The appearance of a new coronavirus disease called COVID-19 at the end of 2019 and its pandemic expansion in the world has changed the usual practice of the specialty of Otorhinolaryngology (ENT). After a phase of exponential growth of infections, it has been possible to enter a phase of control of the spread of the disease in which the possibility of infection persists, and the appearance of new cases is considered acceptable by the health system.
The aim of this document is to review the available evidence and propose strategies and recommendations for the medical-surgical practice of otorhinolaryngology and head and neck surgery, which allow establishing the usual activity, adapting the safety and efficacy standards to the current situation. Therefore, it is required to identify and classify patients according to criteria of infectious-immunological status, and to establish recommendations for protection in consultations, hospitalization and the operating room, which avoid the transmission of the disease to other users and healthcare personnel, in the specific context of the development of our specialty. This document is the result of the collaboration of all the scientific commissions and the SEORLCCC COVID-19 committee.
La aparición de una nueva enfermedad por coronavirus denominada COVID-19 a finales de 2019 y su expansión pandémica en el mundo ha cambiando la práctica habitual de la especialidad de Otorrinolaringología (ORL). Tras una fase de crecimiento exponencial de los contagios, se ha logrado entrar en una fase de control de la expansión de la enfermedad en la que persiste la posibilidad de contagio, pero la aparición de nuevos casos se considera asumible por el sistema sanitario.
El objetivo del presente documento es revisar la evidencia disponible y proponer estrategias y recomendaciones para la práctica médico-quirúrgica de la otorrinolaringología y cirugía de cabeza y cuello, que permitan establecer la actividad habitual, adecuando los estándares de seguridad y eficacia a la situación actual. Se requiere, por lo tanto, identificar y clasificar a los pacientes en función de criterios de estado infeccioso-inmunológico, y establecer las recomendaciones de protección en consultas, hospitalización y quirófano, que eviten la transmisión de la enfermedad a otros usuarios y al personal sanitario, en el contexto específico del desarrollo de nuestra especialidad. El presente documento es fruto de la colaboración de las comisiones científicas y del comité COVID-19 de la SEORLCCC.
The disease termed coronavirus 2019 (COVID-19), caused by the SARS-CoV-2 virus, has led to millions of infections and thousands of deaths worldwide since an outbreak of pneumonia of unknown aetiology was reported in Wuhan, China, in December 20191 and led to the declaration of a pandemic by the World Health Organization (WHO) on 11 March 2020.2 It is considered a zoonosis, with bats or manis being the most likely source.3 Its main routes of transmission are direct contact from contaminated hands or fomites with the oral and nasal mucous membranes, and Flügge drops or microparticles that are inadvertently expelled through the mouth and nose and reach the respiratory mucous membrane.4 After an incubation period of 5 days on average (2–14 days) the disease manifests itself with fever, dry cough, dyspnoea, myalgia, fatigue, headache, expectoration, diarrhoea, general malaise, pharyngeal pain, anosmia, hyposmia and dysgeusia.5,6 In most cases it is mild but may evolve into bilateral pneumonia, reaching, in the most severe forms, respiratory distress with a fatality rate of between 1% and 12%.
Depending on the country, healthcare providers can reach 20% of the infected population.7 Of the medical and surgical specialties, the special risk of infection in ENT specialists, anaesthetists, dentists and ophthalmologists is particularly evident, due to the proximity to the upper airway of the patient as they undertake their activity, and it is noteworthy that the first deaths of doctors caused by COVID-19 were ENT specialists and ophthalmologists.7,8 ENT assessment requires physical examination at a distance of less than 30 cm and using specific instruments (otological microscope, nasofibrolaryngoscopy, nasal endoscopy) that could enable or encourage transmission of the virus. We must also bear in mind that ENT services attend patients of all ages, children and people over the age of 60 being patients with special characteristics, both because of the way the disease manifests and because of contagiousness, risk and accessibility (accompaniment), which implies the need to take specific protective measures in these groups.9,10
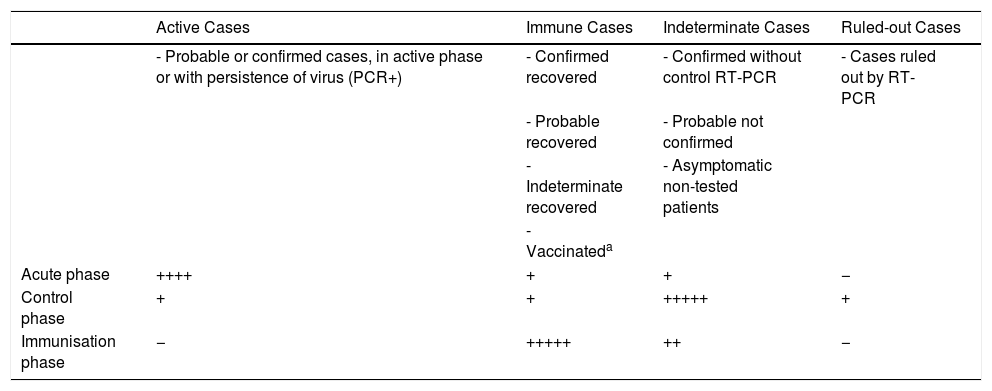
However, no studies have been carried out on the seroprevalence of existing immunity (real percentage of infected population), therefore we do not know the immune status of most patients attended.11 Bearing in mind the evidence of the risk of infection by asymptomatic patients, until sufficient data are available these patients should be considered a group at high risk of transmission of the virus12 (Table 1).
Summary of the Phases of the COVID-19 Pandemic and Their Relationship With The Infectious-immunological Status of Patients Attending Consultation or Surgery.
| Active Cases | Immune Cases | Indeterminate Cases | Ruled-out Cases | |
|---|---|---|---|---|
| - Probable or confirmed cases, in active phase or with persistence of virus (PCR+) | - Confirmed recovered | - Confirmed without control RT-PCR | - Cases ruled out by RT-PCR | |
| - Probable recovered | - Probable not confirmed | |||
| - Indeterminate recovered | - Asymptomatic non-tested patients | |||
| - Vaccinateda | ||||
| Acute phase | ++++ | + | + | − |
| Control phase | + | + | +++++ | + |
| Immunisation phase | − | +++++ | ++ | − |
Since control of the spread of the disease began, health institutions have been developing plans to enable the resumption of the health activity established before the pandemic. Due to the specific characteristics of ENT, the SEORLCCC, through the commission on protocols, standard clinical guidelines and nomenclature, the COVID committee and the scientific commissions, has drawn up this document with strategies and recommendations aimed at providing guidance in the daily management of patients during the control phase of COVID-19, in which we currently find ourselves.
Material and MethodA review of the literature available in PubMed, Embase and Scopes in English and Spanish was carried out using the MeSH terms "COVID disease", "preventive measures in COVID", "diagnosis COVID", "serological study in COVID", as well as all the official journals and reports of the Ministry of Health, Consumer Affairs and Social Welfare (MSCBS), and of the scientific societies.
Diagnosis of COVID-19The diagnostic tools available allow us to search for the presence of the virus in the respiratory mucosa, as well testing for antibodies in serum13:
- 1
Based on demonstrating the presence of specific SARS-CoV-2 virus genes. The search for RNA in samples of different origin in the airway is performed by reverse transcriptase polymerase chain reaction (RT-PCR). This is the test of choice for determining the presence of the virus. It is based on the detection of the virus’s RNA. It is highly reliable provided that sample collection and analysis is carried out by trained personnel, and probes that look for at least two genes of the virus are used. Its presence does not indicate that there is transmissibility of the virus, only that it is present in the sample secretions. In patients with asymptomatic or mild forms of presentation, positive RT-PCR does not indicate the ability to transmit the virus but neither does it rule it out, and therefore precautionary measures should be taken until the recovery phase of the disease.10,11 Until more information is available, to consider the absence of virus at transmissible levels, it is advisable to repeat RT-PCR at least twice with a 48 h–72 h interval.
In RT-PCR positive patients, and in those who have been in contact with confirmed cases, confinement of at least 14 days is recommended before requesting repeat RT-PCR. The aim is twofold: to wait for the test to be negative and for patients to move to a phase of the disease that lacks the capacity to transmit the virus.14
- 2
Based on the search for the presence of virus antigens (S, E and M antigens) in airway secretions. These are rapid tests with a high number of false negatives depending on the stage of the disease that is being studied. Therefore, they do not have sufficient sensitivity and, at present, are not recommended for evaluating patients.
- 3
Study of antibodies in blood-serum. In patients with severe forms of COVID-19, the detection of IgM antibodies 7 days from the onset of symptoms has been described, considering this and their progressive rise a sign of the patient's immune response to neutralise the presence of the virus.15 On the other hand, up to 20% of patients with mild forms of COVID-19, who are not admitted, do not present antibodies in serum.11,16 At the same time, in most hospitalised patients, IgG antibodies are produced 14 days from the onset of symptoms; their interest lies in their possible long-lasting response effect, which has not yet been demonstrated. Cases where IgG and IgM are detected, both of which elevated, could imply intermediate situations of infection and immunisation.
The antibody study can be performed by:
- •
Rapid tests for the presence of antibodies. These do not require specialist personnel and are low cost. They are based on immunochromatography (lateral flow technique) and have become popular as "rapid tests" for detecting antibodies.17,18 They are unreliable and are not recommended for clinical use. They are used in studies of care homes and in some risk groups to determine their state of immunity, and are recommended for this purpose by the Spanish Society of Immunology (SEI).13
- •
Laboratory tests to measure the level of antibodies. Technique using enzyme-linked immunosorbent assay (ELISA) or chemiluminescence assay (CLIA). These are quantitative tests (in particular CLIA has a sensitivity of 1 pg/mL in serum) and have higher sensitivity and specificity. They are standardised, easy to interpret, but require specialist personnel to perform them, and their cost is greater.13
Testing for the presence of the virus must be by RT-PCR since its diagnostic accuracy is superior to the tests that detect the presence of the viral capsid antigens. None of the SARS-CoV-2 antibody tests can completely assure the transmission status of the virus and therefore we must advise against their interpretation in isolation from RT-PCR. On the other hand, rapid tests, either antigen or antibody, are not recommended in the clinical setting because of their low predictive value.
The presence of antibodies in COVID-19 patients should always be interpreted taking the RT-PCR result into account and the clinical phase of disease progression. A positive RT-PCR should be considered a potentially infectious patient. The presence of IgM and IgG antibodies should provide guidance on the host response to the virus, which could be related to specific immunity, but we cannot consider the infectious status of the patient if the RT-PCR result is not available.19,20
Results from studies of subjects from the general population (seroprevalence) who have not had or have had mild forms of the disease are needed for information on the true meaning of the presence of antibodies in serum and to explain whether immune responses are truly protective, as well as the duration of their effect.11
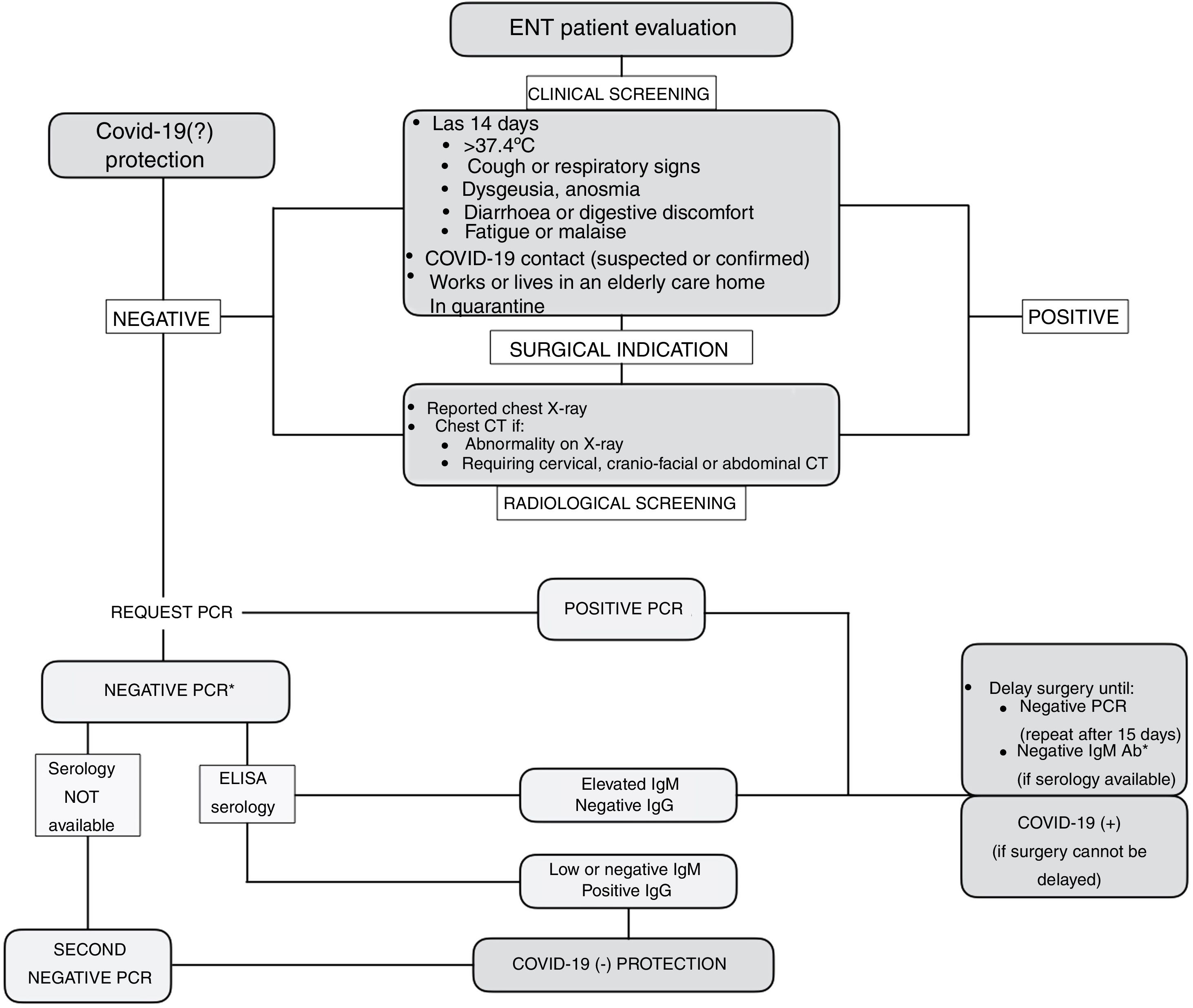
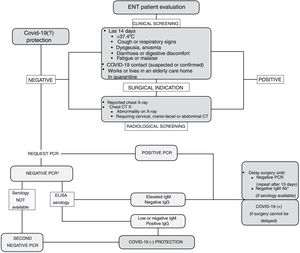
Strategy for Identifying Patients at Risk of InfectionIn light of the limited resources available, a strategy is proposed that combines patient safety and the safety of healthcare personnel in detecting patients who are at risk or carriers of COVID-19. To this end, clinical screening is recommended based on questions about occupation, the most prevalent symptoms of the disease and possible risk contacts. In a study of a population of more than 800 health professionals with suspicion of SARS-CoV-2 infection and presenting mild symptoms, it was found that clinical screening based on fever, anosmia, poor general condition, myalgia, headache, eye pain and extreme fatigue had a moderate predictive value for the presence of the disease, with a sensitivity of 91%.21 Clinical screening will allow us to select patients with high suspicion of the disease, sufficient for their evaluation in consultation (Fig. 1).
In patients who are to undergo surgery, given the increased surgical risk during the incubation period of COVID-19,14 it is recommended that clinical screening be supplemented with an RT-PCR test and a conventional chest X-ray reported by the radiologist. If the X-ray shows an abnormality or if the patient, due to his or her underlying disease, is to undergo a computed tomography (CT) scan, it is recommended that the study be extended and a chest CT scan be performed (Fig. 1).
Depending on the clinical-radiological screening and the RT-PCR result, patients will be classified as:
- •
Confirmed case: patient who meets laboratory criteria (RT-PCR positive for SARS-CoV-2 genes).
- •
Probable case: patient with clinical (symptoms) or radiological (bilateral interstitial pneumonia compatible with a diagnosis of COVID-19) criteria who has not had a microbiological diagnostic test, or whose laboratory result for SARS-CoV-2 is not conclusive.
- •
Ruled out case: patient with a negative clinical-radiological study and negative RT-PCR. COVID-19(−).
- •
Indeterminate case: asymptomatic patient who has not been tested (RT-PCR or X-ray).
Therefore, with the evidence currently available it is not possible to ensure "zero risk" in patient selection. The combination of the "best available study" together with prevention and protection measures is considered the current recommended strategy to resume clinical activity ensuring the highest safety of patients and health care providers (Fig. 1).
Preventive Measures Against Transmission of SARS-CoV-2Specific measures will be established according to the characteristics of the patients treated and the risks to be assumed by health personnel. In this sense, all our actions must be subject to and adapted to our local setting, according to the guidelines received from the MSCBS of the Spanish Government, from the Health Department of each Autonomous Region, the Management and Medical Direction.
For practical purposes, we consider classifying patients into 3 categories on which the strategies will be based:
- 1)
Confirmed or probable: COVID-19(+).
- 2)
Ruled out: COVID-19(−).
- 3)
Indeterminate: COVID-19(?).
The possibility of excluding elderly ENT patients or those with chronic medical comorbidities from interacting with patients who are highly suspicious or positive for COVID-19 should be considered,22 by means of an evaluation request to each centre’s Occupational Risk Prevention Unit.
Hand washing with soap and water, and disinfection with hydroalcoholic gels, will be carried out based on WHO recommendations, and following the 5 Moments for Hand Hygiene.23,24
The type of mask will depend on the distance from the patient and the type of procedure being performed, with or without risk of aerosolization. In general, at distances greater than 2 m, the use of a non-surgical mask is recommended. Between 1 m and 2 m, a surgical mask should be used and at distances less than 1 m, which are those that correspond to ENT examination, N95 or FFP2 type masks (filtration >94%) is recommended if no aerosols are being generated, or FFP3 (filtration >99%) if there is a risk of aerosol generation.25,26 Only in hearing impaired patients may the specially designed lip-reading masks be used, provided that there is no potential risk to the practitioner or if procedures with risk of aerosol generation are being performed. In this case, the procedure will be explained with the reading-lip mask and a face shield, and then the practitioner will put on the appropriate type of mask.
The use of gloves, eye protection, face protection, caps and gowns is recommended depending on the risk of contagion. Footwear and clothing should be worn exclusively for work, and just as for patients, health professionals should not wear jewellery at work.4 During surgical procedures, personal protective equipment (PPE) should be used according to the risk of infection with each patient. PPE should be put on and taken off strictly following its specific instructions.27–30
Specific Measures in ConsultationOn the Physical SpaceIt is recommended that visual information (posters, brochures, etc.) be placed in strategic locations to provide the population with instructions on hand hygiene and respiratory hygiene.31 Dispensers with hydroalcoholic solution and waste containers with pedal-operated lids should be available for both patient and health-care users.
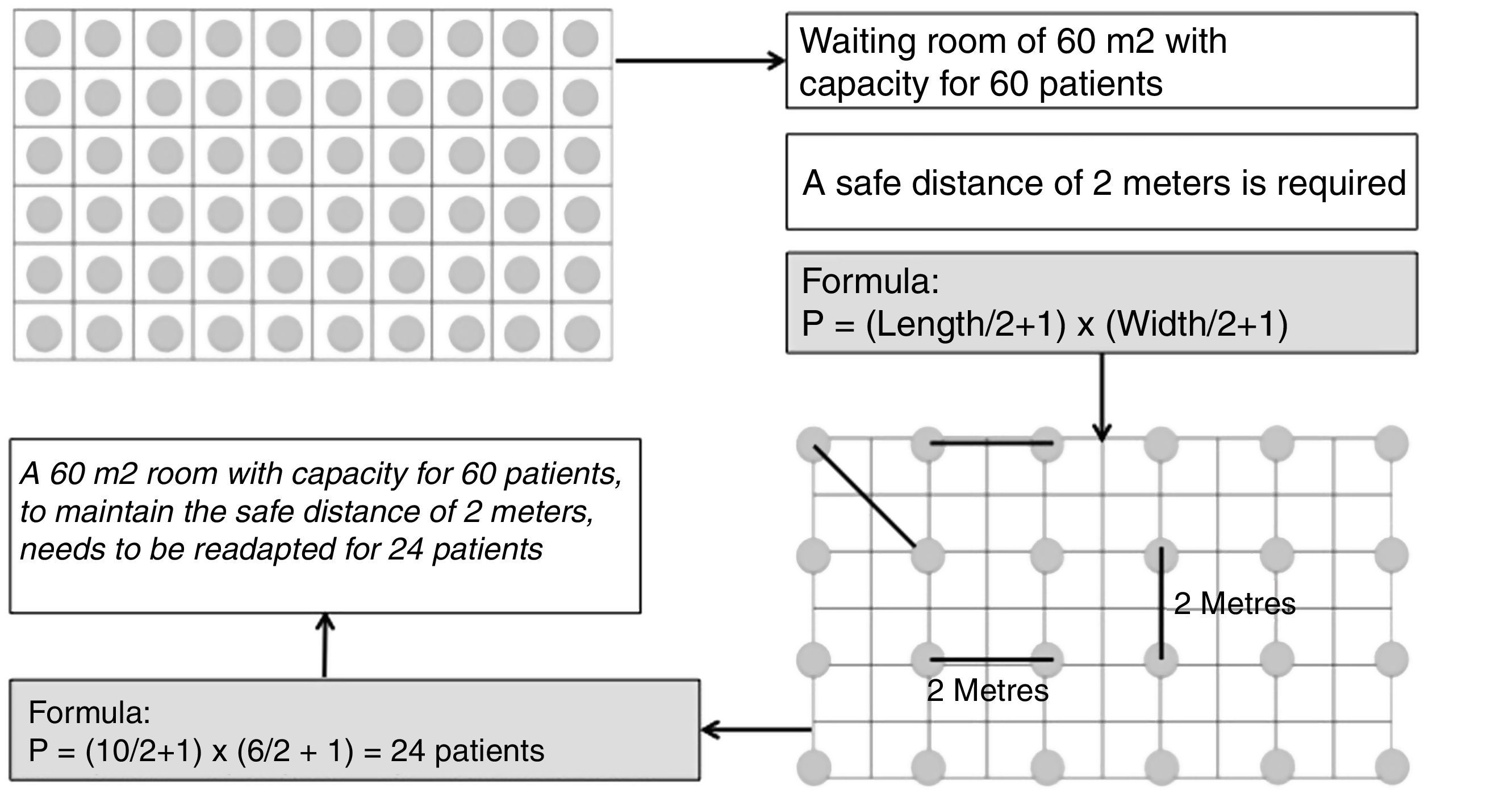
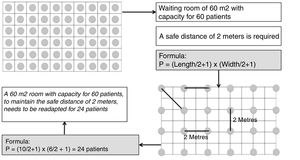
It is recommended that the waiting room be readapted so that patients can keep a safe distance of 2 m (Fig. 2). In the event that, due to the physical characteristics of these rooms, it is foreseen that the safe distance will not be kept, a double waiting room is recommended, with an interior capacity for 3 patients and their companions simultaneously (for immediate access to consultations) and an exterior capacity for the next patients to wait.
In the consultation room, it is recommended that unnecessary objects be removed, so as to facilitate flow, order, subsequent cleaning and disinfection; as well as to make provision for the material that will be used.
On Access and the Appointment SystemIt is recommended to adapt the system of appointments and consultations and attempt to minimize the number of simultaneous appointments. In order to resolve any delays, extending opening hours will be considered and preference will be given to telecare.
Patients will be advised to attend the consultation punctually to avoid crowds that make it difficult to comply with the rules (safety distance of at least 2 m), especially where corridors cross and in waiting rooms.
Patients will be advised to come to the clinic alone, wearing a mask without a valve, and avoiding the use of jewellery.4 If accompaniment is required, patients will be asked to remain in the waiting room, whenever possible. For minors, hearing impaired patients and dependent persons, only one companion will be allowed.
On ProceduresBefore the start of the consultation, clinical screening is recommended. If possible, it is recommended that this be done remotely (Fig. 1). In the case of triage with the patient in attendance, a safe distance from the administrative and auxiliary personnel should be respected at all times. In addition to using masks, these personnel should be separated from the patients by means of transparent methacrylate screens.
During the medical consultation, the door will remain closed and at the end of the consultation, the room will be kept aired for at least 15 min.32
A distinction can be made in the consultation with regard to the use of specific ENT instruments:
- •
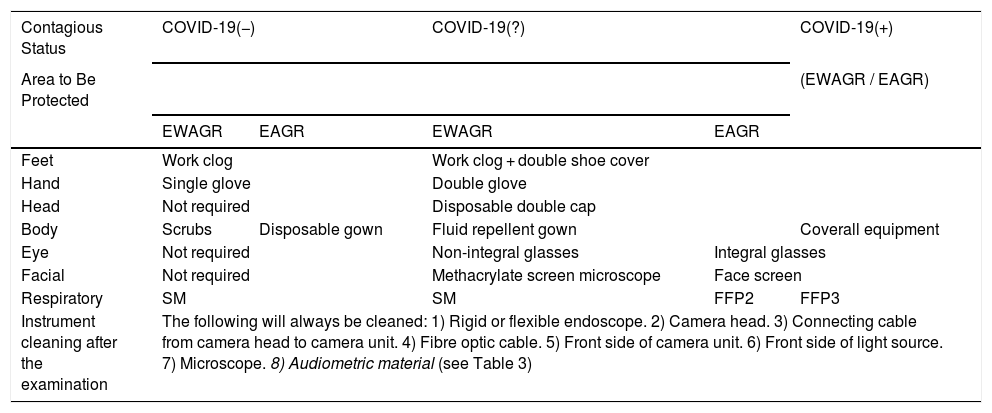
Rigid endoscope or flexible nasofibrolaryngoscope: due to the high nasal viral load of SARS-CoV-2,6,33 it is recommended to increase the distance between the ENT specialist and the patient as much as possible by using a tower with camera, screen and light source instead of using a direct view eyepiece. Care should be taken to ensure that the patient does not remove the mask, but only partially lowers it, or that some system is used to seal the nose during the examination (Fig. 3). In addition, manipulations such as removal of scabs or secretions should be limited if possible, and the use of atomised local anaesthetic should be replaced by cotton wicks or patties soaked in anaesthetic.34,35 Phenolic compounds, peracetic acid, or sodium hypochlorite are useful for disinfecting endoscopes.36 As an alternative, protective sheaths may be used (Table 2).
Table 2.Protective Measures for the Professional in the ENT Outpatient and in-patient Departments (Admissions for Non-surgical Procedures).
Contagious Status COVID-19(−) COVID-19(?) COVID-19(+) Area to Be Protected (EWAGR / EAGR) EWAGR EAGR EWAGR EAGR Feet Work clog Work clog + double shoe cover Hand Single glove Double glove Head Not required Disposable double cap Body Scrubs Disposable gown Fluid repellent gown Coverall equipment Eye Not required Non-integral glasses Integral glasses Facial Not required Methacrylate screen microscope Face screen Respiratory SM SM FFP2 FFP3 Instrument cleaning after the examination The following will always be cleaned: 1) Rigid or flexible endoscope. 2) Camera head. 3) Connecting cable from camera head to camera unit. 4) Fibre optic cable. 5) Front side of camera unit. 6) Front side of light source. 7) Microscope. 8) Audiometric material (see Table 3) EAGR: Examinations with aerosol generation risk (performed at less than 1 m or with direct inspection of the aerodigestive tract); EWAGR: Examinations without aerosol generation risk (performed further than 1 m); SM: Surgical mask; FFP2: FFP2 Mask; FFP3: FFP3 Mask.
- •
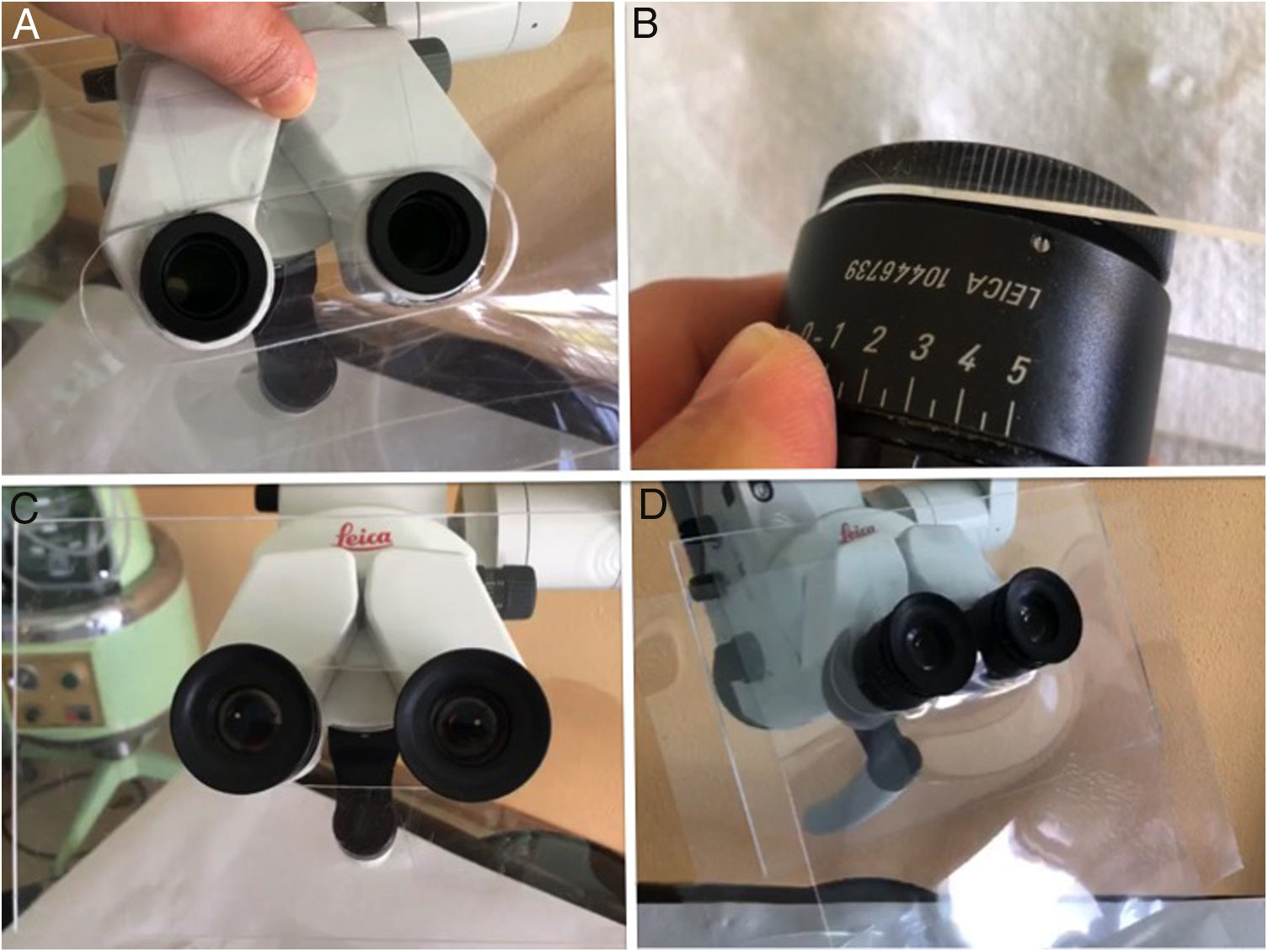
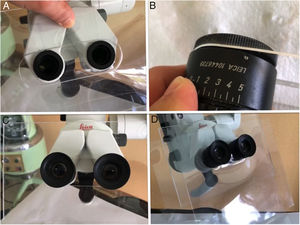
Microscope: The use of disposable material is recommended. Although these examinations have an intermediate risk, measures can be taken between the microscope and the patient such as methacrylate screens adapted to the binoculars or disposable plastic covers (Fig. 4). After the examination under the microscope, the areas close to or in contact with the patient, the lens and the binocular area of the device should be cleaned and disinfected, following the disinfection and hygiene measures in audiology recommended by the SEORLCCC audiology commission (Table 2).37
Figure 4.A) Methacrylate screen fitted to the microscope after removal of binoculars. B) Adaptation of binoculars to the methacrylate allowing adjustment of the interpupillary distance. C) Adjustment of the binoculars to the microscope together with the methacrylate screen. D) Interposed and replaceable plastic screen to improve the sealing of the methacrylate.
(0.17MB). - •
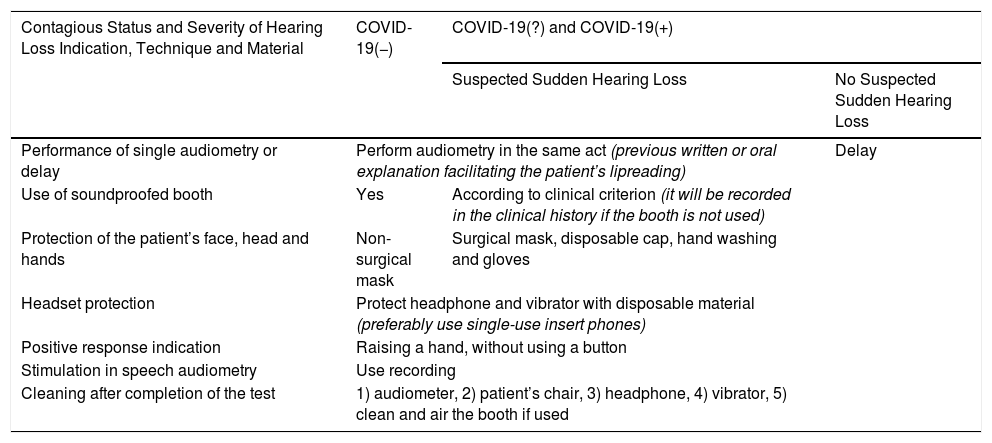
Tests in audiometry booths: These are considered closed spaces with a high risk of accumulation of viral load, and difficult to disinfect, and extreme precautions should be taken during these examinations. It is recommended that the patient and auxiliary personnel enter the booth wearing a mask, having washed their hands with hydroalcoholic solution and wearing gloves. The patient should sit in the chair without touching anything and the vocal tests should be done using a recording, never live voice (Table 3).
Table 3.Performance of Audiometry; Indication and Protective Measures for Professional and Patient.
Contagious Status and Severity of Hearing Loss Indication, Technique and Material COVID-19(−) COVID-19(?) and COVID-19(+) Suspected Sudden Hearing Loss No Suspected Sudden Hearing Loss Performance of single audiometry or delay Perform audiometry in the same act (previous written or oral explanation facilitating the patient’s lipreading) Delay Use of soundproofed booth Yes According to clinical criterion (it will be recorded in the clinical history if the booth is not used) Protection of the patient’s face, head and hands Non-surgical mask Surgical mask, disposable cap, hand washing and gloves Headset protection Protect headphone and vibrator with disposable material (preferably use single-use insert phones) Positive response indication Raising a hand, without using a button Stimulation in speech audiometry Use recording Cleaning after completion of the test 1) audiometer, 2) patient’s chair, 3) headphone, 4) vibrator, 5) clean and air the booth if used - •
Vestibular tests: The recommendations for general consultation and audiometry should be followed (Table 3).
When the patient leaves the consultation, the disposable material should be removed from the container and surfaces, and areas in contact with the patient should be carefully cleaned and disinfected with a disposable cloth or non-sterile swab, leaving the product to act for a few minutes. The usual cleaning and disinfection products are effective against coronaviruses that can remain active on plastic and stainless steel surfaces between 2–3 days,38 and even up to 9 days on non-porous surfaces.32 Among the most commonly used are sodium hypochlorite solution at .1%, and hydroalcoholic solution applied preferably with a single-use paper towel. Alternatively, ethanol can be used (62%-71% concentration).36 By keeping these products on surfaces for an exposure time of 1 min, other types of coronavirus can be eradicated, although this has not been demonstrated in SARS-CoV-2.32
In relation to disinfection using ultraviolet light (UV-C), its effectiveness for mask sterilization seems to have been demonstrated.39 Also on sanitary surfaces, although in this sense, there is consensus on improved results when surface disinfection and UV-C are combined.40 The Spanish Society of Environmental Health (SESA) advises against the use of chlorine dioxide to disinfect the air. The use of ozone for air and surface disinfection requires high concentrations with the consequent risk of toxicity, and therefore the rest times of the disinfected rooms must be maintained, reducing the efficiency of the method. To date, there is no certainty that the increase in temperatures with the arrival of spring, together with the greater ultraviolet radiation to the earth’s surface, can eradicate coronavirus.41
Specific Measures for the ENT Patient Admitted to the WardIn general, it is recommended to avoid hospitalisation and prioritise the regime of major outpatient surgery and day hospital to reduce hospital stays. The perioperative pathways will follow the proposed algorithm (Fig. 1).
In the case of hospital admission of a patient from the emergency department, clinical screening will be performed, and the patient will be classified as probable or indeterminate depending on the result. RT-PCR and X-ray will be requested. The management of an ENT patient admitted to hospital, who does not require surgery, will be the same as that of an outpatient. In the case of an emergency patient admitted for surgery that cannot be delayed, or for whom surgery is indicated during admission, the scheduled surgery protocol will be followed.42,43
Special Inpatient Situations- •
Patients who have undergone nasosinusal endoscopic surgery: it is preferable to avoid endonasal manipulation. The use of resorbable packing is recommended, and removal of scabs and secretions should be avoided as much as possible. Nasal packing removal should follow the equipment measures according to the patient’s classification.
- •
Post-tracheotomy care: it is recommended to keep the cuff inflated, use an in-line suction system and delay changing the tracheotomy tube until the RT-PCR is negative.44,45
- •
Patients with CPAP or BiPAP devices for obstructive sleep apnoea/hypoapnoea syndrome (OSAH): these are devices that generate aerosols due to incomplete sealing, which could increase the risk of spreading SARS-CoV-2 by air. The use of helmet type CPAP masks is recommended to reduce the risk of transmission.46,47
- •
Paediatric population: in order to avoid admissions for post-tonsillectomy bleeding, in the event of having to perform this type of intervention during the control phase, the use of the technique with the lowest risk of postoperative complications (intracapsular tonsillectomy) is recommended).48
A high risk of spread has been demonstrated in surgery of the aerodigestive tract, due to the high viral load detected in the nasopharynx, associated with potential aerosol generation and dissemination of viral particles during surgical instrumentation, through the use of drills, microdebriders, and / or electric or ultrasonic scalpels, often used in ENT surgery.19,38,43,46,49,50
It is recommended that all patients who are to undergo surgery of the aerodigestive tract are screened clinically and radiologically, and that RT-PCR studies and immunological tests are carried out to determine their infectivity and immunity status, respectively (Fig. 1). Thus, the best prevention and protection measures can be adapted. In addition, it is advisable that explicit informed consent is signed, informing of the risk that surgical intervention could entail during any of the phases of the pandemic.
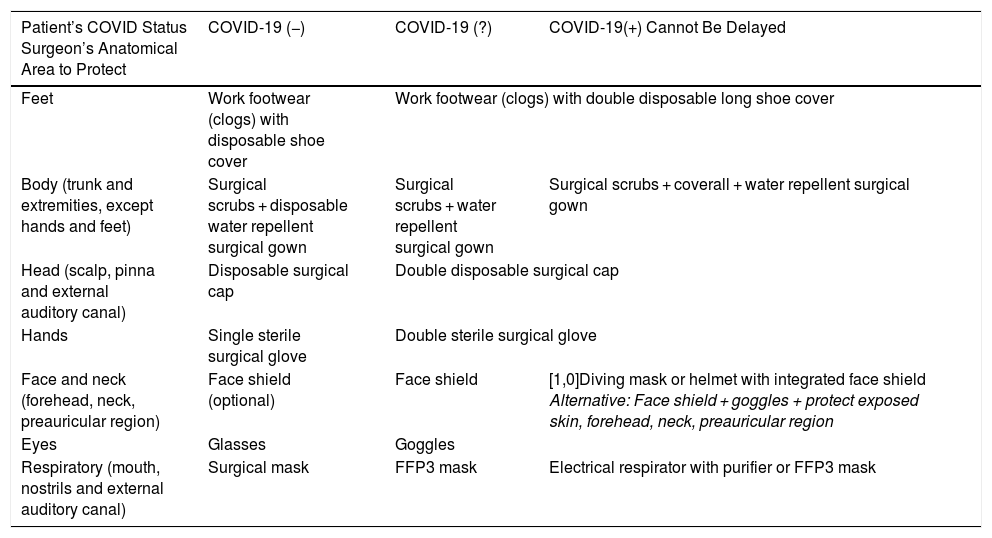
With regard to cross-sectional measures of management in the operating room, a negative pressure surgical environment is recommended to reduce the risk of spread of SARS-CoV-2, through high frequency of air changes (25 per hour) that reduces viral load inside the operating theatre.7,51 The same operating theatre and anaesthesia equipment should be used for all probable or confirmed COVID-19 patients.7,52 It is also recommended that the number of surgical team members be restricted during the monitoring phase.35 At the time of induction of general anaesthesia, endotracheal intubation and air-purifying respirators will be mandatory,53 and the patient’s anaesthetic depth should be sufficient during the operation to avoid intraoperative awakening. The use of electrical instruments such as micro-debriders, high-speed motors such as drills, electric scalpels, radio frequency, piezoelectric and ultrasonic systems should be avoided whenever possible. Individual protective measures should be adapted according to the classification of the patient (Table 4).
Protective Measures for the Professional in the Operating Theatre and During Risky Procedures Outside the Operating Theatre: Change of Cannula or Voice Prosthesis, Dressing in a Patient With Tracheal Stoma, Biopsy or Puncture in Oropharyngeal Region.
| Patient’s COVID Status Surgeon’s Anatomical Area to Protect | COVID-19 (−) | COVID-19 (?) | COVID-19(+) Cannot Be Delayed |
|---|---|---|---|
| Feet | Work footwear (clogs) with disposable shoe cover | Work footwear (clogs) with double disposable long shoe cover | |
| Body (trunk and extremities, except hands and feet) | Surgical scrubs + disposable water repellent surgical gown | Surgical scrubs + water repellent surgical gown | Surgical scrubs + coverall + water repellent surgical gown |
| Head (scalp, pinna and external auditory canal) | Disposable surgical cap | Double disposable surgical cap | |
| Hands | Single sterile surgical glove | Double sterile surgical glove | |
| Face and neck (forehead, neck, preauricular region) | Face shield (optional) | Face shield | [1,0]Diving mask or helmet with integrated face shield Alternative: Face shield + goggles + protect exposed skin, forehead, neck, preauricular region |
| Eyes | Glasses | Goggles | |
| Respiratory (mouth, nostrils and external auditory canal) | Surgical mask | FFP3 mask | Electrical respirator with purifier or FFP3 mask |
In the immediate post-operative period, the relatives will be informed, preferably remotely to reduce the movement of health personnel in the hospital.54 The operated patient will go to the resuscitation room, where the measures proposed by the anaesthesiology service will be taken.
ConclusionsDuring the control phase of the COVID-19 pandemic, prevention measures should continue to be maintained towards a progressive and safe return to normal ENT activity. In this document, studies have been contrasted to provide information about the strategies and recommendations to be followed, and a classification of patients agreed before assessment by ENT (COVID-19 classification), based on the results of clinical-radiological screening and diagnostic tests. RT-PCR is considered the test of choice for detecting the presence of SARS-CoV-2, supplemented, if possible, by quantitative serological techniques (ELISA and CLIA). All tests should be assessed in the context of the patients’ clinical phase and never in isolation. Asymptomatic patients who have not been tested (COVID-19(?)) should be treated as carriers until the actual status of the patient is known. Therefore, to avoid a new outbreak of the disease through cross transmission from patient to health care provider and vice versa, extreme protective precautions are recommended in the different medical-surgical ENT settings.
All the proposals drafted may be modified as the COVID-19 pandemic and immunological status of the general population (group immunity) is known, since this information will allow decisions to be made on future prevention measures, until an effective vaccine is developed. Meanwhile, for the appropriate selection of patients who can be assessed in consultation, or candidates for surgery during the control phase, a clinical, radiological and immunological algorithm is proposed along with protective measures for patients, ENT and Head and Neck Surgery specialists and the professional setting.
AuthorshipJuan Manuel Maza-Solano and Juan Carlos Amor-Dorado share the same authorship in this work: design, text writing, methodology, revision and editing. All the authors have participated and reviewed the final text as well as contributed comments, Figures and references.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Fernando López Álvarez, Isam Alobid, Luis Lassaletta Atienza, Manuel Bernal Sprekelsen, María José Lavilla Martín de Valmaseda, Pedro Díaz de Cerio Canduela, Alejandro Lowy Benoliel, Serafín Sánchez Gómez, Pedro Rafael Cabrera Morín, José Miguel Villacampa Aubá, Carmelo Morales Angulo, Isabel García López, Francisco Javier Aguilar Vera and Gonzalo de los Santos Granados for their collaboration in revising the document.
Please cite this article as: Maza-Solano JM, Plaza-Mayor G, Jiménez-Luna A, Parente-Arias P, Amor-Dorado JC. Estrategias para la práctica de la otorrinolaringología y cirugía de cabeza y cuello durante la fase de control de la COVID-19. Acta Otorrinolaringol Esp. 2020. https://doi.org/10.1016/j.otorri.2020.05.001