Metalloproteases (MMPs) and tissue inhibitor of metalloprotease-3 (TIMP-3) have been associated to the risk of having cancer and tumor aggressiveness. When facing the difficulties of prostate cancer diagnosis, the expression of MMPs and TIMP-3 in negative biopsies could be helpful to evaluate a diagnostic suspicion. Our objective is to carry out a comparative study of the expression of MMPs and TIMP-3 in previous negative biopsies and radical prostatectomies (RP).
Material and methodsRetrospective analysis of a hospital-based cohort including 21 patients with suspicion of prostate carcinoma, whose expressions of MMP-2, 9, 11 and 13 and TIMP-3 were evaluated by immunohistochemistry in the tumor area from previous negative biopsies and RP.
ResultsImmunohistochemical staining values (Score) for MMPs (-11 and -13) and TIMP-3 showed no significant differences when comparing the areas of negative biopsies where tumors subsequently developed with those of the RP. However, we did observe a significant difference in the increased expression of MMP-2 (P=0.002) and MMP-9 (P=0.001) in the tumor area of the RP with respect to the corresponding area of the previous negative biopsy.
ConclusionsOur data indicate a higher overall expression of MMP-2 and MMP-9 in the tumor area of the RP compared to the corresponding areas of the negative previous biopsy, which seems to be associated to the process of malignant transformation.
Las metaloproteasas (MMPs) y el inhibidor tisular de metaloproteasas-3 (TIMP-3) se han relacionado con el riesgo de padecer cáncer y con la agresividad de varios tumores. En ocasiones, existen muchas dificultades para diagnosticar el cáncer de próstata (CaP), la expresión de MMPs y del TIMP-3 en biopsias negativas nos podría ayudar a realizar una sospecha diagnóstica en estos casos. El objetivo es realizar un estudio comparativo de la expresión de MMPs y TIMP-3 en las biopsias previas negativas y las prostatectomías radicales (PR).
Material y métodosAnálisis retrospectivo de una cohorte de base hospitalaria que incluye 21 pacientes con sospecha de carcinoma prostático en los que se analizaron por técnica inmunohistoquímica las expresiones de MMP-2, 9, 11 y 13 y el TIMP-3 en la zona tumoral, tanto de las biopsias previas negativas como de las PR.
ResultadosLos valores de tinción inmunohistoquímicos Score para las MMPs -11 y -13 y TIMP-3 no mostraron diferencias significativas al comparar las áreas de las biopsias negativas donde luego se desarrolló tumor con las de la PR. Sin embargo, si que observamos una diferencia significativa aumentando la expresión de la MMP-2 p=0,002 y MMP-9 p=0,001 en la zona tumoral de la PR con respecto al área correspondiente de la biopsia previa negativa.
ConclusionesNuestros datos indican una mayor expresión global de la MMP-2 y MMP-9 en la zona tumoral de la PR en comparación con las áreas correspondientes de la biopsia previa negativa, lo que parece estar en relación con el proceso de transformación maligna.
Prostate cancer (PCa) is the most frequently diagnosed tumor in male population in Spain.1 However, an adequate diagnosis is often complicated, and patients are submitted to numerous diagnostic tests which are regularly not exempt from complications.
Prostate biopsies have high false negative rates which can reach up to 47%.2 This is why imaging techniques have become interesting in this diagnosis, reducing the number of unnecessary biopsies.
Multiparametric magnetic resonance imaging of the prostate is a non-invasive procedure which, due to its higher spatial resolution, can provide more accurate diagnoses, providing great anatomical detail of the prostate by precisely locating carcinomas in those patients with clinical suspicion. With its more accurate 3D image of the prostate, it guides the insertion of the needle to obtain punctures of the suspicious areas.3 Fusion biopsies are useful in the selection of clinically significant cancers; by reducing the detection of low-risk tumors, over-treatments and their corresponding side effects may be avoided. There are no doubts regarding the advantages provided by image fusion biopsy for the diagnosis of PCa. However, we must take into account several of its limitations such as high price, low accessibility, technical difficulty, among others.
Therefore, it would be of great interest to find a biomarker containing information about the progression of the disease that could help us identify patients with high-risk tumors.
Currently, many histopathological diagnoses of disease are based on the interaction between the tumor cell and the extracellular matrix (ECM).4 Tumors result from mutations in genes that lead to uncontrolled growth of cell tissue, with metalloproteases (MMP) being the main mediators during tumor progression.5 The ECM performs vital functions for the cell and tissues regarding their multiplication and preservation which are essential for their survival,6 and it protects the tissues from tumor cell infiltration.7
The MMPs of the ECM have gained important interest in cancer research due to their role in the degradation of the basement membrane, promoting tumor invasion and metastasis. MMPs play an essential role in the degradation of connective tissue in the stroma and basement membrane: they are involved in carcinogenesis, in the invasion and production of tumor metastases and regulate the signaling pathways that control cell growth, inflammation and angiogenesis.8
The expression of MMP 2, 9, 11 and 13 has been found in many human tissues, especially in fibroblasts and endothelial/epithelial cells. Patients with PCa have a greater MMP-9 expression of both serum and tissue levels.9 Being a secreted protein that binds strongly to the ECM, the tissue inhibitor of metalloproteases 3 (TIMP-3) inhibits the proteolytic activity of the MMPs.
The objective of our study is to perform a comparison of the overall expression of MMPs and TIMP-3 in the areas where tumors will develop, between previous negative biopsies and radical prostatectomies (RP) of patients diagnosed with PCa.
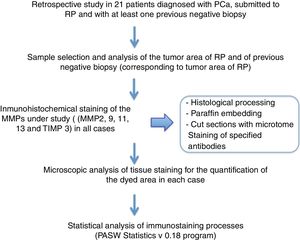
Material and methodsA retrospective analysis was carried out after selecting 21 patients diagnosed with PCa who underwent RP and had at least one previous negative biopsy.
In all cases, the main characteristics of the patients were analyzed using the patient's filiation data (name, age, numbers of medical record, biopsy and RP specimen) and the prognostic factors before (PSA) and after (PSA, clinical stage, Gleason score of prostatectomy specimen and tumor location) treatment were evaluated.
The histological material used in this study has been obtained from negative prostate biopsies in patients submitted to RP once diagnosed with PCa by a subsequent positive biopsy in cases when the disease was in a localized stage.
With the objective of determining the expression of MMPs -2, -9, -11 and -13 and TIMP-3 in the tumor area of the patients' samples in previous negative biopsy and in the neoplastic area of subsequent RP we have processed 1320 determinations; 1056 of the MMP-2, -9, -11 and -13 expression and 264 of the TIMP-13 expression. To do this, we routinely analyzed the tissue samples in the Anatomic Pathology department by fixing them in 4% buffered formalin for a minimum of 24h, after which a macroscopic study was carried out, including the representative sections in paraffin for anatomopathological diagnosis. For inclusion, the following process was carried out: formalin 15min- alcohol 70°C for 30min - 96°C for 30min- 100°C for 30min- 100°C for 30min - 100°C for 45min- xylene for 60min - xylene for 60min.
After obtaining tissue blocks, 3μm sections were cut with the help of a microtome and subsequently placed in paraffin blocks that were introduced in an oven at 65° to favor the correct binding of the tissue to the paraffin blocks.
The immunohistochemical study has been performed on these sections, using specific antibodies against MMP-2, -9, -11 and -13 and TIMP-3.
This technique follows the immunological principles and techniques for the study of cells and tissues with the objective of their identification, in situ localization and quantification.
It is not enough to perform the previously mentioned immunostaining steps correctly in order to obtain a reliable and successful result with these techniques. In addition, the samples must be handled carefully and appropriately fixed and processed.
Staining was performed with the antibody under study and were contrasted with hematoxylin, since each type of stain has different optical thresholds.
All 4 samples of each patient were analyzed at 100X-400X, and the 4 previously selected areas were analyzed for positive staining of the protein under study.
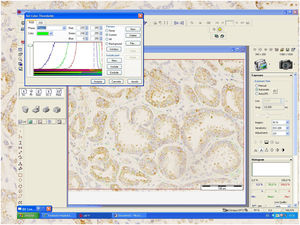
A percentage of dyed area of each field has been obtained from the relationship between the positively stained area (brown) and the negative area (whitish) (Fig.1). The staining value, overall expression or score has been calculated by multiplying its intensity level by the percentage of stained area in a spreadsheet.
A certified pathologist who is completely blind to the clinical outcome of the patients, performed the histological examination and the assessment of the expression of each protein by the different cell types.
The results obtained from immunostaining have been statistically analyzed using the PASW statistics 18 program (analySIS, Soft Imaging System). Staining values for each protein have been expressed as medians, with a minimum and maximum range. To determine the differences in the overall expression of the factors (score), we used the Mann-Whitney or Kruskal-Wallis U test. Differences between groups were considered significant when the p value was equal to or less than 0.05 (Fig. 2).
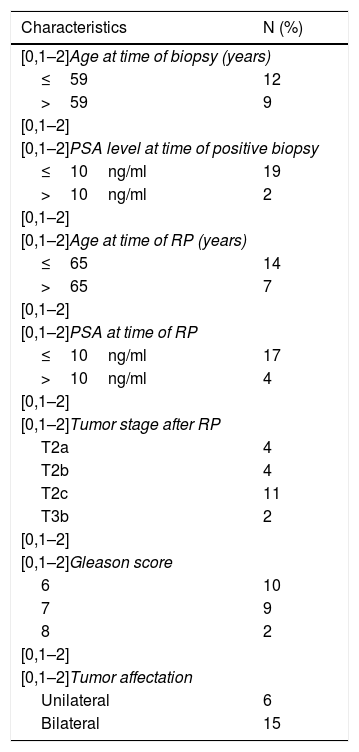
ResultsThe patients included had a median age at the time of negative biopsy of 59 years (range of 52–70 years) with a median PSA of 5,04ng/dl (range of 4.14–10.84). The median age of the patients when submitted to RP was 65 years (range 53–71) with a median PSA of 7,24ng/ml (range 4.92–13.75). Tumor stage after RP was: 4 T2a, 4 T2b, 11 T2c and 2 T3b; the Gleason score in the RP specimen was 10Gl. 6; 9Gl. 7 and 2Gl. 8, and the affectation after RP was 6 unilateral and 15 bilateral.
Table 1 shows the distribution of tumor stages, histological grades and extent of prostate involvement of the RP specimen.
Clinicopathological characteristics of 21 patients with prostate cancer undergoing radical prostatectomy and their relation to the expression of the different factors.
| Characteristics | N (%) |
|---|---|
| [0,1–2]Age at time of biopsy (years) | |
| ≤59 | 12 |
| >59 | 9 |
| [0,1–2] | |
| [0,1–2]PSA level at time of positive biopsy | |
| ≤10ng/ml | 19 |
| >10ng/ml | 2 |
| [0,1–2] | |
| [0,1–2]Age at time of RP (years) | |
| ≤65 | 14 |
| >65 | 7 |
| [0,1–2] | |
| [0,1–2]PSA at time of RP | |
| ≤10ng/ml | 17 |
| >10ng/ml | 4 |
| [0,1–2] | |
| [0,1–2]Tumor stage after RP | |
| T2a | 4 |
| T2b | 4 |
| T2c | 11 |
| T3b | 2 |
| [0,1–2] | |
| [0,1–2]Gleason score | |
| 6 | 10 |
| 7 | 9 |
| 8 | 2 |
| [0,1–2] | |
| [0,1–2]Tumor affectation | |
| Unilateral | 6 |
| Bilateral | 15 |
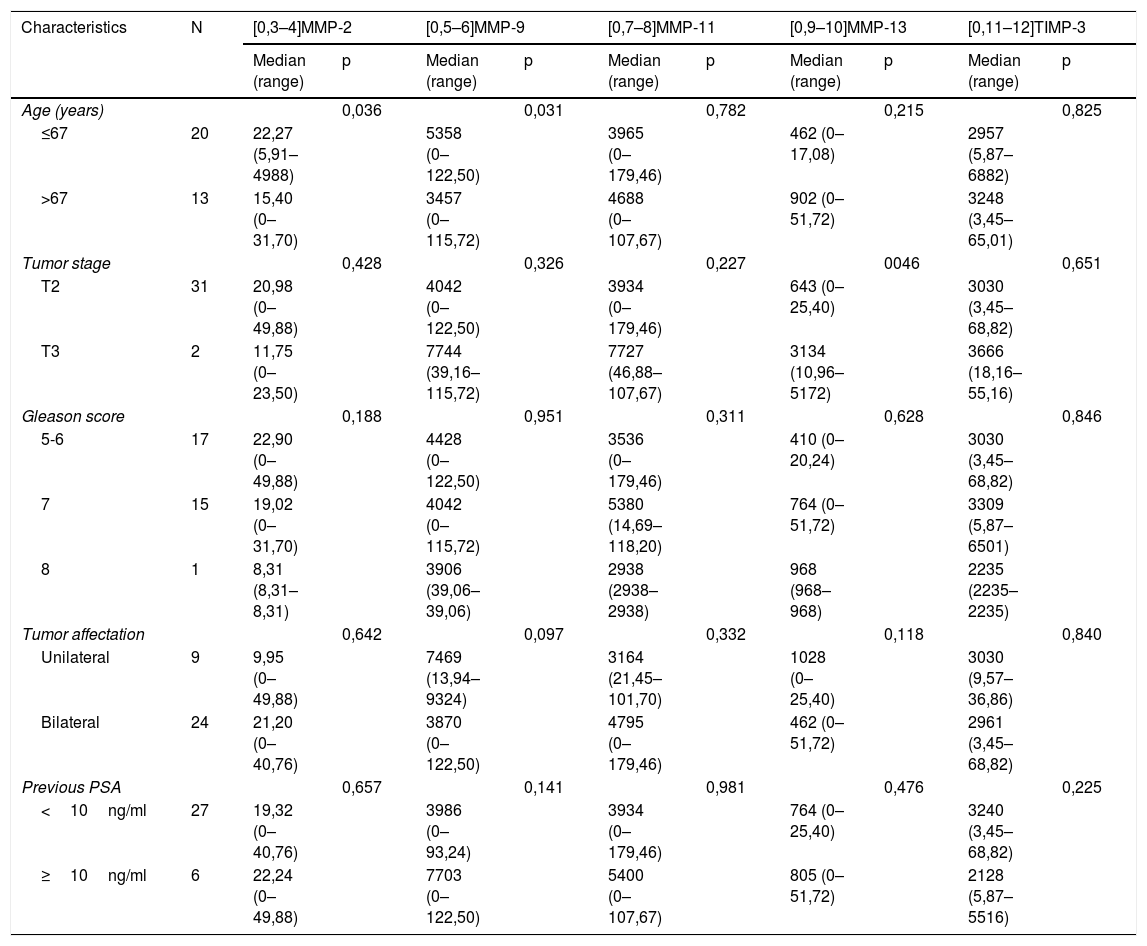
When analyzing the relationship between the scores of the different factors with the clinicopathological characteristics, we only found a significant association between the expression of MMP-13 with tumor stage; tumors in more advanced stages showed higher MMP expressions p=0,046 Table 2).
Relationship of the overall expression of MMP and TIMP-3 (score) in radical prostatectomy tumor area with the clinicopathological characteristics of the patients.
| Characteristics | N | [0,3–4]MMP-2 | [0,5–6]MMP-9 | [0,7–8]MMP-11 | [0,9–10]MMP-13 | [0,11–12]TIMP-3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (range) | p | Median (range) | p | Median (range) | p | Median (range) | p | Median (range) | p | ||
| Age (years) | 0,036 | 0,031 | 0,782 | 0,215 | 0,825 | ||||||
| ≤67 | 20 | 22,27 (5,91–4988) | 5358 (0–122,50) | 3965 (0–179,46) | 462 (0–17,08) | 2957 (5,87–6882) | |||||
| >67 | 13 | 15,40 (0–31,70) | 3457 (0–115,72) | 4688 (0–107,67) | 902 (0–51,72) | 3248 (3,45–65,01) | |||||
| Tumor stage | 0,428 | 0,326 | 0,227 | 0046 | 0,651 | ||||||
| T2 | 31 | 20,98 (0–49,88) | 4042 (0–122,50) | 3934 (0–179,46) | 643 (0–25,40) | 3030 (3,45–68,82) | |||||
| T3 | 2 | 11,75 (0–23,50) | 7744 (39,16–115,72) | 7727 (46,88–107,67) | 3134 (10,96–5172) | 3666 (18,16–55,16) | |||||
| Gleason score | 0,188 | 0,951 | 0,311 | 0,628 | 0,846 | ||||||
| 5-6 | 17 | 22,90 (0–49,88) | 4428 (0–122,50) | 3536 (0–179,46) | 410 (0–20,24) | 3030 (3,45–68,82) | |||||
| 7 | 15 | 19,02 (0–31,70) | 4042 (0–115,72) | 5380 (14,69–118,20) | 764 (0–51,72) | 3309 (5,87–6501) | |||||
| 8 | 1 | 8,31 (8,31–8,31) | 3906 (39,06–39,06) | 2938 (2938–2938) | 968 (968–968) | 2235 (2235–2235) | |||||
| Tumor affectation | 0,642 | 0,097 | 0,332 | 0,118 | 0,840 | ||||||
| Unilateral | 9 | 9,95 (0–49,88) | 7469 (13,94–9324) | 3164 (21,45–101,70) | 1028 (0–25,40) | 3030 (9,57–36,86) | |||||
| Bilateral | 24 | 21,20 (0–40,76) | 3870 (0–122,50) | 4795 (0–179,46) | 462 (0–51,72) | 2961 (3,45–68,82) | |||||
| Previous PSA | 0,657 | 0,141 | 0,981 | 0,476 | 0,225 | ||||||
| <10ng/ml | 27 | 19,32 (0–40,76) | 3986 (0–93,24) | 3934 (0–179,46) | 764 (0–25,40) | 3240 (3,45–68,82) | |||||
| ≥10ng/ml | 6 | 22,24 (0–49,88) | 7703 (0–122,50) | 5400 (0–107,67) | 805 (0–51,72) | 2128 (5,87–5516) | |||||
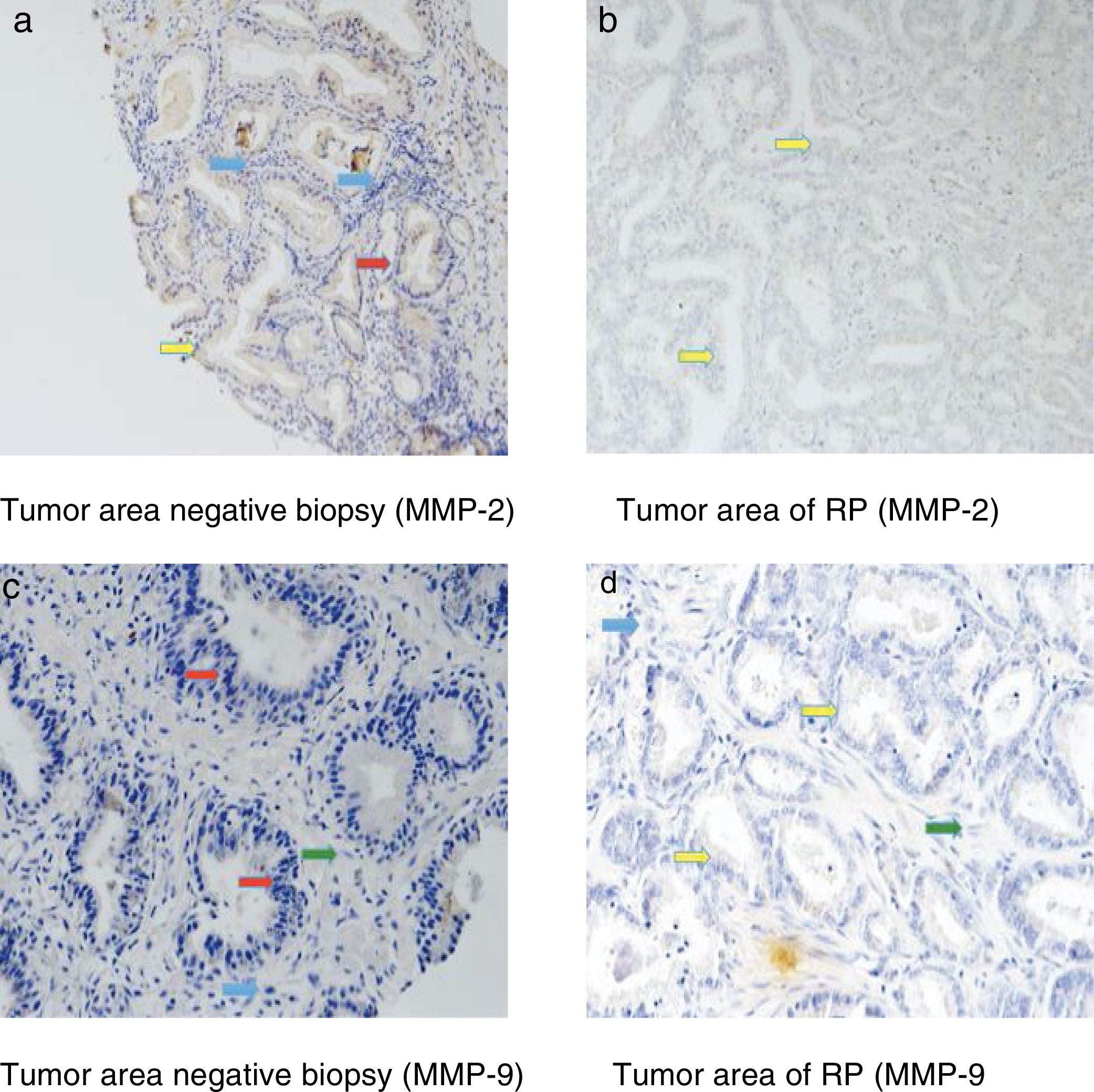
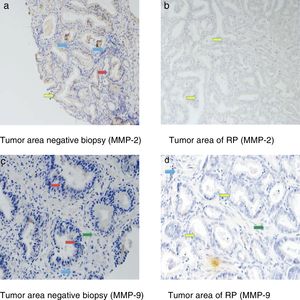
Fig. 3 shows examples of immunohistochemical stains for the various factors in which we found differences in our study. The dyed area of each MMP in all the analyzed groups is indicated by arrows: epithelial cells (red arrow), tumor cells (yellow arrow), fibroblasts (green arrow) and mononuclear inflammatory cells (blue arrow).
Immunohistochemical stains were located in the epithelial cells of the negative biopsies and in the tumor cells of RP, but also in the fibroblasts and in the mononuclear inflammatory cells in both tissue samples.
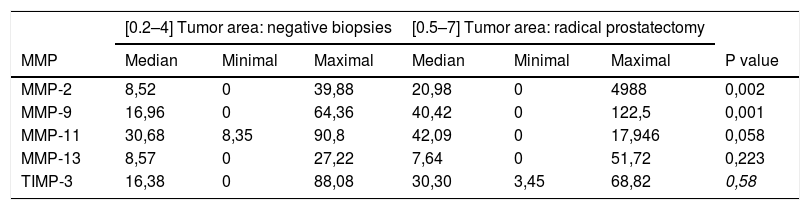
Table 3 shows the global staining values score for each factor in the tissue samples corresponding to the negative biopsies and in the tumor area of the RP specimens. As can be seen in this table, there were no significant differences in the expression of MMP-11, MMP-13 or TIMP-3 between these 2 differentiated tissue samples. However, we observed that the expressions of MMP-2 and MMP-9 were significantly higher in the tumor area of RP than in negative prostate biopsies p=0,002 y p=0,001, respectively.
Comparison of the factor expressions (score) between the tumor area in negative biopsies and radical prostatectomies.
| [0.2–4] Tumor area: negative biopsies | [0.5–7] Tumor area: radical prostatectomy | ||||||
|---|---|---|---|---|---|---|---|
| MMP | Median | Minimal | Maximal | Median | Minimal | Maximal | P value |
| MMP-2 | 8,52 | 0 | 39,88 | 20,98 | 0 | 4988 | 0,002 |
| MMP-9 | 16,96 | 0 | 64,36 | 40,42 | 0 | 122,5 | 0,001 |
| MMP-11 | 30,68 | 8,35 | 90,8 | 42,09 | 0 | 17,946 | 0,058 |
| MMP-13 | 8,57 | 0 | 27,22 | 7,64 | 0 | 51,72 | 0,223 |
| TIMP-3 | 16,38 | 0 | 88,08 | 30,30 | 3,45 | 68,82 | 0,58 |
Due to the comorbidities that may occur to patients (need for repeat biopsies with their possible complications, anxiety in patients and their families before a clear diagnosis since a tumor cannot be ruled out), an adequate diagnosis of PCa -especially in patients with low PSA- is currently a complicated task. Therefore, we have investigated the relevance of MMP analysis in the tumor area of previous negative biopsies and RP, in case there were changes that could indicate possible tumor development in these patients.
Factors that were previously found high in PCa were analyzed. The expression of MMP-2 is increased in PCa,10 associating in most cases a worse prognosis,11 increasing tumor aggressiveness12 and creating a favorable environment for the development and growth of tumor cells.13 We have found differences when comparing the previous negative biopsy and the PR, observing an increased MMP-2 expression in tumor cells in RP with respect to the epithelial cells of the biopsy p=0,002.
PCa patients have a higher proportion of serum and tissue levels of MMP-9.9 In addition to being associated with increased activity in tumor prostate tissue versus hyperplastic tissue,14 its expression was higher in patients with metastatic carcinoma in comparison to localized carcinoma.15 Same as with MMP-2, we found a greater expression of MMP-9 in the tumor area of the RP specimen compared to previous negative biopsies.
MMP-11 is expressed primarily by peritumoral stromal cells,16 this being its main differentiation with other MMPs. The expression of MMP-11 has been related to aggressive prostate tumors17 and a decrease in cancer-specific survival18 but has not showed relation to the diagnosis of the tumor. We did not find differences in the expression of MMP-11 between the tumor area and the negative biopsies in our study.
MMP-13 is expressed by different PCa cell lines, PCa tissue and BPH.19 However, we have not obtained differences when comparing the expression of MMP-13 between the neoplastic and non-neoplastic tissue analyzed.
Both MMP-11 and MMP-13 can be very good therapeutic targets for PCa inhibition, as described in previous studies.20
TIMP-3 is capable of inhibiting growth, invasion and metastasis of various types of cancers21 such as brain, colon and non-small-cell lung. No differences were found in the expression of TIMP-3 between tumor and non-tumor samples when conducting the study.
Taking into account the relevance that PCa has in our usual clinical practice, we need biomarkers that can more accurately predict the risk of suffering significant PCa and thereby prevent patients from undergoing unnecessary biopsies.
Prostate carcinogenesis is complex, which is why it is difficult to analyze and study the molecular genetics of PCa. The increased knowledge about prostate cancer genetics has provided us with a better understanding of the mechanisms that produce it, being very useful to reach a deeper knowledge of the biology underlying the conversion of "benign" tumors from the histological point of view in aggressive processes in the clinical aspect, with the aim of being able to develop new preventive and therapeutic strategies in this disease. With this objective, new biomarkers that may be useful for these purposes have been showed in recent years. PTEN is a gene that encodes a protein/lipid phosphatase and undergoes significant mutations or deletions, in localized as well as metastatic prostate tumors.22 Ki67 is a cell proliferation antigen, which is expressed in both benign and malignant prostate tissue. In addition, its increased expression in PCa has been related to Ki67 and p53 expressions.23 P27 is a protein that suppresses the cell cycle, slowing down cell division. The loss of its expression can accelerate tumorigenesis by enabling the progress of cells throughout the cell cycle and has been associated with biochemical recurrence in patients with PCa undergoing RP.24 Bcl-2 is an essential regulator of apoptosis and can act as an oncogene and a tumor suppressor gene. It can be expressed in healthy and tumor prostate tissue, it prevents apoptosis, and its overexpression has been related to high Gleason score values, development of hormone refractory prostate cancer and worse prognosis.25 P53 is one of the most important hormone suppression genes in cancer, it plays an important role in apoptosis and cell cycle control. Its decreased expression is associated with uncontrolled cell proliferation, reduction of apoptosis, and relevantly active in the progression of locally advanced and metastatic cancer.26 However, none of these biomarkers is routinely employed in the current clinical practice. MMPs have a multifactorial dimension, as they are involved in different paths of tumor development.
The results of the present study indicate a variability in the expression of MMP/TIMP in prostate tissues with suspected cancer, the overexpression of MMP-2 and -9 may be useful to show the presence of a tumor even in the case of a negative biopsy. In the future, the combination with other markers may facilitate the determination of molecular profiles to select patients with negative biopsies who may require additional biopsies and the area in which the biopsy should be performed. The recent application of multiparametric magnetic resonance imaging and fusion biopsies has facilitated this process, but the association of biomarkers such as the evaluated MMPs can make it even more reliable.
Therefore, we consider that the study of MMPs expression with some of the factors indicated in our work can contribute to greater diagnostic and/or prognostic accuracy in PCa, in addition to showing an effectiveness that should be explored in subsequent studies.
We have encountered several limitations when carrying out the study. The main one derived from the difficulties in finding a larger sample size, since patients were required to present a PCa in a localized stage and had undergone RP and at least one negative biopsy before being diagnosed with PCa. In addition, an adequate histological sample (biopsy) is necessary, and the location of the tissue to be analyzed in the biopsy and in the PR should be determined (a significant number of patients had to be discarded for this reason, limiting the number of cases obtained). Another limitation that we encountered was the wide heterogeneity of our series and the long time required for data collection.
ConclusionsWhen analyzing tumor areas of the negative biopsies and RPs, a greater overall expression of the MMP-2 and MMP-9 was evidenced in the RP specimens compared to negative biopsies. There were no differences between the expressions of MMP-11, MMP-13 and TIMP-3 when analyzing previous negative biopsies with RP specimens. Therefore, according to the data obtained in our study, an increased MMP-2 and MMP-9 expression could favor tumor progression.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Medina-González A. et al. Análisis comparativo de la expresión de metaloproteasas (MMP-2, MMP-9, MMP-11 y MMP-13) y del inhibidor tisular de la metaloproteasa 3 (TIMP-3) entre las biopsias previas negativas y las prostatectomías radicales. Actas Urol Esp. 2020;44:78–85.