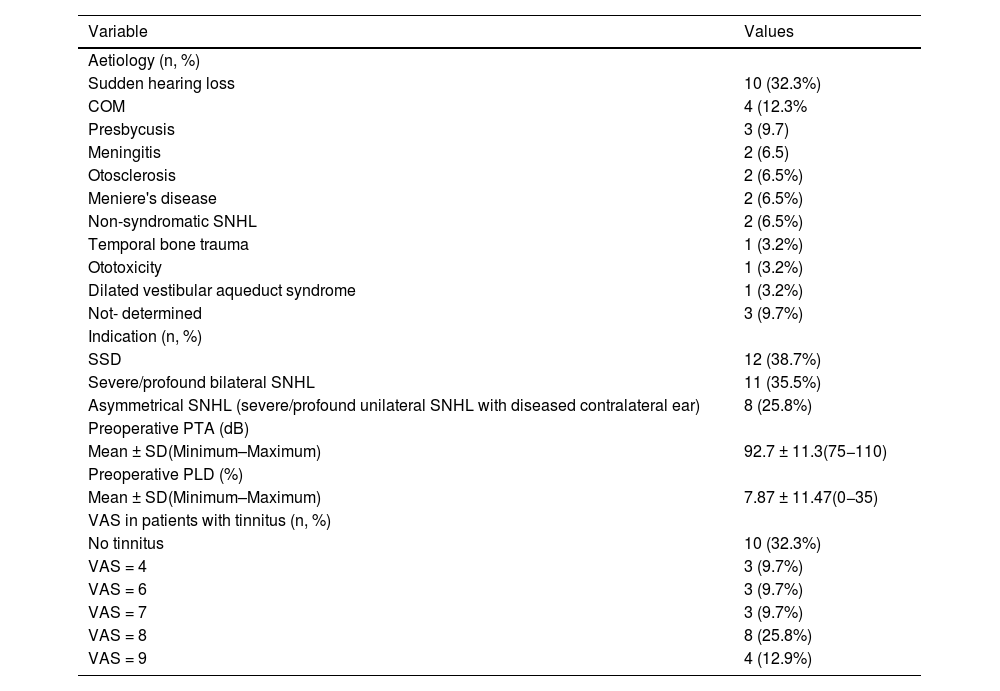
Clinical findings of the study population (n = 31).
| Variable | Values |
|---|---|
| Aetiology (n, %) | |
| Sudden hearing loss | 10 (32.3%) |
| COM | 4 (12.3% |
| Presbycusis | 3 (9.7) |
| Meningitis | 2 (6.5) |
| Otosclerosis | 2 (6.5%) |
| Meniere's disease | 2 (6.5%) |
| Non-syndromatic SNHL | 2 (6.5%) |
| Temporal bone trauma | 1 (3.2%) |
| Ototoxicity | 1 (3.2%) |
| Dilated vestibular aqueduct syndrome | 1 (3.2%) |
| Not- determined | 3 (9.7%) |
| Indication (n, %) | |
| SSD | 12 (38.7%) |
| Severe/profound bilateral SNHL | 11 (35.5%) |
| Asymmetrical SNHL (severe/profound unilateral SNHL with diseased contralateral ear) | 8 (25.8%) |
| Preoperative PTA (dB) | |
| Mean ± SD(Minimum–Maximum) | 92.7 ± 11.3(75−110) |
| Preoperative PLD (%) | |
| Mean ± SD(Minimum–Maximum) | 7.87 ± 11.47(0−35) |
| VAS in patients with tinnitus (n, %) | |
| No tinnitus | 10 (32.3%) |
| VAS = 4 | 3 (9.7%) |
| VAS = 6 | 3 (9.7%) |
| VAS = 7 | 3 (9.7%) |
| VAS = 8 | 8 (25.8%) |
| VAS = 9 | 4 (12.9%) |
COM = Chronic otitis media; PLD = Percentage of language discrimination; SD = Standard deviation; SNHL = Sensory neural hearing loss; SSD = Single sided deafness; VAS = Visual analogue scale.
Regarding the surgical findings, in 25 patients (80.6%) the anatomy of the RW and basal region turn of the cochlea was normal, in four patients (12.9%) obliteration of the RW niche and/or lower segment of the basal region turn of the cochlea was found and in two patients (6.5%) there was complete cochlear ossification. In 27 patients (87.1%) the insertion was done by RW, in 2 cases (6.5%) the lower segment of the basal turn of the cochlea had to be reamed due to partial obliteration and in 2 patients radical cochleostomy was required (6.5%) due to complete ossification of the cochlea. During surgery, the surgeon reported complete insertion of the electrode array (24 electrodes) in 24 patients (77.4%) and partial insertions in the remaining 7 patients. The lowest number of active electrodes was observed in the two patients who required radical cochleostomy: 7 and 10, respectively.
Regarding intraoperative complications, there were two cases (6.5%) of cerebrospinal fluid (CSF) fistula, which were the two patients with complete cochlear ossification that required radical cochleostomy. In both cases the CSF fistula occurred due to entry into the internal auditory canal (IAC) during cochlear drilling. They were managed by obliterating the fistula site with a temporal muscle-fascia graft, sealing the Eustachian tube with bone grafts (cortical mastoid) and temporal muscle, closing the external auditory canal (part of the radical cochleostomy technique), obliterating the tympanomastoid cavity with abdominal fat (after placing the CI) and leaving a compressive bandage for three days postoperatively. In both cases there was control of the CSF fistula intraoperatively and no additional measures were required postoperatively. No intraoperative complications were reported in the remaining 29 patients (93.5%).
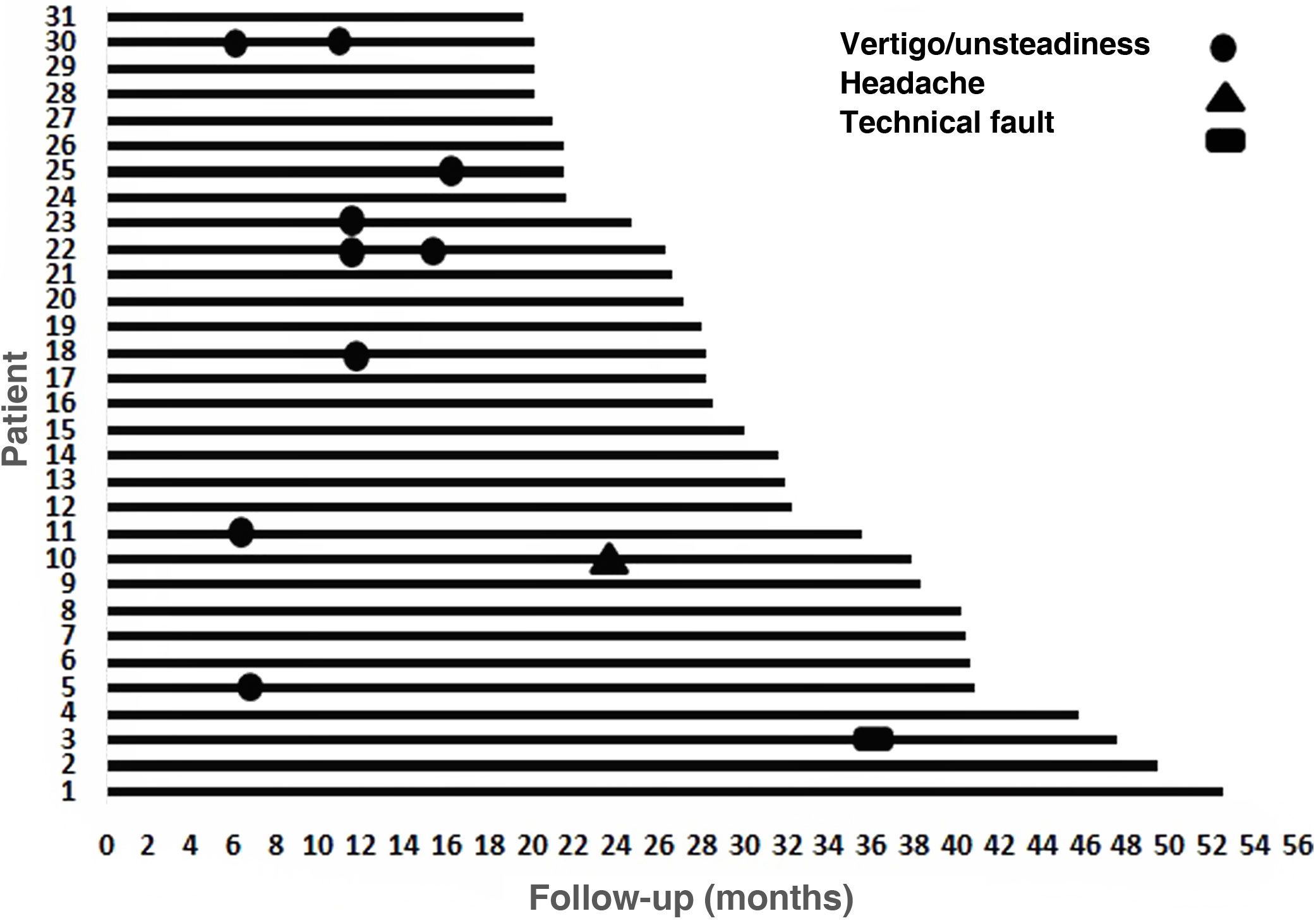
During follow-up, one patient presented technical failure (3.23%) that required explantation and reimplantation with a new device at the same time. The manufacturer's report states that the explanted device had no damage to its coverage, it passed the airtightness test (helium test) and the alteration found was a failure in the current source compatible with damage due to electrostatic discharge (ESD). Once said component was replaced, proper functioning of the stimulator receiver was verified, as well as its communication with the sound processor. This was a 75-year-old patient with severe/profound bilateral SNHL with a follow-up of 48 months, whose best PLD before explantation was 80% and after reimplantation the PLD was 60% (6 months post-reimplantation). Ten events were reported as minor complications, of which 9 were vertigo/unsteadiness and one was headache (Fig. 4). The vertigo/unsteadiness events were managed with Epley repositioning manoeuvers and/or vestibular therapy. The patient who reported headache received acetaminophen; all events were resolved satisfactorily from a symptomatic point of view.
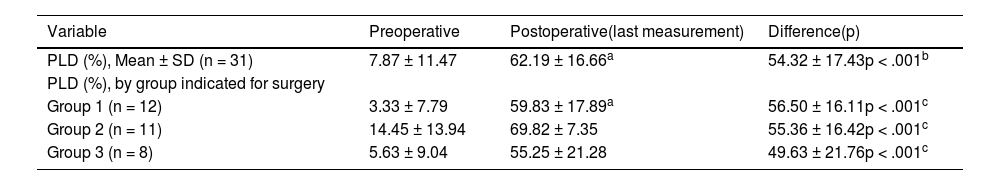
The audiometric results are shown in Table 3, both for the total group and discriminated by the three groups according to the indication for surgery. At the preoperative audiometric baseline, a significant difference in PLD was observed between Group 1 (3.33 ± 7.79) vs Group 2 (14.45 ± 13.94), (p = .019, ANOVA); but not between Group 1 vs Group 3 (p = .641, ANOVA), nor between Group 2 vs Group 3 (p = .085, ANOVA). Postoperative language discrimination results showed a clear improvement compared to preoperative, both for the total group and for the three surgery indication subgroups (p < .001, for all comparisons). There were no differences in postoperative PLD results between the three groups (p > .05, for all comparisons).
Language discrimination in patients from the study.
| Variable | Preoperative | Postoperative(last measurement) | Difference(p) |
|---|---|---|---|
| PLD (%), Mean ± SD (n = 31) | 7.87 ± 11.47 | 62.19 ± 16.66a | 54.32 ± 17.43p < .001b |
| PLD (%), by group indicated for surgery | |||
| Group 1 (n = 12) | 3.33 ± 7.79 | 59.83 ± 17.89a | 56.50 ± 16.11p < .001c |
| Group 2 (n = 11) | 14.45 ± 13.94 | 69.82 ± 7.35 | 55.36 ± 16.42p < .001c |
| Group 3 (n = 8) | 5.63 ± 9.04 | 55.25 ± 21.28 | 49.63 ± 21.76p < .001c |
Group 1: patients with single-sided deafness (SSD). Group 2: patients with bilateral severe/profound SNHL. Group 3: Patients with asymmetric SNHL.
PDL = Percentage of language discrimination, list of disyllables in free field at 65 dB SPL.
Regarding the suppression of tinnitus, the analysis was carried out with the 21 patients who presented it preoperatively (Table 4). A marked reduction in tinnitus was observed with the use of the CI, statistically significant (p < .001) for all follow-up points analysed (up to 24 months). Furthermore, tinnitus reduction increased significantly (p < .05) over time up to 18 months, after which a plateau effect was observed.
Outcomes regarding tinnitus in the study population.
| Measurement | Preoperative(n = 21) | 3 months (n = 21) | 6 months (n = 21) | 12 months (n = 21) | 18 months (n = 20) | 24 months (n = 14) | 36 months (n = 3) |
|---|---|---|---|---|---|---|---|
| VAS Mean ± SD | 7.2 ± 1.6 | 3.7 ± 1.1 | 3.1±.9 | 2.5 ± .7 | 1.7 ± 1.3 | 1.6 ± 1.2 | .7 ± 1.2 |
| pa | <.001 | <.001 | <.001 | <.001 | <.001 |
VAS: Visual analogue scale.
Analysis of variance (ANOVA) of repeated measures. Each follow-up point is compared with the preoperative measurement. Post hoc analyses (Bonferroni test) showed that the tinnitus suppression effect continued to increase over time up to 18 months and that these differences were statistically significant: 3 months vs 6 months p = .025, 6 months vs 12 months p = .05, 12 months vs 18 months p = .031. After 18 months the tinnitus suppression effect stabilized: 18 months vs 24 months p = .41. Comparisons made with the total number of subjects (n) available at each follow-up point.
At the time of activation there were an average of 21.8 functional electrodes; at 3 months = 21.9, 6 months = 21.9, 12 months = 21.8, 18 months = 21.3 and at 24 months = 21.3; differences that were not significant (p > .05. ANOVA of repeated measures). Regarding the dynamic range of the active electrodes, a statistically significant increase was observed up to 6 months (p < .05. ANOVA of repeated measures; post hoc analysis Bonferroni test): upon activation = 42.9 ± 6.0UI, 3 months = 55.8 ± 8.1IU, 6 months = 60.1 ± 7.9IU. From 12 months onwards, a plateau effect was observed, with stabilization of the dynamic range: 12 months = 62.7 ± 8.9 IU, 18 months = 65.7 ± 8.7 IU, 24 months = 62.9 ± 10.6 IU and 36 months = 68.3 ± 7.6 IU (p > .05. ANOVA of repeated measures).
Two cases that require special description are those that had complete cochlear obliteration, detected on preoperative imaging. In both cases, the cause of hearing loss was sequelae of chronic otitis media, with a history of ∼4 and 5 years of profound loss. In one case the indication was for SSD (PTA healthy ear = 13 dB, PLD = 100%) and the other for asymmetric SNHL (PTA better ear = 55 dB, air-bone gap = 30 dB, PLD = 100%, hearing aid user). The two patients had significant tinnitus (VAS = 6 and 8, respectively), were active at work (the first case of a driving profession) and after reviewing the rehabilitation options, it was considered, together with the patients, that the best alternative was CI. At the time of surgery, the first patient was 44 years old and the second was 47 years old. There was a CSF fistula that was controlled intraoperatively and by adapting the sound processors, 7 and 10 electrodes could be activated, respectively. Although in both cases the PLD was low, 20% and 30%, tinnitus suppression was significant (VAS = 2 and 1, respectively), both patients used the CI routinely (>12 h/day, every day of the week) and permanently during their work.
DiscussionCochlear implantation in the study population produced a favourable outcome (PLD = 62.2% at 65 dB SPL) comparable to those published in the literature for postlingual adults with similar indications.1,8 The series of patients was relatively uniform with respect to outcome prognostic factors: there were no major malformations of the inner ear (only one case with dilated vestibular aqueduct), the mean time of hearing deprivation was not prolonged = 49.5 months (∼4 years) and there were no cases with pathology of the auditory nerve. On the other hand, there were two cases of complete cochlear ossification that required radical cochleostomy for placement of the electrode array. The need for a radical cochleostomy can negatively affect the result of cochlear implantation, since only a part of the electrodes remains in contact with the cochlear nerve, with the said contact not being very stable, resulting in this ultimately leading to fewer functional electrodes.12,14 In our series, these two patients with complete cochlear ossification have extreme postoperative PLD values (PLD = 20% and PLD = 30%). If we exclude these two cases from the analysis, language discrimination for the total group increases to 64.8%±13.8% (n = 29).
In the initial clinical study with the Nurotron™ CI Zeng et al.8 reported a sentence recognition percentage of 68% at 4 months post-activation, with a maximum of 89% at 36 months of follow-up and with a plateau effect in performance at 6 months. That study included 60 postlingual patients with bilateral severe/profound SNHL, native Mandarin speakers.8 In our study, the group of patients who had a similar indication (group 2, n = 11) showed a disyllable recognition percentage of 69.8%, with a mean follow-up of 31.6 months. Although the results are not directly comparable, since sentences have more redundancy of information than disyllables and therefore higher recognition percentages are expected, the measurements in these two studies show a clear benefit with the CI. In another study with postlingual adults, they report a percentage of recognition of monosyllables (“consonat-nucleus-consonat”, CNC) of 58% at 12 months, in a population of 259 adults (277 ears) operated on between 2016 and 2019.1 In our series of 31 patients, the PLD = 62% is slightly higher than the reference study, although we must take into account that the redundancy of the information is greater with disyllables than with monosyllables. In a study whose speech evaluation was performed with disyllables (in Korean), a PLD = 70% was found at 12 months for 15 patients with sudden deafness,15 a value slightly higher than 61.1% (±19.1%) among the 12 patients who were implanted by SSD in our study (Group 1). In a clinical trial among patients with SSD, Galvin et al.5 reported increases in monosyllable recognition (CNC) of 66.8%, 76% and 84% at 1, 3 and 6 months post-activation, respectively. This study also showed that patients with SSD had clear benefits in terms of sound localization, speech understanding, tinnitus suppression and improvement in quality of life5; results that are in accordance with other studies published on the matter.4,16,17 Regarding results in Spanish, in a recent report using the Nurotron™ cochlear implant, Pacheco-López et al.18 report a PLD = 50% among seven patients over 65 years of age who received the device. Although the PLD of this series is lower than that found in ours, we must take into account that this series only included the elderly population and that there was a case of cochlear reimplantation (on two occasions), which also had a poor outcome with the two previous brands used.
We found a clear benefit with CI in suppressing tinnitus (Table 4). Although in CI candidates, tinnitus can occur in patients with SSD, severe/profound bilateral SNHL, and asymmetric SNHL, tinnitus suppression results are more frequently reported in patients with SSD. In the clinical trial by Galvin et al., among patients with SSD, there was a statistically significant reduction in tinnitus, assessed using the VAS; until reaching a value of 2.3 at 6 months post-activation.5 Similar results have been published consistently by other authors.16,17,19,20 In a technology evaluation study among adults and children with SSD (CI and bone conduction implants), the benefit was greater with cochlear implantation.16 Although the two systems showed benefits in hearing thresholds (functional gain), language understanding in noisy backgrounds and improvement in quality of life; only CI showed a clear reduction in tinnitus and a clearly favourable profile in the cost-effectiveness analyses when comparing CI vs no treatment.16 This study reports that, on average, the increase in the cost-effectiveness ratio was between $17,783 and $18,148/year adjusted for quality of life (QALY) and with a willingness to pay of $100,000 per QALY. The authors found that 70% of simulations were considered profitable for cochlear implantation.16
In an implantable medical device, clinical safety with its use and the replicability of the results are very important aspects for patients, clinical care centres and regulatory authorities. The present study had a mean follow-up = 31.6 months, so it can be considered a medium-term report. It was found that there were two patients with CSF fistula (2/31 = 6.5%), which was satisfactorily controlled in the same operation. Both cases had complete cochlear ossification, required radical cochleostomy, and it was during said drillimg that the IAC was entered and the fistula occurred. Therefore, this situation is directly related to the surgical technique that was required and not to the brand of the device. Some authors consider this as a complication when it occurs postoperatively and not intraoperatively. In a summary of complications among studies with more than 500 patients (10 studies), a prevalence of postoperative CSF fistula = .2% (9/5985)1 is reported. Regarding minor postoperative complications (Fig. 4), we had 10 events in 8 patients, that is, a prevalence = 25.8%; of them, 9 events were vertigo/unsteadiness (in 7 patients, prevalence = 22.6%). Carlson et al., in their summary of studies, report a prevalence of minor complications of 8.4% (482/5771); vertigo/unsteadiness being the most frequent, in 102/4664(2.2%) patients.1 In our study, this frequency was higher in part because both the symptom and the sign were reported as a positive finding. In a meta-analysis that specifically investigated this symptom, it was found that 9.3% of patients (1283/13783) reported vertigo after cochlear implantation.21 However, as a new symptom in the postoperative period, in the 31 studies that reported it, the symptom was present in 17.4%.21 This value is close to the one we found in our series. Even when investigating the symptom preoperatively, the researchers found that, among 32 studies that reported it, 24.7% of patients (451/1827) reported vértigo.21 This finding is interesting and can be explained because the same pathology that causes hearing loss generates an active lesion or permanent damage to the vestibular receptors, leading to persistent vestibular symptoms. Although we did not record preoperative vertigo/unsteadiness, it is expected that a group of patients had it, since there were cases of sudden deafness, Meniere's disease, and ototoxicity and they may have had an active vestibular lesion or permanent damage to the vestibular receptors. Another factor that may also explain the high frequency of vertigo/unsteadiness in our series is that it is a population with a relatively high average age of implantation (49.8 ± 18.8 years), with 24 patients >30 years old and 18 patients >50 years old. In the adult population, there are several factors that contribute to vestibular symptoms after cochlear implantation, apart from surgical trauma, such as previous damage to the vestibular receptors due to otological pathology and degeneration of systems related to balance (presbivertigo).21 In patients >18 years of age, the meta-analysis by Hänsel et al., shows a prevalence of postoperative vertigo of 16.8% (446/2651) and a clear correlation was found between the presence of vertigo and a higher age of implantation.21 These results are comparable to those found in our series.
As a major postoperative complication, we found a patient with hard failure of the implant, equivalent to 3.23% (1/31). In Carlson's review, a prevalence of major complications of 2.7% (207/7542) is reported; with a mean frequency of hard failure of CI = 1.9% (125/6461), among the 7 articles that report this information (range = .2%–6.0%).1 When evaluating failure in CI, the follow-up period is a relevant factor, since a greater frequency of failures will be expected as follow-up is prolonged. For example, in a group of patients operated on over a period of 30 years (median follow-up = 4.8 years), the frequency of CI failures was 4.8% (136/2827); 3.9% being technical failures that do not allow the implant to function (“hard failure”) and .9% being “soft” technical failures.22 These failures occurred ∼5 years after surgery (children = 5.5 years, adults = 5.3 years),22 which reinforces the importance of the follow-up period when assessing this complication. Therefore, we see that with the medium-term follow-up that we report, the frequency of technical failure of 3.23% is higher than that reported in studies with comparable follow-up and is an aspect that must continue to be observed.
Regarding complications using the specific brand Nurotron™, there is neither clear nor sufficient information available, given that it is a relatively new brand on the market (11 years of clinical use) and the published studies are limited. Zeng et al. did not record information about complications or device failures.8 Li et al.23 and Yu et al.24 vaguely report that during follow-up (2 and 3 years, respectively), there was no infection, no extrusion, no complications or adverse effects related to the Nurotron™ CI. However, methodological systematicity to collect information on complications is not observed in these studies. Rebscher et al., only mention complications during electrode insertion in tests on temporal bones, with a frequency of severe trauma with insertion of 12.5% (8 specimens)25 Other clinical studies also do not describe complications with the use of this brand of CI, nor do they mention device failures.18,26–30 The analysis of the implant that presented a technical failure by the manufacturer reported electronic damage. In the review of 136 cochlear implants that failed, Wang et al.22 found damage to the electronic components in 19.5% of the 77 devices that were analysed by the manufacturer. In this study, the most frequent cause of device failure was damage to the electrode array with 48.1%.
The availability of an additional brand of CI generates greater supply and can improve access, especially in developing countries where the cost of treatment is an important factor in its achievement. This is well exemplified in a bidding process, frequently used for the purchase of these devices, where “bidding” with one more bidder can lead to more devices being obtained for a fixed amount of money. In Colombia we have the option of using five brands of CI (Advanced Bionics™, Cochlear™, Medel™, Nurotron™ and Oticon™); we found that Nurotron™ is ∼20% lower in cost than the average of other brands.
In conclusion, we found that among postlingual Spanish-speaking adult patients, the Nurotron™ CI presents satisfactory audiological results that are consistent with those reported with other CI brands in similar population groups. Our findings replicate the results of studies with native Mandarin speakers that have been published with Nurotron™. We also found a significant reduction in tinnitus with the use of the CI. Regarding clinical safety, with a medium-term follow-up, we found that the minor complications are consistent with those reported in the literature, although the percentage of technical failure must be carefully monitored as it was higher than that reported in other series with a period of comparable monitoring. Postoperative vertigo/unsteadiness was slightly more common, due in part to the clinical characteristics of the study population. As limitations of the present study we must mention the descriptive nature of its design, the lack of audiological measurements in noisy background and quality of life measurements; which are currently very important when evaluating a treatment such as CI. Studies are required in other population groups and with longer follow-up periods, which corroborate the audiological and clinical safety results, both from our study and those reported with native Mandarin-speaking patients.
Conflicts of interestsNone.
To Doctor Jorge Medina from the research department of Unisanitas-Clínica Universitaria Colombia for his support during the preparation process of this study.
Partial financial funding was received from Fundación Universitaria Sanitas (Unisanitas, Bogotá, Colombia), specifically for investigation committee approval process.
Preliminary outcomes presented at: 11th Asia Pacific Symposium on Cochlear Implants and Related Sciences. Cyprus – September 19–22, 2017. Abstract published in: Ordóñez-Ordóñez LE, Angulo-Martínez ES, Rodriguez SR, Vanegas SC. Clinical Safety and Outcomes with Nurotron™ Cochlear Implant in Spanish Speaking Patients. J Otolaryngol ENT Res. 2017;9(2): 00280. DOI:10.15406/joentr.2017.09.00280.