In recent years, different urinary markers such as the Bladder Epicheck® have been developed in an attempt to reduce the number of cystoscopies in the follow-up of non-muscle invasive bladder cancer (NMIBC).
AimTo provide a systematic review of Bladder Epicheck® and its current clinical utility in the follow-up and detection of recurrence of NMIBC.
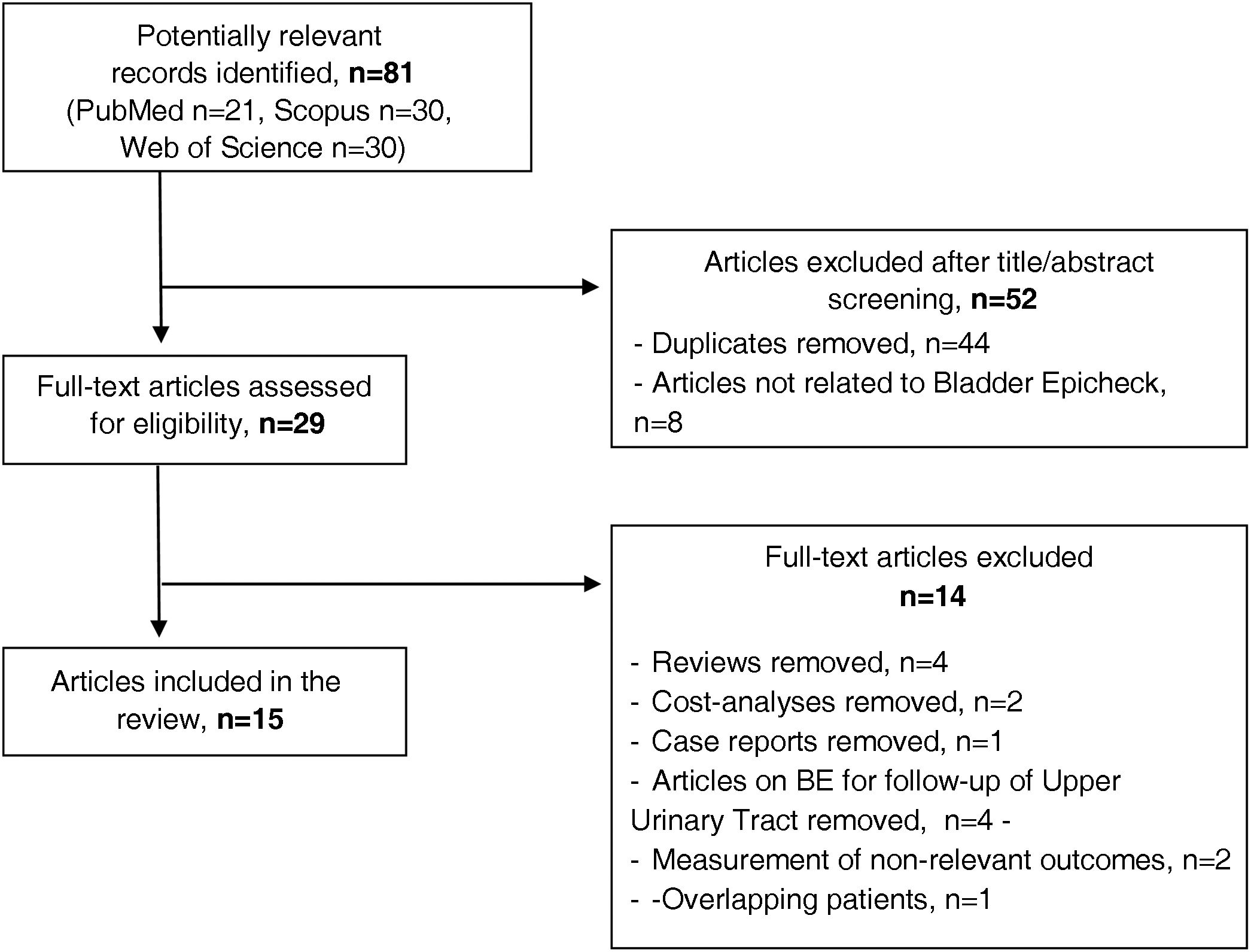
Material and methodsSystematic review based on a literature search of PubMed, Web of Science and Scopus databases until October 2023, according to PRISMA and Quadas-2 criteria. Sensitivity (Se), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) of the marker were calculated. Diagnostic performance was evaluated by the area under the curve (AUC).
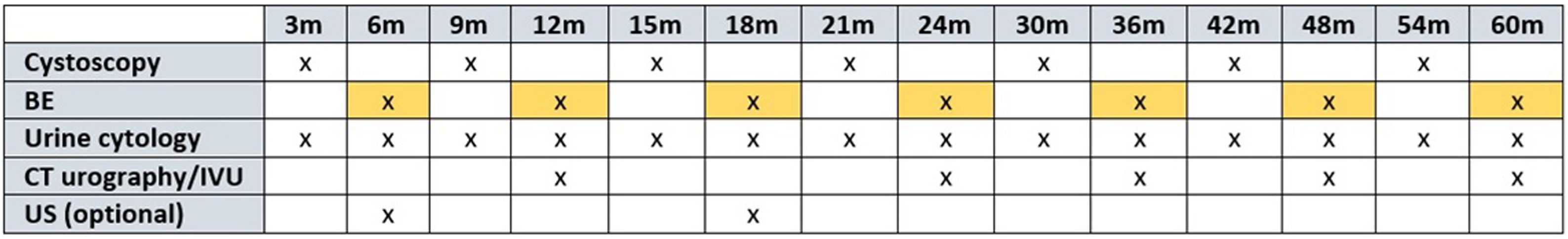
ResultsFifteen studies were analyzed (n = 3761) including 86.7% prospective studies. Of the patient series, 53.2% had received previous intravesical instillations. The mean Se of the biomarker in the detection of recurrence varied according to tumor grade (87.9%-high grade/HG vs. 44.9%-low grade/LG, respectively). Their weighted mean Se and Sp were 71.6% and 84.5%, respectively. The mean recurrence rate was 29.1%. The weighted mean PPV and NPV were 56.4% and 92.8% (97.7% non-LG), respectively. The mean AUC was 85.63%.
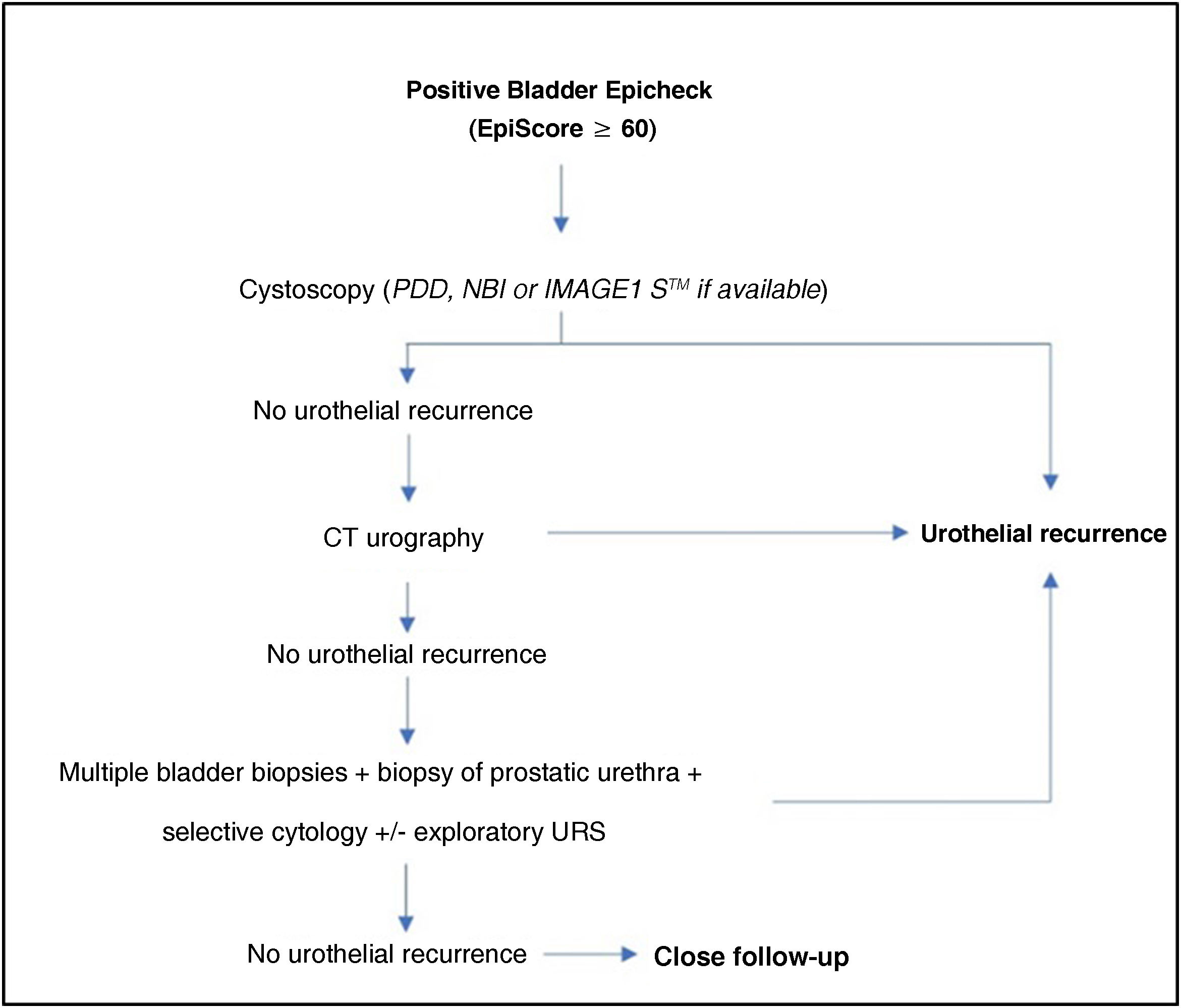
ConclusionBladder Epicheck® is a useful urinary marker in the follow-up of NMIBC, with significantly high Se and NPV in the detection of recurrences, especially in cases of HG disease. Its use can reduce the number of cystoscopies required in the follow-up of NMIBC, improving the quality of life of patients and potentially increasing health economic savings.
En los últimos años se han desarrollado diferentes biomarcadores urinarios,entre ellos Bladder Epicheck®,con el fin de disminuir el número de cistoscopias en el seguimiento de los tumores vesicales no músculo infiltrantes(TVNMI).
ObjetivoProporcionar una revisión sistemática sobre Bladder Epicheck® y su aplicabilidad clínica actual en el seguimiento y detección de recurrencia de los TVNMI.
Material y métodosRevisión sistemática basada en la búsqueda bibliográfica a través de PubMed, Web of Science y Scopus hasta octubre de 2023, acorde a los criterios PRISMA y Quadas-2. Se calcularon la sensibilidad(S), especificidad(E) y valores predictivos positivo(VPP) y negativo(VPN) del biomarcador. El rendimiento diagnóstico se evaluó mediante el área bajo la curva(AUC).
ResultadosSe analizan 15 estudios(n = 3761), siendo el 86,7% prospectivos. El 53,2% de la serie había recibido instilaciones intravesicales previas. La S media del biomarcador en la detección de recurrencia varió según el grado tumoral(87,9%-alto grado (high grade/HG) vs 44,9%-bajo grado(low grade/LG, respectivamente). Su S y E media ponderada fue del 71,6% y 84,5%, respectivamente. La tasa media de recidivas fue del 29.1%. Los VPP y VPN medios ponderados fueron de 56,4% y 92,8% (97,7% no-LG), respectivamente. El AUC medio fue del 85.63%.
ConclusiónBladder Epicheck® es un biomarcador urinario útil en el seguimiento de los TVNMI, destacando su elevada S y VPN en la detección de recurrencias, sobre todo en casos de HG.Su uso puede reducir el número de cistoscopias necesarias a realizar en dicho seguimiento, mejorando la calidad de vida de los pacientes y con un probable impacto en el ahorro económico sanitario.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora