This study aimed to analyze the behavior of acute invasive fungal rhinosinusitis (AIFRS) associated with COVID-19 infection as there has been an increase in the rate of AIFRS cases in the last two years, and many reports connected this rising with the COVID-19 infection. We studied most factors that may impact the prognosis as a trial to find the most affecting factors to improve the outcomes.
MethodsIt was a retrospective observational study that included cases from four tertiary referral institutions between November 2020 to February 2022. We included sixty-six patients who suffered from AIFRS associated with confirmed COVID-19. We observed the prognosis of all included patients with a six-month follow-up. We correlated the prognosis with many factors, such as demographic data, medical conditions, blood investigations, the features of fungal infections, and management.
ResultsForty-two patients (64%) survived after the AIFRS associated with COVID-19, and twenty-two patients (36%) died. High doses of corticosteroids with prolonged use were the main factors that affected the behavior of the AIFRS associated with COVID-19. HbA1c was a good predictor of the prognosis; a level less than 9.35% may indicate survival with 87.5% sensitivity.
ConclusionsAccording to this multi-center study, the mortality of the AIFRS associated with COVID-19 was high. The behavior was affected by glycemic control, the type of fungal species, and the type of antifungal therapy. Early surgical debridement, a combination of Amphotericin B with Voriconazole, and anticoagulants helped improve the prognosis.
Durante el periodo de la pandemia de COVID19, ha habido un aumento en la tasa de casos de rinosinusitis fúngica invasiva aguda (RSFIA), siendo cada vez más evidente la asociación entre ambas entidades. EL objetivo de este estudio ha sido analizar la evolución de los pacientes con rinosinusitis fúngica invasiva aguda asociado con la infección por COVID-19, analizando los factores determinantes en la evolución y pronóstico de la enfermedad.
MétodosFue un estudio observacional retrospectivo que incluyó casos de cuatro instituciones de referencia de tercer nivel entre noviembre de 2020 y febrero de 2022. Se incluyeron sesenta y seis pacientes que padecían RSFIA asociado a COVID-19 confirmado. Observamos el pronóstico de todos los pacientes incluidos con un seguimiento de seis meses. Correlacionamos el pronóstico con muchos factores, como los datos demográficos, las condiciones médicas, las investigaciones de sangre, las características de las infecciones fúngicas y el manejo.
ResultadosCuarenta y dos pacientes (64%) sobrevivieron después de la RSFIA asociada a COVID-19, y veintidós pacientes (36%) fallecieron. Las dosis altas de corticoides con uso prolongado fueron los principales factores que afectaron el comportamiento de la RSFIA asociada a la COVID-19. HbA1c fue un buen predictor del pronóstico; un nivel inferior al 9,35 % puede indicar supervivencia con una sensibilidad del 87.5%.
ConclusionesSegún este estudio multicéntrico, la mortalidad de la RSFIA asociada a la COVID-19 fue alta. El comportamiento se vio afectado por el control glucémico, el tipo de especie fúngica y el tipo de terapia antifúngica. El desbridamiento quirúrgico temprano, una combinación de Amphotericin B con Voriconazole y anticoagulantes ayudaron a mejorar el pronóstico.
The recent worldwide pandemic of Coronavirus disease-19 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has become at the forefront of the interest of all health service givers and institutes.1
COVID-19 affects immunity by depressing the neutrophilic response, causes overexpression of inflammatory cytokines (cytokine storm), and impairs cell-mediated immunity by decreasing cluster differentiation of CD4+ T and CD8+ T-helper cells. These factors cause a rapid increase in co-existing infections, especially fungal infections.2
The fungal hyphae are usually present in the nasal mucosa as commensals and only become infectious and develop pathology if the host’s immunity is impaired. In these cases, the hyphae invade the blood vessels and mucous membranes, causing invasive fungal rhino-sinusitis (IFRS). So, IFRS is usually presented in immunosuppression, as in cases with hematological malignancies, acquired immunodeficiency syndrome, cases on chemotherapy, and patients with organ transplants.3
Clinically, acute IFRS can be presented by signs and symptoms similar to complicated sinusitis, such as nasal blockage, crustations, proptosis, facial pain, facial edema, ptosis, chemosis, ophthalmoplegia, and various neurological signs and symptoms of intracranial extension.4 During the COVID-19 pandemic, there has been an increase in the rate of acute IFRS cases. Additionally, many reports connected the rising incidence of the IFRS with the COVID-19 infection.5
The aim of this study was to analyze the behavior, course, and prognosis of the acute IFRS associated with COVID-19. We also aimed to find the most significant prognosis predictors that may help improve the outcomes.
Patients and methodsEthicsBefore starting our research, we got the Institutional Review Board of Tanat University’s approval to conduct this study (approval code: 35920). Signed informed consent for using the patient’s data in our research was obtained from the patient or the relatives. All included procedures were performed according to the Declaration of Helsinki.
Study designIt was a retrospective observational study.
Sample collectionWe included 66 patients from four tertiary referral hospitals in the period between November 2020 to February 2022.
SubjectsWe included patients who presented with acute invasive fungal rhinosinusitis (AIFRS) with a proven COVID-19 infection within the prior three months. We excluded patients who didn't complete the six-months follow-up (four cases). We also excluded patients with a history of acquired immunodeficiency syndrome (one patient), malignancies (six patients), chemotherapy (three patients), bone marrow depression (two patients), organ transplant (one patient), and non-diabetic patients (six patients). So, we included 66 patients out of 89 patients. We excluded patients with immunosuppression causes other than DM to unify the cause of the immunosuppression and focus on the role of COVID-19 on the prognosis.
COVID-19 infectionCOVID-19 infection was proved by the clinical presentation and symptoms, chest computed tomography (CT) scan, and positive polymerase chain reaction (PCR) test. The patients were classified into mild (mild symptoms and normal imaging), moderate (pneumonia manifestations on radiology associated with symptoms &/Or leucopenia or lymphopenia and the blood oxygen saturation >92%), and severe (blood oxygen saturation <92%, the respiratory rate >30 breaths per minute, and the lung infiltrates >50%) according to COVID-19 severity.6 They were managed according to the protocol of each category with or without antibiotics and corticosteroids.
AIFRSThe diagnosis of AIFRS was confirmed by the clinical presentation (recent sinonasal manifestations), endoscopic nasal examination, CT scan with or without magnetic resonance imaging (MRI), histopathological assessment of nasal biopsies, and a fungal culture for identification of fungal species.
Blood workupOn admission into the hospital, the following blood investigations were done; hemoglobin (Hb) level, glycosylated hemoglobin (HbA1c) level, C-reactive protein (CRP) test, D-dimer test, and serum ferritin level. The patients who received anticoagulants were monitored by activated partial thromboplastin time (APTT). Kidney functions were closely monitored during the antifungal course.
AIFRS managementAll patients underwent glycemic control and surgical debridement of the necrotic gangrenous tissues. The debridement was tailored according to the findings of each case. The surgical intervention was done when the patients became generally stable with complete personal safety protection procedures. The patients received an antifungal regimen (with or without anticoagulant) according to the treating institute protocol and the histopathology results.
Outcome measuresWe observed the prognosis of patients with AIFRS after COVID-19 infection. Improvement was confirmed by clinical examination and imaging. The survived cases underwent a six-month follow-up to assess any relapse. We correlated the prognosis of the included patients with different variables as a trial to get the most important factors that impacted the prognosis of the AIFRS after COVID-19 infection. We divided the patients into two groups according to the prognosis: group A included forty-two patients (64%) who survived, and group B included twenty-four patients (36%) who did not survive.
Statistical analysisStatistical analysis was done using SPSS v22 (IBM© Inc., Chicago, IL, USA). Numerical variables were presented as mean and standard deviation (SD). Categorical variables were presented as frequency and percentage (%). We used the Mann–Whitney test to compare the means of numerical variables. We used the Chi-square test to compare categorical variables. To assess the correlation of the prognosis with the other variables, we used the Spearman correlation coefficient test. P-value < 0.05 was considered significant. We used the Kaplan-Meier curve to assess the effect of corticosteroids on the time interval between the COVID-19 infection and the start of AIFRS. We held the receiver operating characteristic (ROC) curve between the prognosis and the HbA1c blood level.
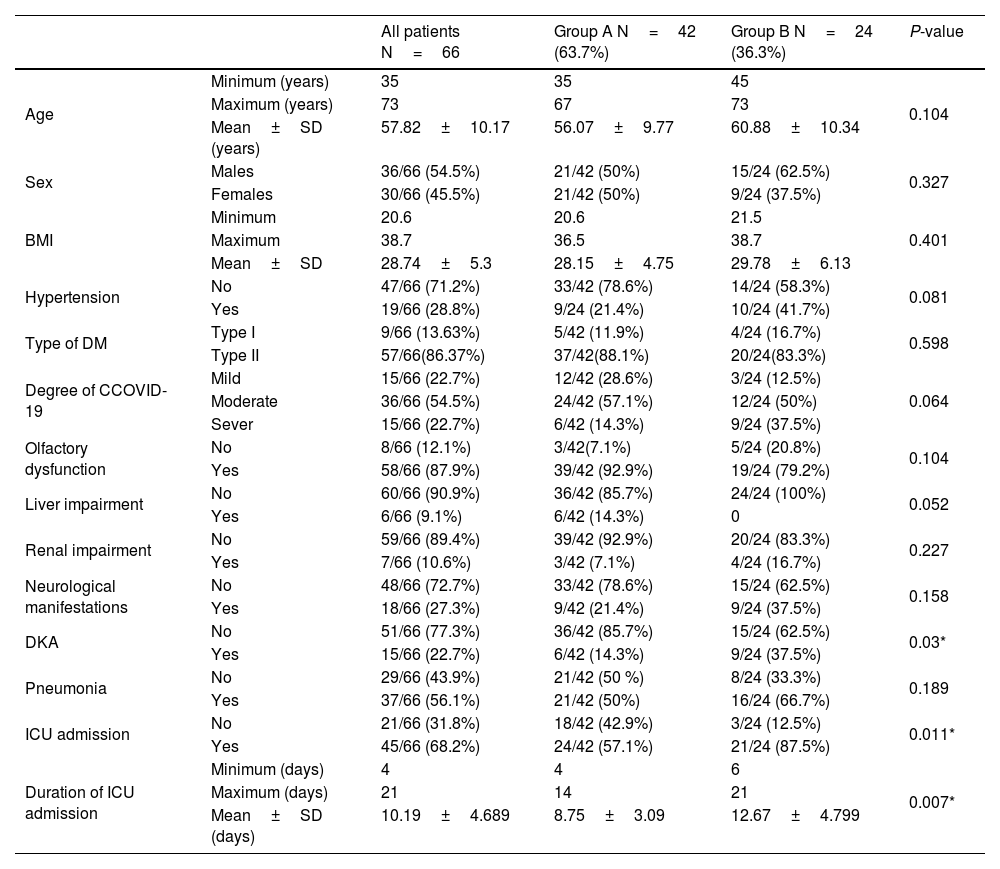
ResultsDemographic resultsThe patientsʹ age, sex, and BMI didn't show statistically significant differences between both groups (P-values > 0.05) (Table 1).
The results of the demographic data and medical conditions.
| All patients N=66 | Group A N=42 (63.7%) | Group B N=24 (36.3%) | P-value | ||
|---|---|---|---|---|---|
| Age | Minimum (years) | 35 | 35 | 45 | 0.104 |
| Maximum (years) | 73 | 67 | 73 | ||
| Mean±SD (years) | 57.82±10.17 | 56.07±9.77 | 60.88±10.34 | ||
| Sex | Males | 36/66 (54.5%) | 21/42 (50%) | 15/24 (62.5%) | 0.327 |
| Females | 30/66 (45.5%) | 21/42 (50%) | 9/24 (37.5%) | ||
| BMI | Minimum | 20.6 | 20.6 | 21.5 | 0.401 |
| Maximum | 38.7 | 36.5 | 38.7 | ||
| Mean±SD | 28.74±5.3 | 28.15±4.75 | 29.78±6.13 | ||
| Hypertension | No | 47/66 (71.2%) | 33/42 (78.6%) | 14/24 (58.3%) | 0.081 |
| Yes | 19/66 (28.8%) | 9/24 (21.4%) | 10/24 (41.7%) | ||
| Type of DM | Type I | 9/66 (13.63%) | 5/42 (11.9%) | 4/24 (16.7%) | 0.598 |
| Type II | 57/66(86.37%) | 37/42(88.1%) | 20/24(83.3%) | ||
| Degree of CCOVID-19 | Mild | 15/66 (22.7%) | 12/42 (28.6%) | 3/24 (12.5%) | 0.064 |
| Moderate | 36/66 (54.5%) | 24/42 (57.1%) | 12/24 (50%) | ||
| Sever | 15/66 (22.7%) | 6/42 (14.3%) | 9/24 (37.5%) | ||
| Olfactory dysfunction | No | 8/66 (12.1%) | 3/42(7.1%) | 5/24 (20.8%) | 0.104 |
| Yes | 58/66 (87.9%) | 39/42 (92.9%) | 19/24 (79.2%) | ||
| Liver impairment | No | 60/66 (90.9%) | 36/42 (85.7%) | 24/24 (100%) | 0.052 |
| Yes | 6/66 (9.1%) | 6/42 (14.3%) | 0 | ||
| Renal impairment | No | 59/66 (89.4%) | 39/42 (92.9%) | 20/24 (83.3%) | 0.227 |
| Yes | 7/66 (10.6%) | 3/42 (7.1%) | 4/24 (16.7%) | ||
| Neurological manifestations | No | 48/66 (72.7%) | 33/42 (78.6%) | 15/24 (62.5%) | 0.158 |
| Yes | 18/66 (27.3%) | 9/42 (21.4%) | 9/24 (37.5%) | ||
| DKA | No | 51/66 (77.3%) | 36/42 (85.7%) | 15/24 (62.5%) | 0.03* |
| Yes | 15/66 (22.7%) | 6/42 (14.3%) | 9/24 (37.5%) | ||
| Pneumonia | No | 29/66 (43.9%) | 21/42 (50 %) | 8/24 (33.3%) | 0.189 |
| Yes | 37/66 (56.1%) | 21/42 (50%) | 16/24 (66.7%) | ||
| ICU admission | No | 21/66 (31.8%) | 18/42 (42.9%) | 3/24 (12.5%) | 0.011* |
| Yes | 45/66 (68.2%) | 24/42 (57.1%) | 21/24 (87.5%) | ||
| Duration of ICU admission | Minimum (days) | 4 | 4 | 6 | 0.007* |
| Maximum (days) | 21 | 14 | 21 | ||
| Mean±SD (days) | 10.19±4.689 | 8.75±3.09 | 12.67±4.799 | ||
Group A, survived patients; Group B, dead patients; SD, standard deviation; BMI, body mass index; PCR, polymerase chain reaction; DKA, diabetic ketoacidosis; DM, diabetes mellitus; ICU, intensive care unit.
*Significant as the P-value was < 0.05.
There was no statistically significant difference between both groups regarding the COVID-19 severity. All the patients were not vaccinated against COVID-19 (Table 1).
Medical co-morbiditiesOn hospital admission, six patients (14.3%) in group A and nine patients (37.5%) in group B had diabetic ketoacidosis (DKA), with a statistically significant difference between both groups. On the other hand, both groups did not differ significantly regarding the other co-morbidities such as hypertension, bronchopneumonia, type of DM, olfactory dysfunction, liver impairment, and renal impairment. Additionally, eighteen patients (27%) suffered from other neurological manifestations such as vertigo (3 patients in group A and six patients in group B) and peripheral neuritis (4 patients in group A and five patients in group B) without a statistically significant difference between both groups. On the other hand, 24 patients (57.1%) in group A and 21 patients (87.5%) in group B were admitted to the ICU, with a statistically significant difference between both groups and a statistically significant longer ICU admission duration in group B (Table 1).
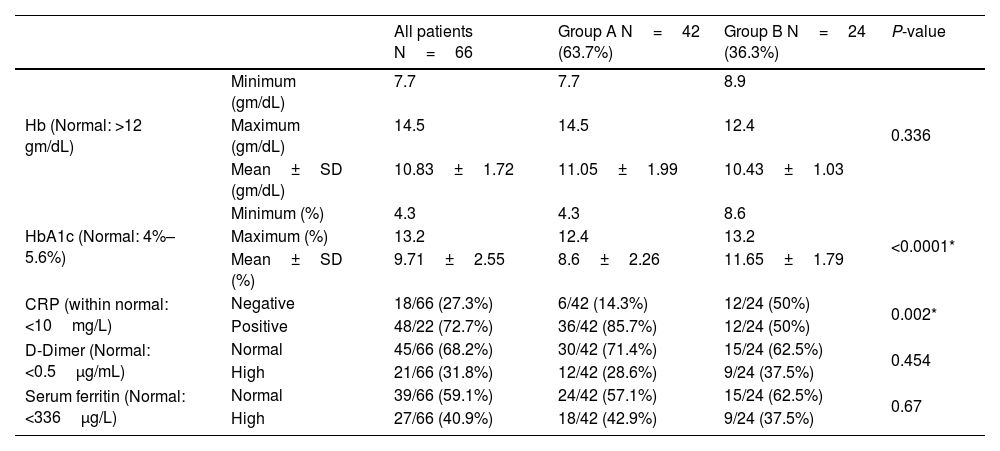
InvestigationsOn admission, the HbA1c ranged from 4.3%–12.4% in group A and 8.6%–13.2% in group B, with a statistically significant difference between both groups (P-value < 0.0001). The level of HbA1c at the six-month follow-up of the forty-two patients improved patients (group A) ranged from 4.1%–6.2%, with a mean of 5.03±0.62. The CRP was positive in thirty-six patients (85.7%) in group A and twelve patients (50%) in group B, with a statistically significant difference between both groups. The Hb, serum ferritin, and D-dimer levels didn't show statistically significant differences between both groups (Table 2).
The blood investigations results.
| All patients N=66 | Group A N=42 (63.7%) | Group B N=24 (36.3%) | P-value | ||
|---|---|---|---|---|---|
| Hb (Normal: >12 gm/dL) | Minimum (gm/dL) | 7.7 | 7.7 | 8.9 | 0.336 |
| Maximum (gm/dL) | 14.5 | 14.5 | 12.4 | ||
| Mean±SD (gm/dL) | 10.83±1.72 | 11.05±1.99 | 10.43±1.03 | ||
| HbA1c (Normal: 4%–5.6%) | Minimum (%) | 4.3 | 4.3 | 8.6 | <0.0001* |
| Maximum (%) | 13.2 | 12.4 | 13.2 | ||
| Mean±SD (%) | 9.71±2.55 | 8.6±2.26 | 11.65±1.79 | ||
| CRP (within normal: <10mg/L) | Negative | 18/66 (27.3%) | 6/42 (14.3%) | 12/24 (50%) | 0.002* |
| Positive | 48/22 (72.7%) | 36/42 (85.7%) | 12/24 (50%) | ||
| D-Dimer (Normal: <0.5μg/mL) | Normal | 45/66 (68.2%) | 30/42 (71.4%) | 15/24 (62.5%) | 0.454 |
| High | 21/66 (31.8%) | 12/42 (28.6%) | 9/24 (37.5%) | ||
| Serum ferritin (Normal: <336μg/L) | Normal | 39/66 (59.1%) | 24/42 (57.1%) | 15/24 (62.5%) | 0.67 |
| High | 27/66 (40.9%) | 18/42 (42.9%) | 9/24 (37.5%) | ||
Group A, survived patients; Group B, dead patients; SD, standard deviation; Hb, hemoglobin level; CRP, c-reactive protein.
*Significant as the P-value was < 0.05.
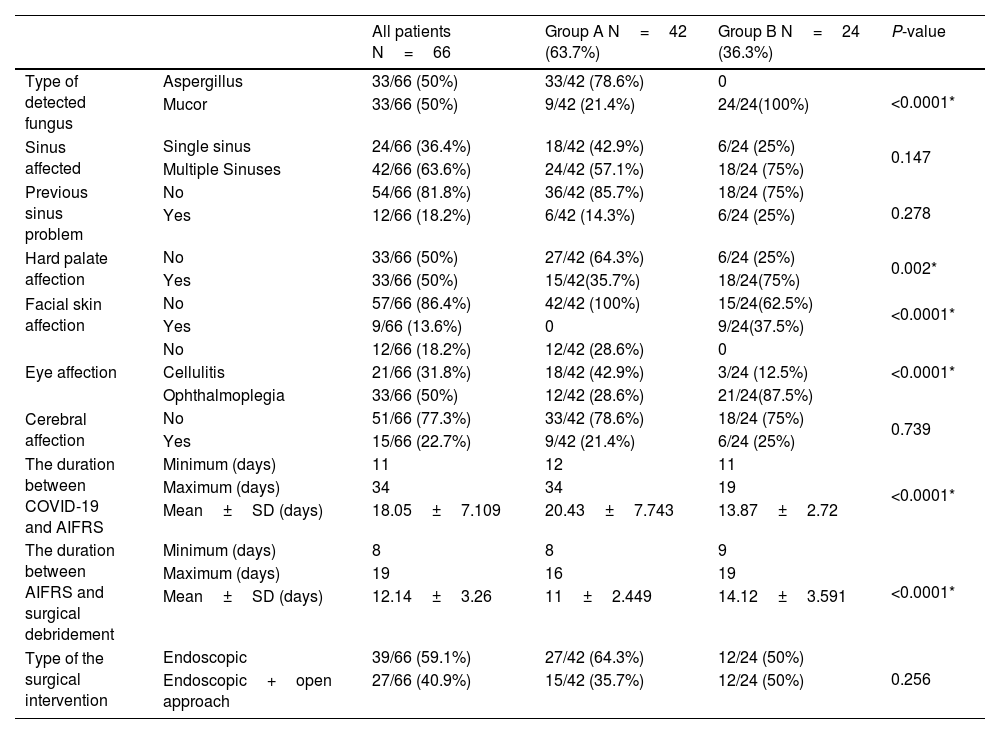
In group A, thirty-three patients (79%) had Aspergillus, and nine patients (21%) had Mucormycosis. In contrast, the causative fungus in group B was Mucormycosis in all patients. The causative fungus type showed a high statistically significant difference between both groups (P-value < 0.0001). The duration between the first positive COVID-19 PCR and the appearance of AIFRS ranged from 12 days to 34 days in group A and 11 days to 19 days in group B, with a statistically significant difference between both groups (P-value < 0.0001).
The hard palate was affected in fifteen patients (35.7%) in group A and eighteen patients (75%) in group B. The facial skin was involved in nine patients (37.5%) in group B, and no patient was affected in group A. In groups A, eighteen patients (42.5%) had orbital cellulitis, and twelve (28.6%) suffered from ophthalmoplegia. While in group B, orbital cellulitis was present in three (12.5%) patients, and twenty-one (87.5%) had ophthalmoplegia. Both groups showed statistically significant differences regarding hard palate, facial skin, and orbital affection.
The included patients underwent surgical debridement of the necrotic tissues by either; a transnasal endoscopic approach in thirty-nine patients (59%) or an endoscopic approach combined with the open approach in twenty-seven patients (41%). The time interval between the start of AIFRS and the surgical debridement ranged from eight days to sixteen days in group A and nine days to nineteen days in group B, with a statistically significant difference between both groups. This time-lapse differed according to the general condition of every patient and the COVID-19 condition (Table 3).
The results of the AIFRS.
| All patients N=66 | Group A N=42 (63.7%) | Group B N=24 (36.3%) | P-value | ||
|---|---|---|---|---|---|
| Type of detected fungus | Aspergillus | 33/66 (50%) | 33/42 (78.6%) | 0 | <0.0001* |
| Mucor | 33/66 (50%) | 9/42 (21.4%) | 24/24(100%) | ||
| Sinus affected | Single sinus | 24/66 (36.4%) | 18/42 (42.9%) | 6/24 (25%) | 0.147 |
| Multiple Sinuses | 42/66 (63.6%) | 24/42 (57.1%) | 18/24 (75%) | ||
| Previous sinus problem | No | 54/66 (81.8%) | 36/42 (85.7%) | 18/24 (75%) | 0.278 |
| Yes | 12/66 (18.2%) | 6/42 (14.3%) | 6/24 (25%) | ||
| Hard palate affection | No | 33/66 (50%) | 27/42 (64.3%) | 6/24 (25%) | 0.002* |
| Yes | 33/66 (50%) | 15/42(35.7%) | 18/24(75%) | ||
| Facial skin affection | No | 57/66 (86.4%) | 42/42 (100%) | 15/24(62.5%) | <0.0001* |
| Yes | 9/66 (13.6%) | 0 | 9/24(37.5%) | ||
| Eye affection | No | 12/66 (18.2%) | 12/42 (28.6%) | 0 | <0.0001* |
| Cellulitis | 21/66 (31.8%) | 18/42 (42.9%) | 3/24 (12.5%) | ||
| Ophthalmoplegia | 33/66 (50%) | 12/42 (28.6%) | 21/24(87.5%) | ||
| Cerebral affection | No | 51/66 (77.3%) | 33/42 (78.6%) | 18/24 (75%) | 0.739 |
| Yes | 15/66 (22.7%) | 9/42 (21.4%) | 6/24 (25%) | ||
| The duration between COVID-19 and AIFRS | Minimum (days) | 11 | 12 | 11 | <0.0001* |
| Maximum (days) | 34 | 34 | 19 | ||
| Mean±SD (days) | 18.05±7.109 | 20.43±7.743 | 13.87±2.72 | ||
| The duration between AIFRS and surgical debridement | Minimum (days) | 8 | 8 | 9 | <0.0001* |
| Maximum (days) | 19 | 16 | 19 | ||
| Mean±SD (days) | 12.14±3.26 | 11±2.449 | 14.12±3.591 | ||
| Type of the surgical intervention | Endoscopic | 39/66 (59.1%) | 27/42 (64.3%) | 12/24 (50%) | 0.256 |
| Endoscopic+open approach | 27/66 (40.9%) | 15/42 (35.7%) | 12/24 (50%) | ||
Group A, survived patients; Group B, dead patients; SD, standard deviation.
*Significant as the P-value was < 0.05.
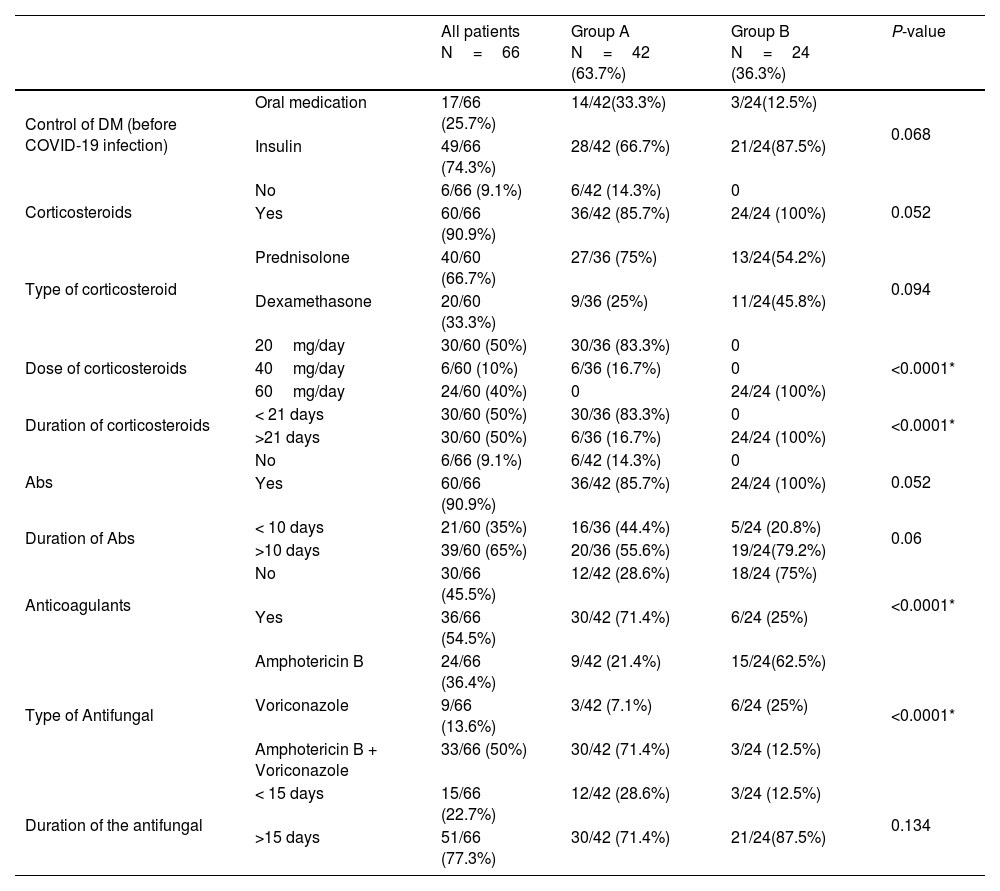
The dose and the course duration of the given corticosteroids differed significantly between both groups. Sixty cases (91%) received antibiotics without a statistically significant difference between both groups. In group A, nine patients (21.4%) received Amphotericin B, three patients (7.1%) received Voriconazole, and thirty patients (71.4%) received a combination of Amphotericin B and Voriconazole. While in group B, fifteen patients (62.5%) received Amphotericin B, six patients (25%) received Voriconazole, and three patients (12.5%) received a combination of Amphotericin B and Voriconazole. The type of the given antifungal drug showed a high statistically significant difference between both groups (P-value < 0.0001). Thirty patients (71.4%) in group A and six patients (25%) in group B received anticoagulants, with a statistically significant difference between both groups (P-value < 0.0001) (Table 4).
The results of the given medications.
| All patients N=66 | Group A N=42 (63.7%) | Group B N=24 (36.3%) | P-value | ||
|---|---|---|---|---|---|
| Control of DM (before COVID-19 infection) | Oral medication | 17/66 (25.7%) | 14/42(33.3%) | 3/24(12.5%) | 0.068 |
| Insulin | 49/66 (74.3%) | 28/42 (66.7%) | 21/24(87.5%) | ||
| Corticosteroids | No | 6/66 (9.1%) | 6/42 (14.3%) | 0 | 0.052 |
| Yes | 60/66 (90.9%) | 36/42 (85.7%) | 24/24 (100%) | ||
| Type of corticosteroid | Prednisolone | 40/60 (66.7%) | 27/36 (75%) | 13/24(54.2%) | 0.094 |
| Dexamethasone | 20/60 (33.3%) | 9/36 (25%) | 11/24(45.8%) | ||
| Dose of corticosteroids | 20mg/day | 30/60 (50%) | 30/36 (83.3%) | 0 | <0.0001* |
| 40mg/day | 6/60 (10%) | 6/36 (16.7%) | 0 | ||
| 60mg/day | 24/60 (40%) | 0 | 24/24 (100%) | ||
| Duration of corticosteroids | < 21 days | 30/60 (50%) | 30/36 (83.3%) | 0 | <0.0001* |
| >21 days | 30/60 (50%) | 6/36 (16.7%) | 24/24 (100%) | ||
| Abs | No | 6/66 (9.1%) | 6/42 (14.3%) | 0 | 0.052 |
| Yes | 60/66 (90.9%) | 36/42 (85.7%) | 24/24 (100%) | ||
| Duration of Abs | < 10 days | 21/60 (35%) | 16/36 (44.4%) | 5/24 (20.8%) | 0.06 |
| >10 days | 39/60 (65%) | 20/36 (55.6%) | 19/24(79.2%) | ||
| Anticoagulants | No | 30/66 (45.5%) | 12/42 (28.6%) | 18/24 (75%) | <0.0001* |
| Yes | 36/66 (54.5%) | 30/42 (71.4%) | 6/24 (25%) | ||
| Type of Antifungal | Amphotericin B | 24/66 (36.4%) | 9/42 (21.4%) | 15/24(62.5%) | <0.0001* |
| Voriconazole | 9/66 (13.6%) | 3/42 (7.1%) | 6/24 (25%) | ||
| Amphotericin B + Voriconazole | 33/66 (50%) | 30/42 (71.4%) | 3/24 (12.5%) | ||
| Duration of the antifungal | < 15 days | 15/66 (22.7%) | 12/42 (28.6%) | 3/24 (12.5%) | 0.134 |
| >15 days | 51/66 (77.3%) | 30/42 (71.4%) | 21/24(87.5%) | ||
Group A, survived patients; Group B, dead patients; SD, standard deviation; Abs, antibiotics.
*Significant as the P-value was < 0.05.
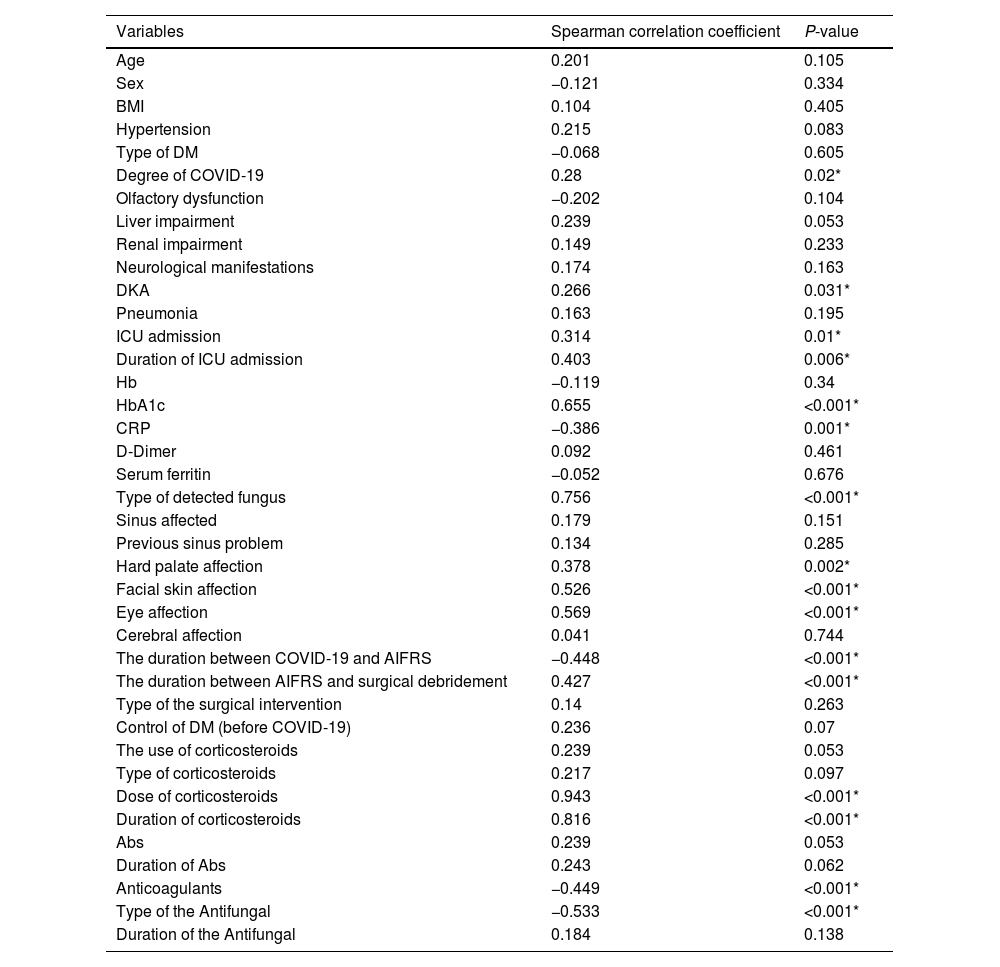
The bivariate correlation analysis revealed that the patient prognosis (survived or not) was significantly correlated with the following variables: the severity of COVID-19, DKA, ICU admission, HbA1c blood level, CRP blood level, the dose of the given corticosteroids, duration of the given corticosteroids, use of anticoagulants, type of antifungal drug, type of the causative fungus, facial skin affection, hard palate affection, ocular affection, the duration between the positive COVID-19 PCR to the start of AIFRS, and the time interval between the incidence of AIFRS to the surgical debridement (Table 5).
The correlation of the prognosis with the variables.
| Variables | Spearman correlation coefficient | P-value |
|---|---|---|
| Age | 0.201 | 0.105 |
| Sex | −0.121 | 0.334 |
| BMI | 0.104 | 0.405 |
| Hypertension | 0.215 | 0.083 |
| Type of DM | −0.068 | 0.605 |
| Degree of COVID-19 | 0.28 | 0.02* |
| Olfactory dysfunction | −0.202 | 0.104 |
| Liver impairment | 0.239 | 0.053 |
| Renal impairment | 0.149 | 0.233 |
| Neurological manifestations | 0.174 | 0.163 |
| DKA | 0.266 | 0.031* |
| Pneumonia | 0.163 | 0.195 |
| ICU admission | 0.314 | 0.01* |
| Duration of ICU admission | 0.403 | 0.006* |
| Hb | −0.119 | 0.34 |
| HbA1c | 0.655 | <0.001* |
| CRP | −0.386 | 0.001* |
| D-Dimer | 0.092 | 0.461 |
| Serum ferritin | −0.052 | 0.676 |
| Type of detected fungus | 0.756 | <0.001* |
| Sinus affected | 0.179 | 0.151 |
| Previous sinus problem | 0.134 | 0.285 |
| Hard palate affection | 0.378 | 0.002* |
| Facial skin affection | 0.526 | <0.001* |
| Eye affection | 0.569 | <0.001* |
| Cerebral affection | 0.041 | 0.744 |
| The duration between COVID-19 and AIFRS | −0.448 | <0.001* |
| The duration between AIFRS and surgical debridement | 0.427 | <0.001* |
| Type of the surgical intervention | 0.14 | 0.263 |
| Control of DM (before COVID-19) | 0.236 | 0.07 |
| The use of corticosteroids | 0.239 | 0.053 |
| Type of corticosteroids | 0.217 | 0.097 |
| Dose of corticosteroids | 0.943 | <0.001* |
| Duration of corticosteroids | 0.816 | <0.001* |
| Abs | 0.239 | 0.053 |
| Duration of Abs | 0.243 | 0.062 |
| Anticoagulants | −0.449 | <0.001* |
| Type of the Antifungal | −0.533 | <0.001* |
| Duration of the Antifungal | 0.184 | 0.138 |
BMI, body mass index; PCR, polymerase chain reaction; DKA, diabetic ketoacidosis; DM, diabetes mellitus; ICU, intensive care unit; Hb, hemoglobin level; CRP, c-reactive protein; Abs, antibiotics.
*Significant if the P-value was less than 0.05.
Kaplan-Meier curve indicated that the duration between the COIVID-19 and the start of the AIFRS had a significant association with the dose (P-value < 0.0001) and the duration (P-value < 0.001) of the corticosteroid treatment; when the dose and the duration increased, the AIFRS had an earlier presentation.
The ROC curveThe ROC curve between the patient prognosis and the HbA1c blood level showed a potent correlation as the area under the curve was 0.893. The best cut-off point was 9.35 with 87.5% sensitivity and 78.6% specificity.
PrognosisThe improvement time in group A ranged from 34 to 118 days with a mean of 73 .21±25.61 days. On the other side, the time of death in group B ranged from 33 to 69 days with a mean of 50±11.07 days. The olfactory dysfunction didn’t improve in 18/39 cases (46%).
DiscussionAlthough AIFRS has a low incidence rate, the number of AIFRS patients showed triple-fold increases in the last two years. This jumping increase may be attributed to the COVID-19 pandemic.7 This resembles the increase of fungal infections during the SARS-CoV pandemic in 2003, which was considered the leading cause of death in 25–73.7% of infected patients. SARS-COV and SARS-COV-2 were proven to belong to the same species with similar biological and clinical characteristics.8 Many recent reports connected the AIFRS with COVID-19. Song et al. studied the association between COVID-19 and invasive fungal sinusitis. They concluded that many patients affected by or recovered from COVID-19 are at increased risk of developing invasive fungal diseases.9 White et al. studied 135 adults with COVID-19 infection and reported an incidence of 26.7% for invasive fungal infections.7
The COVID-19 patient is more liable to the AIFRS due to the impairment in the cell-mediated immunity caused by decreased CD4+ and CD8+ T-helper cells.10 Also, there is a defect in the complement system with an increase of IL-2, IL-6, IL-10, TNF-alpha, and other inflammatory markers, which is known to cause a cytokine storm.11 Besides the alveolar damage with an increase in the inflammatory exudate, the liability for air-born coinfection (as fungal infections) increases. All these factors cause tissue inflammation and damage with necrosis and thrombosis, which may increase complications.12
The main risk factor of AIFRS and its poor prognosis is primarily due to immunosuppression which can be present in many conditions such as DM, malignancies, chemotherapy, organ transplantation, and corticosteroid administration.13 We found that 84% (75 out of 89 patients) of the patients had diabetes, 70 patients had only DM, and five patients had DM with another cause of immunosuppression. This followed the studies by Kursun et al. and Mohammadi et al., which concluded that the most common underlying disorders in AIFRS patients were diabetes mellitus and, with fewer implications, hematological malignancies.14,15
Our study included AIFRS cases associated with COVID-19 with DM. We excluded cases with other causes of immunosuppression to focus on the impact of COVID-19 on the behavior of AIFRS in diabetic patients. Poor control of DM played an influential role in the bad prognosis. This was demonstrated in the significantly higher number of initial DKA cases among the no-survival group. Also, there was a significant elevation of the blood level of the HbA1C in the no-survival group at the time of hospital admission. HbA1C is an important marker that reflects blood glucose control in the last few months.16 HbA1c less than 9.35% may indicate a good prognosis with 87.5% sensitivity.
Mucor fungal species was the dominant species among the no-survival group. On the other hand, Aspergillus fungal species was the prevalent fungus among the surviving patients. This followed the EL-Kholy et al. study, which found the Mucor species in 77.8% of the 36 patients with AIFRS associated with COVID-19.17
According to our study (Tables 4 and 5), corticosteroids played an essential role in the bad prognosis of AIFRS associated with COVID-19. At the same time, high doses and prolonged corticosteroids fastened the start of AIFRS in patients associated with COVID-19. This followed the study of Sharma S et al., which included 23 patients with AIFRS associated with COVID-19, and concluded that uncontrolled diabetes and over-zealous use of steroids are two main factors aggravating the illness, and both of these must be checked appropriately.18 Corticosteroids affect immunity badly by impairing phagocyte function and cytokine transcription.19
Although Amphotericin B was the preferable antifungal therapy, especially against the Mucor species, we found that the combination of Amphotericin B and Voriconazole was very effective in improving the prognosis of AIFRS. Amphotericin B was used in its liposomal form with close monitoring of renal functions. This procedure aimed to reduce the nephrotoxic effect of Amphotericin B.20
Surviving from AIFRS associated with COVID-19 was significantly improved with anticoagulants in the medical management protocol. Anticoagulants may decrease the hypercoagulability status caused by COVID-19. They may also prevent thrombosis, one of the main lethal pathologies during the AIFRS.21
The earlier surgical debridement of necrotic tissue was one of the main factors that improved the behavior of AIFRS associated with COVID-19. It may help lower toxins associated with necrotic tissues while decreasing the burden of infectivity. Also, debridement may improve blood supply with better penetration of antifungal drugs.22 This followed the study by El-Kholy et al., which found that early surgical debridement resulted in better outcomes and higher survival.17
University of Pennsylvania Smell Identification Test was used to assess the olfactory functions in all cases.23 The olfactory dysfunction was high among our study sample (88%) compared to the average incidence of olfactory dysfunction rate among COVID-19 patients (33.9%–68%). Also, the improvement rate was low (54%) among the surviving patients compared to the average improvement rate (64.3%). This could be related to the viral infection, the toxic effect of the AIFRS on the nasal olfactory epithelium, the effect of the surgical intervention on the olfactory mucosa, or all these mechanisms together.24,25
Although there are improvements in the medical and surgical management protocols of AIFRS using intravenous antifungal drugs, the prognosis remains poor. AIFRS has high mortality rates ranging from 33.3%–80%, going up to 100 percent in disseminated infections.26 In our study, 24 patients (36%) died because of the AIFRS associated with COVID-19. This was considered a high rate compared to the World Health Organization data on the cumulative number of AIFRS deaths associated with COVID-19, which revealed that the mortality rate is 15.2% (12.5%–17.9%) outside China.27
We observed that the bad prognosis was associated with severe COVID-19, ICU admission, uncontrolled glycemic level, prolonged administration and high dose of corticosteroids, high level of CRP, Mucor fungal infection, single antifungal therapy, absence of anticoagulants, and delay in the surgical debridement.
RecommendationsWe recommend close and proper glycemic control during the management of AIFRS associated with COVID-19. HbA1c may be a good tool for follow-up. We also recommend using a combination of Amphotericin B (liposomal form) and Voriconazole as an antifungal therapy with the need for further clinical trials. We also recommend the addition of monitored anticoagulant therapeutic protocol during medical management. Early surgical debridement is fundamental for improving the prognosis. Minimizing corticosteroids to manage COVID-19 is essential for improving the prognosis of the AIFRS.
Limitations of our studyOur study was a case-series study without a control group. This may be considered the main limitation of our study, together with a relatively small study sample size.
ConclusionsAccording to this multi-center study, the mortality of the AIFRS associated with COVID-19 was high (36%). High doses of corticosteroid treatment and its prolonged usage were the main factors that affected the prognosis, followed by glycemic control, fungal species, and antifungal therapy. Proper glycemic control and early surgical debridement with the addition of anticoagulants helped improve the prognosis.
FundingThis work has not received any funding.
Conflicts of interestThe authors of this manuscript declare no conflict of interest.