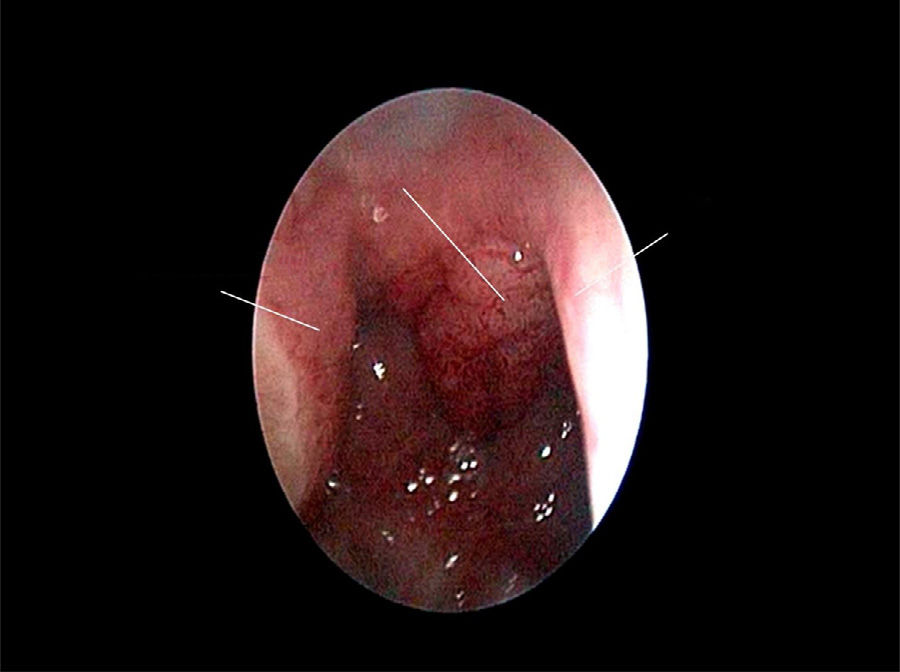
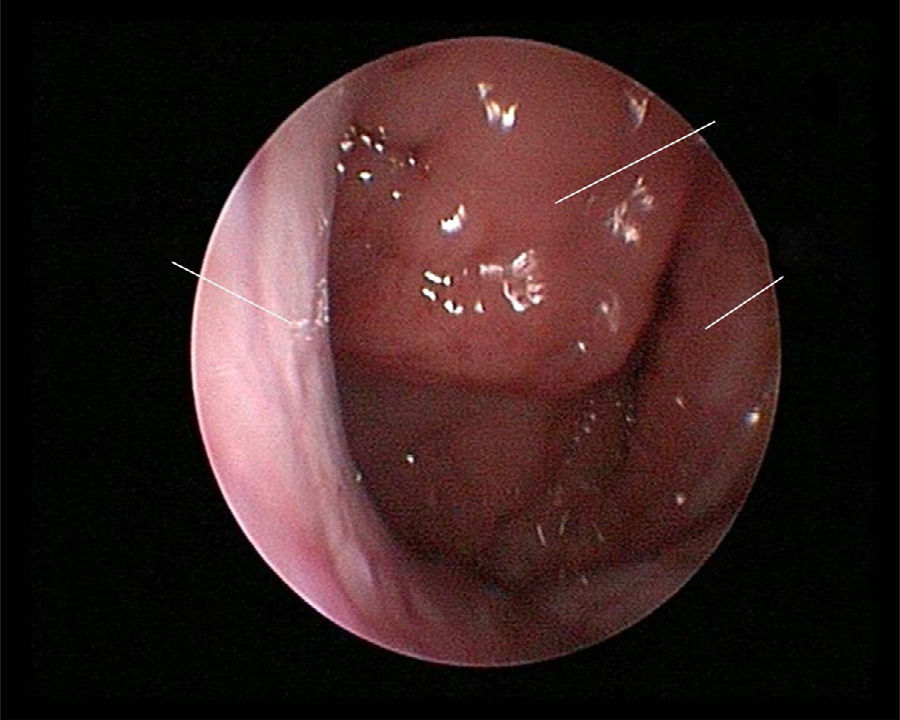
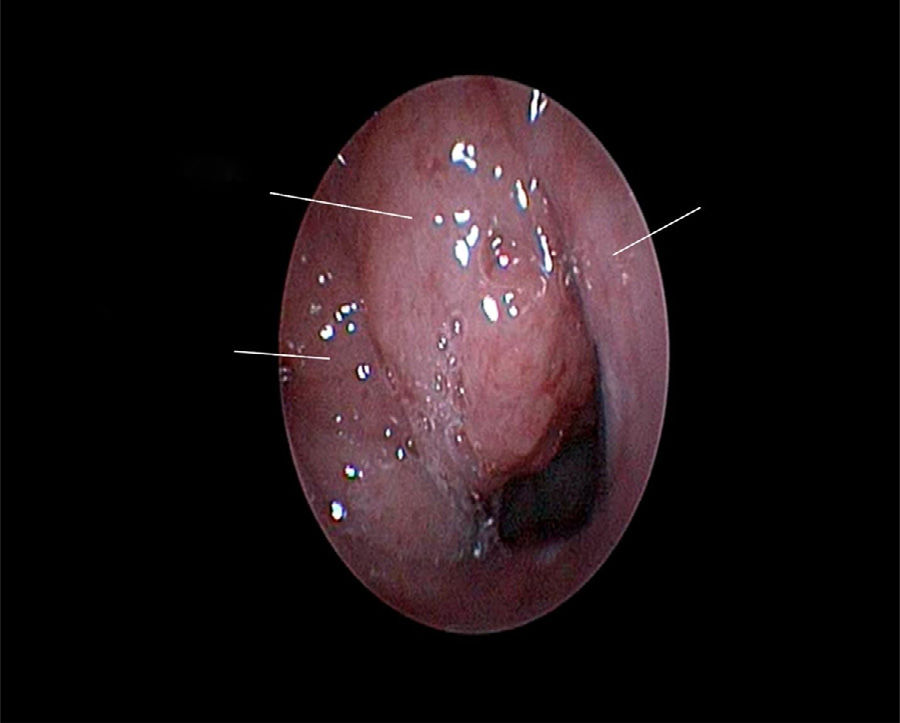
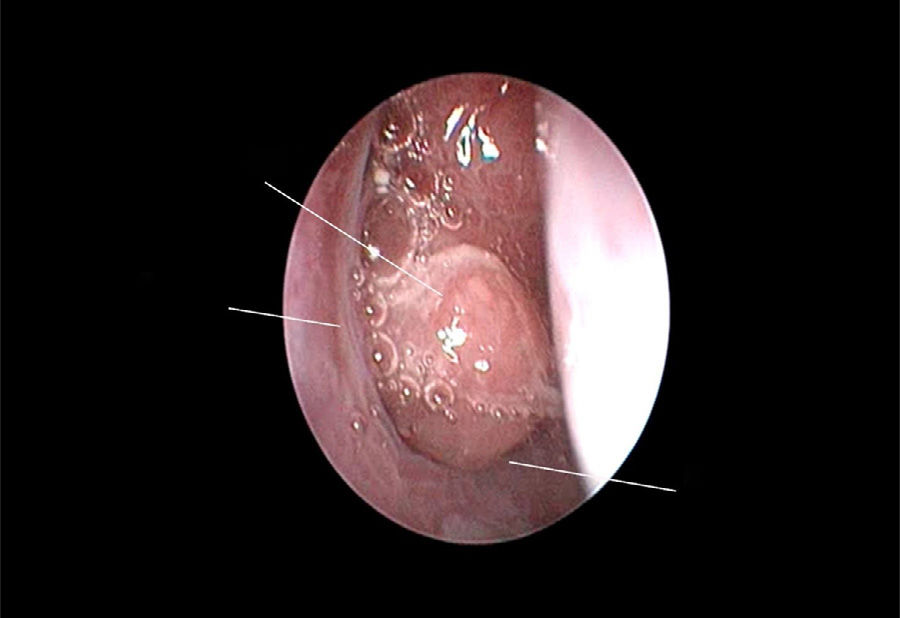
The use of mometasone furoate (MF) intranasal spray in treating adenoid hypertrophy (AH) has a variable outcome due the different methods of adenoid size evaluation. The aim of our study was to evaluate the effect of MF intranasal spray in children and adolescents with AH using a reliable and consistent endoscopic evaluation.
Material and methodA prospective interventional study was conducted. Evaluation took place during the first visit (week 0) and second visit (week 12). Symptoms of nasal obstruction, rhinorrhoea, cough and snoring were assessed, and an overall total symptoms score was obtained. A rigid nasoendoscopic examination using a four-grading system of adenoid size from 1 to 4 was performed. Patients were treated with MF intranasal spray for 12 weeks. Patients’ aged 7–11-years old used 1 spray in each nostril once daily, while patients aged 12–17 used two sprays in each nostril once daily. Reassessment was carried out during the second visit (week 12).
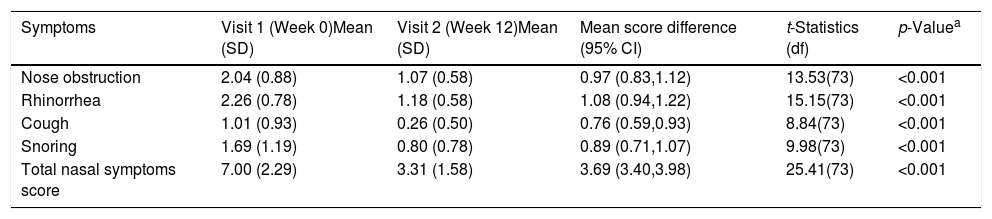
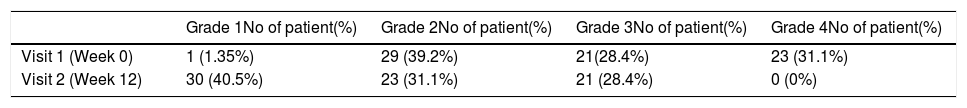
ResultsA total of 74 patients was recruited. There were significant improvements from week 0 to week 12 in the symptoms’ score for nose obstruction, rhinorrhoea, cough, snoring including the total nasal symptoms’ score (p<0.001). AH significantly reduced in size from week 0 (2.89±.87) to week 12 (1.88±.83) (p<0.001).
ConclusionMF intranasal spray is effective in improving the symptoms attributed to AH as well as reducing the adenoid size. MF intranasal spray is advocated as a treatment option before adenoidectomy is considered.
El papel del aerosol nasal de mometasona furoato (MF) para tratar la hipertrofia adenoidea (HA) tiene un resultado variable, debido a los diferentes métodos de evaluación del tamaño de las adenoides. El objetivo de nuestro estudio fue evaluar el efecto del aerosol nasal de MF en niños y adolescentes con HA, utilizando una evaluación endoscópica fiable y consistente.
Material y métodoSe llevó a cabo un estudio prospectivo intervencionista. La evaluación se realizó durante la primera visita (semana 0) y la segunda visita (semana 12). Se valoraron los síntomas de obstrucción nasal, rinorrea, tos y ronquidos, obteniéndose una puntuación de síntomas totales globales. Se realizó un examen nasoendoscópico rígido utilizando un sistema de clasificación del tamaño adenoideo, con valores de 1 a 4. Los pacientes fueron tratados con aerosol intranasal de MF durante 12 semanas. Los pacientes con edades comprendidas entre 7 y 11 años utilizaron 1 pulverización en cada fosa nasal una vez al día, mientras que los pacientes de 12 a 17 años utilizaron 2 pulverizaciones en cada fosa nasal una vez al día. La re-evaluación se realizó durante la segunda visita (semana12).
ResultadosReunimos a un total de 74 pacientes. Se produjeron mejoras significativas de la semana 0 a la 12 en cuanto a puntuación de los síntomas de obstrucción nasal, rinorrea, tos y ronquidos, incluyendo la puntuación total de síntomas nasales (p<0,001). Se redujo significativamente el tamaño de HA de la semana 0 (2,89 ±0,87) a la semana 12 (1,88 ±0,83) (p<0,001).
ConclusiónEl aerosol intranasal de MF es efectivo para mejorar los síntomas atribuidos a HA, así como reducir el tamaño de las adenoides. Se propone el uso de dicho aerosol intranasal como opción de tratamiento, antes de considerarse la adenoidectomía.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora